Abstract
Sutherlandia frutescens is a medicinal plant traditionally used to treat various types of human diseases, including cancer. Previous studies of several botanicals link suppression of prostate cancer growth with inhibition of the Gli/hedgehog (Gli/Hh) signaling pathway. Here we hypothesized the anti-cancer effect of S. frutescens was linked to its inhibition of the Gli/Hh signaling in prostate cancer. We found a dose- and time- dependent growth inhibition in human prostate cancer cells PC3 and LNCaP, and mouse prostate cancer cells TRAMP-C2 treated with S. frutescens methanol extract (SLE). We also observed a dose-dependent inhibition of the Gli-reporter activity in Shh Light II and TRAMP-C2QGli cells treated with SLE. In addition, SLE can inhibit Gli/Hh signaling by blocking Gli1 and Ptched1 gene expression in the presence of a Gli/Hh signaling agonist (SAG). A diet supplemented with S. frutescens suppressed the formation of poorly differentiated carcinoma in prostates of TRAMP mice. Finally, we found Sutherlandioside D was the most potent compound in the crude extract that could suppress Gli-reporter in Shh Light II cells. Together this suggests that the S. frutescens extract may exert anti-cancer effect by targeting Gli/Hh signaling, and Sutherlandioside D is one of the active compounds.
Keywords: Sonic Hedgehog, Sutherlandia frutescens, prostate cancer
1. Introduction
Prostate cancer is the second leading cause of cancer-related deaths in men, behind only lung cancer. The latest American Cancer Society report has estimated 220,800 new cases of prostate cancer in the United States in 2015 and predicted more than 27,540 deaths from prostate cancer in the same year (Siegel et al., 2015). Primary prostate tumors are typically dependent on androgenic steroids and therefore are commonly treated by androgen depletion. However, many tumors recur with hormone therapy-resistance and develop into castration-recurrent prostate cancer that is more difficult to efficiently control and cure. These advanced-stage prostate tumors seem to be systemic androgen-independent but are believed to utilize endogenous androgen and/or other signaling pathways to drive their uncontrolled proliferation (Chen et al., 2011b). In addition, hormone depletion therapy has several adverse side effects due to abrupt change of hormone levels (estrogen and testosterone) in the circulation system. Therefore, novel treatments that can specifically target abnormal signaling pathway(s) in prostate cancer but that have fewer side effects are currently being sought after (Karhadkar et al., 2004, Ng and Curran, 2011, Pasca di Magliano and Hebrok, 2003).
The Hh signaling pathway is composed of ligands (Sonic hedgehog (Shh), Desert hedgehog (Dhh) and Indian hedgehog (Ihh)), the transmembrane receptors Patched (PTCH1) and Smoothened (SMO), and downstream transcription factor Gli. In the absence of a ligand, SMO activity is suppressed by PTCH and thus Gli is sequestered in the cytoplasm (Jiang and Hui, 2008). In the presence of the hedgehog ligand, SMO is relieved from the repression by PTCH and activates the translocation of downstream transcription factor Gli. During embryogenesis, the Hh pathway regulates cell proliferation, differentiation and patterning. However, the Hh pathway activity decreases in most tissues during postnatal development. In adults, the Hh pathway signaling is only active in several tissues and is important for the maintenance of adult stem cell (Han et al., 2008). Dysregulation of Hh signaling during embryonic development causes physical malformations such as fused fingers, and rib and facial abnormalities, whereas increased Hh signaling in adults often leads to cancer, such as medulloblastoma, basal cell carcinoma (Ng and Curran, 2011), small cell lung cancer (Watkins et al., 2003), colorectal cancer (Berman et al., 2003), pancreatic adenocarcinoma (Thayer et al., 2003) and advanced prostate cancer (Chen et al., 2011b, Karhadkar et al., 2004). Specific inhibitors against the Hh signaling pathway shrink tumors in preclinical tumor xenograft models (Datta and Datta, 2006). Several Hh signaling inhibitors are currently in clinical trials with one (Erivedge) recently approved by the US Food and Drug Administration (De Smaele et al., 2010, Lorusso et al., 2011, Ng and Curran, 2011, Rudin et al., 2009, Von Hoff et al., 2009).
In the prostate, Gli/Hh signaling is essential for the regeneration of the epithelium. Enhanced pathway activity transforms progenitor cells into tumorigenic cells and may promote the transition of localized cancer into metastatic prostate cancer (Karhadkar et al., 2004). Blocking the Gli/Hh signaling by cyclopamine or by RNA interference of Gli expression suppresses the proliferation of several human prostate cancer cell lines (Sanchez et al., 2004). However, cyclopamine is not a favorable agent because of its rapid clearance, non-specific toxicity and off-target effects at high concentrations (Lipinski et al., 2008). Therefore, screening novel inhibitors against Gli/Hh pathway may be an effective way to inhibit prostate cancer proliferation and tumorigenesis (Fan et al., 2004, Sanchez et al., 2005, Sheng et al., 2004).
S. frutescens commonly known as the “cancer bush” by South African healers, is a medicinal plant traditionally used to treat many human diseases, including cancer (Chadwick et al., 2007, Grandi et al., 2005, Prevoo et al., 2004, Van Wyk et al., 2002, Van Wyk, 2011, Van Wyk and Albrecht, 2008). Ethanolic extract of S. frutescens showed dose-dependent anti-proliferative and apoptotic effects on several human tumors including the breast cancer cell line MCF-7 (Vorster et al., 2012). A similar inhibition can also be achieved by aqueous extract (Stander et al., 2009, Steenkamp and Gouws, 2006, Tai et al., 2004, Vorster et al., 2012). Chinkwo et al. demonstrated that an aqueous extract induced cellular apoptosis in cervical carcinoma and Chinese Hamster Ovary (CHO) cells (Chinkwo, 2005). Nevertheless, the molecular mechanism and signaling pathway(s) that are perturbed by S. frutescens in human cancers have been largely unclear. Previous studies in our lab demonstrated botanical compounds potentially prevent prostate cancer via inhibition of the Gli/Hh signaling pathway (Slusarz et al., 2010). In this study, we hypothesized that the anti-cancer effects of S. frutescens were due to the inhibition of the Gli/Hh signaling pathway. We found significant inhibition of Gli/Hh signaling by S. frutescens extract in prostate cancer cell lines and suppression of prostatic tumorigenesis in TRAMP mice fed with S. frutescens.
2. Materials and Methods
2.1 Ethics statement
All animal experiments were approved by the Animal Care and Use Committee at the University of Missouri-Columbia. All mice in the colony were checked for overall health daily during feeding and watering. Staff looked for indicators of health such as mobility, general appearance (as judged by hunched posture, piloerection, increased respiratory effort, lethargy, coat condition, weight loss, diarrhea, etc.) and body condition. Mice over 5 weeks of age had weekly body weight and condition assessed.
2.2 Preparation of S. frutescens methanol extract
Dried S. frutescens vegetative material sourced from Big Tree Nutraceutical and Thebe Natural Medicines (Cape Town, South Africa) was extracted with methanol and filtered to remove residual solids. The methanol was removed by rotary evaporation under a vacuum at 70 °C. The components were then dissolved in ethanol to a concentration of 50 mg/mL and referred to as S. frutescens extract (SLE).
2.3 Cell culture
All cell culture reagents were purchased from Life Technologies (Carlsbad, CA, USA) unless indicated. All cell lines were obtained from the American Type Culture Collection (ATCC). Human prostate cancer cell lines PC3 and LNCaP and mouse prostate cancer cell line TRAMP-C2 were cultured in complete RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 4.5 g/mL glucose, 4 mM L-glutamine, 100 μM nonessential amino acids, 10 mM HEPES, 1 mM sodium pyruvate, and 1% penicillin/streptomycin (Pen/Strep).
Shh Light II cells (JHU-68) were derived from the NIH/3T3 cell line that was co-transfected with Gli-responsive firefly luciferase reporter and constitutive Renilla-luciferase reporter (Sasaki et al., 1997). These cells were maintained in DMEM medium supplemented with 10% newborn calf serum, 0.4 mg/mL G418, 0.15 mg/mL zeocin, 4 mM L-glutamine, 0.05% HEPES and 1.5 mg/mL sodium pyruvate.
Conditioned medium (CM) was generated from an HEK293 cell line overexpressing the Shh-N-terminal peptide (HEK293-ShhN, a gift from Dr. Philip Beachy, Stanford University) that binds PTCH and stimulates the Gli/Hh pathway. HEK293-ShhN cells were grown to 80–90% confluence in DMEM medium containing 10% FBS and 40 mg/mL G418. The medium was then replaced with DMEM containing 2% FBS. After 24–30 h, the supernatant was collected, filtered through a 0.22 μm filter and stored at −80 °C.
Cyclopamine, an antagonist of Smoothened (Pasca di Magliano and Hebrok, 2003), was purchased from LC Laboratories (Woburn, MA, USA). In Solution™ Smoothened Agonist (SAG, EMD Millipore, Billerica, MA, USA) served as an agonist of Smoothened that can stimulate the Hh signaling pathway (Chen et al., 2002b).
2.4 Stable transfection of TRAMP-C2 cells with Gli-reporter
TRAMP-C2 cells (ATTC CRL-2731) were maintained in RPMI-1640 medium supplemented with 10 mM HEPES, 5% FBS and 5% newborn calf serum and 1% Pen/Strep. In general 1 × 104 cells per well were seeded in a 96-well plate (Corning Incorporated, Corning, NY, USA) for 18 h prior to transfection. Two vectors, pEGFP-C1 (Clontech, Mountain View, CA, USA) and Cignal Gli reporter/luc (Qiagen, Valencia, CA, USA), were mixed with TransIt Prostate transfection reagent (Mirus Bio, Madison, WI, USA) for 30 min, and then added to the cells at 20 and 200 ng per well of a 96-well plate, respectively. After 2 d, the cells were transferred into culture medium with 100 μg/mL G418. The surviving colonies were expanded and sorted by flow cytometry. The selected cells were then replated at low density to obtain separate colonies. The single colonies were transferred into a 96-well plate, expanded and tested for the Gli activity by using the Dual Luciferase Assay system (Promega, Madison, WI, USA). We referred to the stably transfected cells as TRAMP-C2QGli cells.
2.5 Gli-reporter assay in Shh Light II cells
The Gli reporter assay was performed with Shh Light II cells. Confluent cells were treated for 48 h with vehicle, CM, SAG or SLE in phenol red-free DMEM medium supplemented with 0.5% charcoal-stripped newborn calf serum. The Gli-reporter activity was then measured by the Dual Luciferase Assay System.
2.6 Protein assay
The cells were seeded in 24-well plates and grown to 50% confluence. The medium was then replaced with phenol red-free RPMI 1640 supplemented with 10% charcoal-stripped FBS with treatments. After 72 h, the medium was removed and the cells were washed twice with PBS. The cells were then lysed with 1 N NaOH overnight. The IC50 (the concentration required to inhibit cell growth by 50%) was calculated relative to the vehicle treated control. A time-course assay with intervals of 24, 48 and 72 h was also conducted for each IC50. Total protein concentration was measured by using a DC protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA).
2.7 RNA isolation and real-time qPCR
Total RNA was extracted with the RNeasy Mini kit (Qiagen) following the manufacturer’s instruction. RNA concentration was determined by using a NanoDrop 1000 Spectrophotometer v3.1. The cDNA was prepared with a High Capacity cDNA Reverse Transcription Kit (Life Technologies, Grand Island, NY, USA). Real-time qPCR was performed by using iQ™ SYBR Green Supermix and reactions were run in an iCycler IQ Single-Color Real Time PCR Detection System (Bio-Rad). Gradient dilutions (10-fold increments) of control cDNAs (derived from the cells highly expressing target genes) were used to create standard curves and the GAPDH was used as a calibrator gene. The primer sequences are listed in Table 1.
Table 1.
Primer sequence.
| Gene | Sequence |
|---|---|
| hGli1 | 5′ AGCTAGAGTCCAGAGGTTCAA 3′ 5′ TAGACAGAGGTTGGGAGGTAAG 3′ |
| hPTCH1 | 5′ CCTCGGGAAACCAGAGAATATG 3′ 5′ AAACTCCTGTGTAGGTCGTAAAG 3′ |
| hGAPDH | 5′ GGTGTGAACCATGAGAAGTATGA 3′ 5′ GAGTCCTTCCACGATACCAAAG 3′ |
| mGli1 | 5′ GGAAGTCCTATTCACGCCTTGA 3′ 5′ CAACCTTCTTGCTCACACATGTAAG 3′ |
| mPTCH1 | 5′ CTCTGGAGCAGATTTCCAAGG 3′ 5′ TGCCGCAGTTCTTTTGAATG 3′ |
| mGAPDH | 5′ AGCCTCGTCCCGTAGACAAAAT 3′ 5′ CCGTGAGTGGAGTCATACTGGA 3′ |
2.8 TRAMP mouse studies
Male B6FVB-F1 TRAMP mice were bred as previously described following the guidelines approved by Animal Care and Use Committee at the University of Missouri (Animal Welfare Assurance Number A3394-01) (Slusarz, 2010). Mice were fed a semi-purified, casein-based rodent diet (i.e., AIN-93G) until six weeks of age, at which point they were randomly assigned to treatment groups. To examine the effects of S. frutescens on prostatic tumorigenesis, mice were fed either a casein-based “control” diet or an AIN-93G diet containing 0.05%, 0.25%, or 1% (wt/wt) of ground S. frutescens in place of an equal amount of cornstarch. At 5 months of age, mice were euthanized and various tissues were collected. The reproductive tract (testes, vas deferens, urinary bladder, seminal vesicles and prostate lobes) was weighed, sectioned and fixed in neutral buffered formalin and paraffin embedded for histological analysis. The remainders of tissues were snap-frozen in liquid nitrogen and stored at −80 °C for future studies. Tissue sections were stained with hematoxylin and eosin, and examined by light microscopy for cancer stages by a veterinary pathologist who was blind to the treatment (Shappell et al., 2004). The dorsal prostates were staged as normal, hyperplasia (HYP), prostatic intraepithelial neoplasia (PIN), well-differentiated carcinoma (WDC), moderately differentiated carcinoma (MDC), or poorly differentiated carcinoma (PDC) (Kaplan-Lefko et al., 2003, Shenouda et al., 2007). PDC was further divided into large PDC and micro PDC. We classified large PDC as those that could be observed grossly at the time of necropsy whereas micro PDC was those that could only be visualized by microscopy.
2.9 Statistical Analysis
Data were analyzed with GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA, USA). P-values were generated by using an unpaired two-tailed t-test. In vivo experiments were analyzed by using the Chi-squared test to determine significance.
3. Results
3.1 S. frutescens extract (SLE) inhibited the growth of human and mouse prostate cancer cell lines
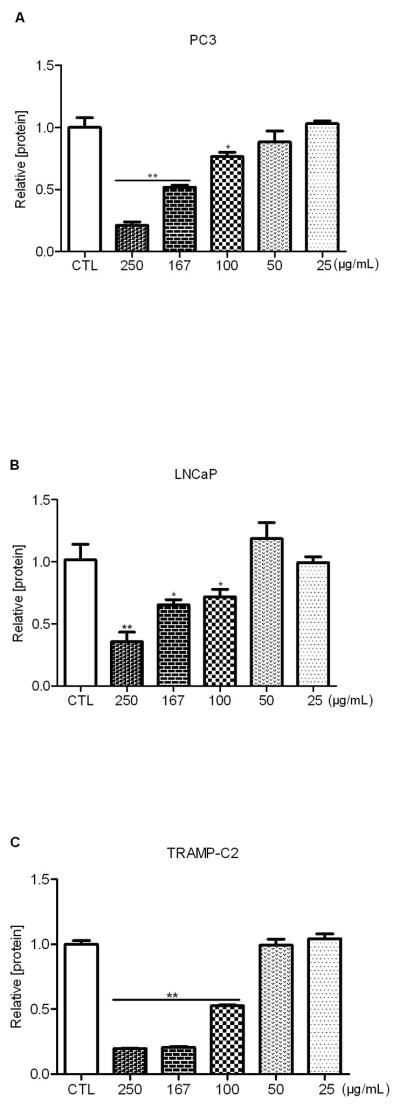
We first tested the inhibitory effect of SLE in three cell lines: human prostate cancer cell lines PC3 (androgen receptor negative) and LNCaP (androgen receptor positive), and mouse prostate cell line TRAMP-C2. We found that SLE suppressed the growth of PC3 at multiple dilutions including 250 μg/mL, 167 μg/mL, and 100 μg/mL with an IC50 of 167 μg/mL (Fig. 1A). The effect of growth inhibition diminished at 50 μg/mL or lower. Similarly, SLE inhibited growth at the above dilutions in LNCaP cells but with an IC50 of 200 μg/mL (Fig. 1B). In addition, mouse prostate cancer cell line TRAMP-C2 showed the sharpest dose-response inhibition with an IC50 of 100 μg/mL (Fig. 1C). However, no inhibition was observed at the concentrations of 50 μg/mL or lower in TRAMP-C2 cells. In addition, normal prostate cell line RWPE-1 showed less cytotoxicity in response to SLE compared to cancer cells (Fig. S1). Together, these data indicate that SLE can inhibit the proliferation of human and mouse prostate cancer cell lines in a dose-dependent manner but have no obvious growth inhibition on normal prostate cells. It appears that the inhibitory effect of SLE is independent of androgen receptors as SLE exhibits growth inhibition in both androgen receptor (AR) positive (LNCaP and TRAMP-C2) and AR negative (PC3) prostate cancer cells.
Figure 1. S. frutescens extract (SLE) inhibited the growth of human and mouse prostate cancer cells.
Human prostate cancer cell lines PC3 (A) and LNCaP (B), and the mouse prostate cancer cell line TRAMP-C2 (C) were treated with multiple concentrations of SLE for 72 h. Total protein was then measured and normalized to vehicle-treated controls (CTL). A dose-dependent inhibition was observed with an IC50 of 167 μg/mL in PC3, 200 μg/mL in LNCaP, and 100 μg/mL in TRAMP-C2, respectively. Each experiment was conducted at least thrice in duplicate. Error bars indicate standard deviation (SD). * P<0.05, ** P<0.01.
3.2 Time-course inhibition of prostate cancer cell proliferation by S. frutescens extract (SLE)
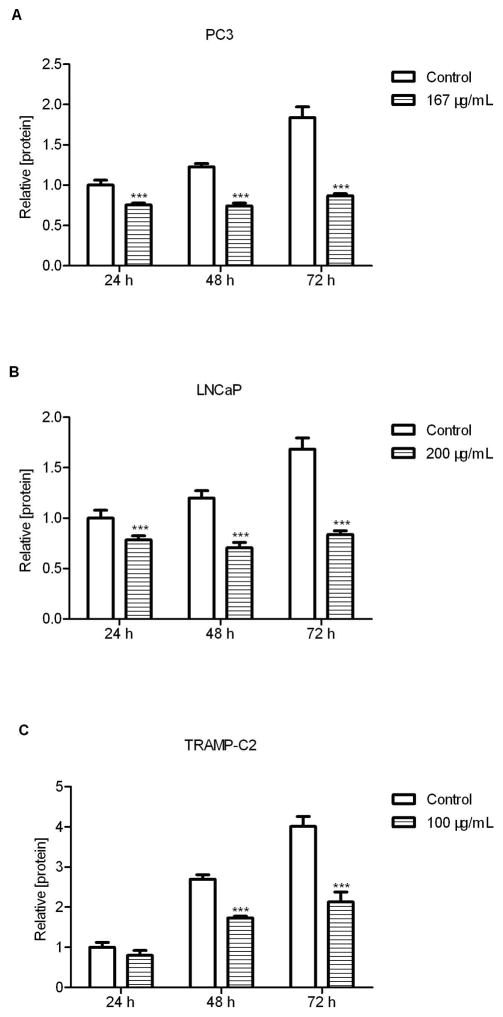
Total proteins from PC3 (Fig. 2A), LNCaP (Fig. 2B), and TRAMP-C2 cells (Fig. 2C) treated with their respective IC50 concentrations of SLE were extracted at different time points (24 h, 48 h and 72 h). The significant growth inhibition occurred within 24 h treatment of PC3 and LNCap cells (P<0.001, Fig. 2A and 2B) and by 48 h in TRAMP-C2 cells (Fig. 2C). The growth activity was reduced by 35%, 41% and 36% at 48 h in PC3, LNCap and TRAMP-C2 cells, respectively. After prolonged incubation (72 h) with SLE, these prostate cancer cells significantly lost the proliferation ability by approximately 50% when compared with the controls (P<0.001). The results highlighted that 72 h of SLE treatment could significantly disrupt the proliferation of prostate cancer cells. However, the inhibitory effects required persistent presence of SLE because SLE withdrawal led to regrowth of prostate cancer cells (data not shown).
Figure 2. Time-course inhibition of cell growth by S. frutescens extract (SLE).
PC3 (A) and LNCaP (B) and TRAMP-C2 (C) were treated with the IC50 of 167 μg/mL, 200 μg/mL, and 100 μg/mL for 24, 48, and 72 h. Then total protein was measured and normalized to the control of 24 h. After 72h, SLE could inhibit the growth of three cell lines by approximately 50%. Each experiment was performed thrice in duplicate. Error bars indicate standard deviation (SD). * P<0.05, ** P<0.01, *** P<0.001.
3.3 S. frutescens extract (SLE) suppressed the Gli/Hh signaling in Shh Light II cell line
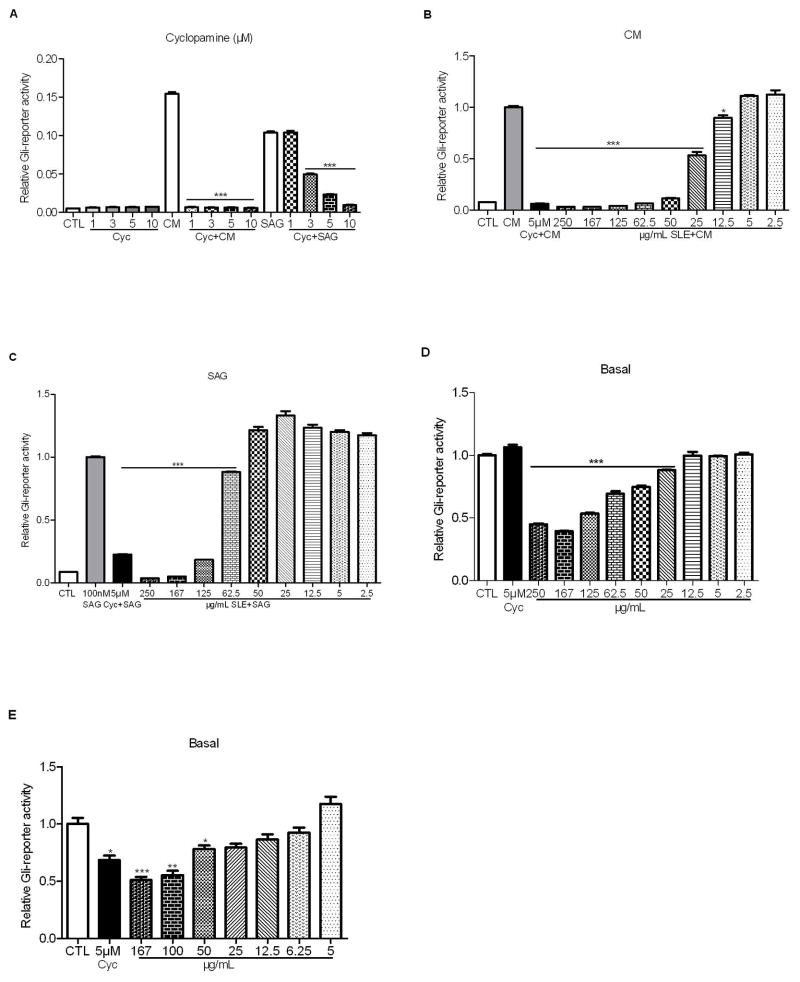
We first tested the effect of cyclopamine on the Gli/Hh pathway activity with or without CM stimulation. As expected, in the absence of Shh ligand cyclopamine couldn’t reduce the basal Gli-reporter activity in Shh Light II cells even at the concentration of 10 μM. However, cyclopamine (1 μM–10 μM) showed significant inhibition (P<0.001) when Shh ligand was present. Similarly, high concentrations of cyclopamine (3 μM–10 μM) overrode the stimulatory effect of SAG in Shh light II cells, though lower concentration (1 μM) failed to block Shh signaling (Fig. 3A). The data suggest that cyclopamine can suppress Gli/Hh pathway when the pathway is activated by Hh ligands or SAG.
Figure 3. S. frutescens extract (SLE) suppressed the Gli/Hh signaling in Shh Light II and TRAMP-C2QGli cells.
A: Cyclopamine (Cyc) inhibited Gli-reporter activity in Shh Light II cells that were stimulated by conditioned medium (CM) or SAG. B: In the presence of CM, SLE significantly blocked Gli-reporter activity. C: In the presence of SAG, SLE significantly inhibited Gli-reporter activity. D: SLE but not cyclopamine inhibited the basal Gli-reporter activity in Shh Light II cells. E: SLE significantly inhibited the basal Gli-reporter activity in a dose-dependent manner in TRAMP-C2QGli cells. Each experiment was conducted at least thrice in triplicate. Error bars indicate standard deviation (SD). * P<0.05, ** P<0.01, *** P<0.001.
To understand whether the anti-proliferation effects of SLE may act through the inhibition of Gli/Hh signaling pathway, we measured Gli reporter activity in Shh Light II cells exposed to multiple concentrations of SLE. The stimulatory effect of CM was counteracted by the SLE at concentrations between 12.5 μg/mL and 250 μg/mL (Fig. 3B). SLE at concentrations higher than 62.5 μg/mL significantly offset the stimulatory effect of SAG (P<0.001). Nevertheless, further dilution of SLE (<62.5 μg/mL) resulted in the loss of the inhibitory capability of SLE against SAG (Fig. 3C). SLE also significantly inhibited basal Gli reporter activity at concentrations between 25–250 μg/mL (P<0.001) (Fig. 3D). In contrast, cyclopamine had no effect on basal Gli/Hh pathway activity. Both cyclopamine and SLE could significantly decrease basal Gli reporter activity in TRAMP-C2QGli cells (Fig. 3E). Together, these results show that SLE blocks both basal and elevated Gli/Hh signaling activity in Shh Light II cells regardless of the presence of CM or SAG. Therefore, this suggests that SLE inhibits Gli/Hh signaling independent of SMO, or perhaps at a site on SMO different from where cyclopamine and SAG bind. Alternatively, SLE may exhibit the inhibition directly on Gli or other components of the Gli/Hh signaling pathway downstream of SMO.
3.4 S. frutescens extract (SLE) suppressed Gli/Hh gene expression
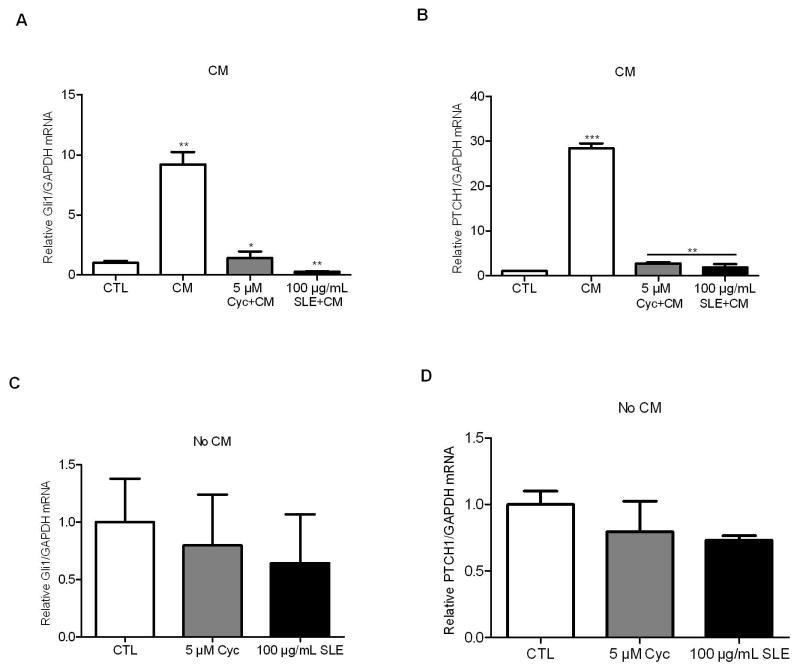
To test whether SLE affects the prostate cancer cells via inhibiting the Gli/Hh gene expression, Gli1 and PTCH1 mRNA abundance was measured by real-time qPCR upon SLE administration. CM resulted in a 10- and 28-fold stimulation of Gli1 and PTCH1 gene expression compared to untreated TRAMP-C2 cells (Fig. 4A and 4B). However, either cyclopamine (5 μM) or SLE (100 μg/mL) significantly inhibited the expression of Gli1 (Fig. 4A) and PTCH1 (Fig. 4B) in the presence of CM. Cyclopamine significantly decreased Gli1 mRNA abundance by 85% (P<0.05) whereas SLE did by 97% (P<0.01). Both cyclopamine and SLE reduced PTCH1 mRNA abundance by 90% in TRAMP-C2 cells. However, in unstimulated TRAMP-C2 cells, neither cyclopamine nor SLE affected the basal mRNA levels of Gli1 and PTCH1 (Fig. 4C and 4D).
Figure 4. S. frutescens extract (SLE) decreased GLI1 and PTCH1 mRNAs in TRAMP-C2 cells.
CM stimulated Gli1 (A) and Ptch1 (B) expression in TRAMP-C2 cells by 10- and 28-fold, respectively. SLE significantly blocked Gli1 (A) and Ptch1 (B) expression in the presence of CM and cyclopamine showed similar effects. However, neither Gli1 (C) nor Ptch1 (D) mRNAs were significantly changed in the presence of cyclopamine or SLE in unstimulated TRAMP-C2 cells. Each experiment was performed three times in duplicate. * P<0.05, ** P<0.01. Error bars indicate SD.
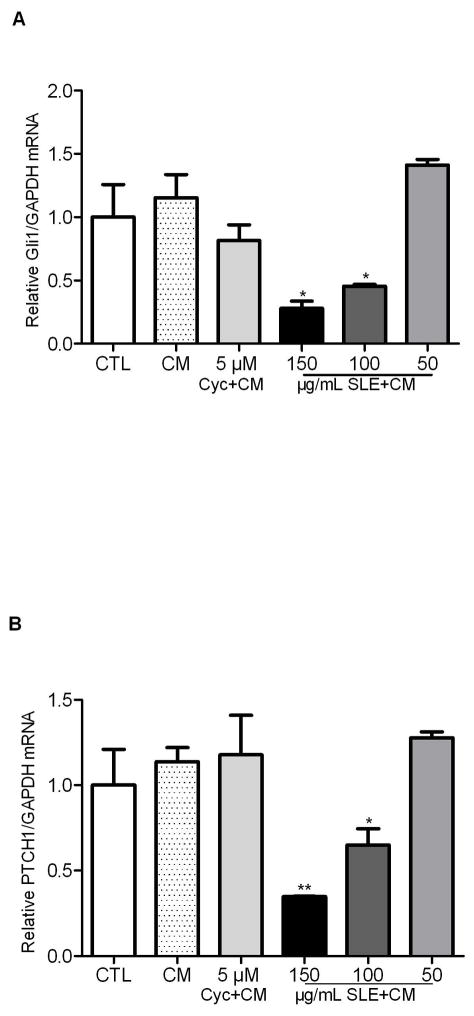
In contrast to TRAMP-C2 cells, CM didn’t significantly stimulate Gli1 or PTCH1 expression, though a trend of slight increase was observed in human PC3 cells (Fig. 5). Cyclopamine didn’t show any inhibition on basal Gli1 or PTCH1 mRNA abundance. However, SLE at concentrations of 150 μg/mL and 100 μg/mL significantly suppressed the Gli1 (P<0.05) and PTCH1 (P<0.01) expression in the presence of CM (Fig. 5A and 5B). The inhibitory effect diminished when the SLE concentration fell below 50 μg/mL. Together, this suggests that SLE could inhibit the CM-stimulated Gli1 and PTCH1 gene expression in TRAMP-C2 cells and the basal GLI1 and PTCH1 mRNA abundance in PC3 cells.
Figure 5. S. frutescens extract (SLE) reduced GLI1 and PTCH1 mRNA abundance in PC3 cells.
Compared with CM groups, 150 μg/mL and 100 μg/mL of SLE significantly decreased GLI1 (A) and PTCH1 (B) mRNA abundance in PC3 cells, although these reductions were not seen at 50 μg/mL of SLE. Neither CM nor cyclopamine could affect GLI1 or PTCH1 expression in PC3 cells. Each experiment was performed three times in duplicate. Error bars indicate SD. * P<0.05, ** P<0.01.
3.5 Sutherlandioside D is the most potent compound in the S. frutescens methanol extract
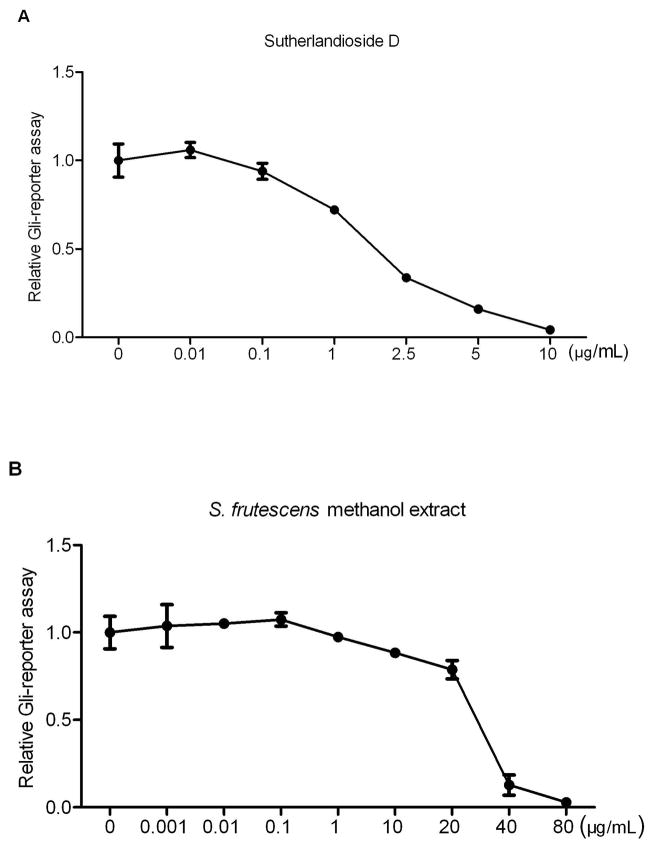
Several fractions of S. frutescens crude extract were separated by high-speed countercurrent chromatography then assessed at concentrations of 10, 1 and 0.1 μg/mL with stimulated Shh Light II cells for 48 h (Fig. S2). At the concentration of 10 μg/mL, the fraction S17 (sutherlandioside D) was the most potent inhibitor which could inhibit the Gli-reporter activity by 89%, followed by S8 (sutherlandiosides A/C/D, 84%), S6 (sutherlandiosides A/B/C/D, 54%), S46 (sutherlandins C&D, 29%), S10 (sutherlandioside B, 22%), S4 (sutherlandins A/B/C/D, 10%) and S31 (sutherlandins A&B, 10%). High-performance liquid chromatography and evaporative light scattering detection (HPLC-ELSD) assay showed about 0.3% of sutherlandioside D in the whole methanol extract. In stimulated Shh Light II cells, the IC50 of sutherlandioside D was 1.8 μg/mL (Fig. 6A) whereas the crude extract required 30 μg/mL for half inhibition (Fig. 6B). Therefore, it appears that sutherlandioside D is the most active compound in the whole S. frutescens extract.
Figure 6. Comparison between Sutherlandioside D and S. frutescens crude extract on the inhibition of Gli-reporter activity in Shh Light II cells.
Sutherlandioside D showed half inhibition of Gli-reporter activity at the concentration of 1.8 μg/mL, but S. frutescens crude extract did at 30 μg/mL. Each experiment was conducted three times in triplicate. Error bars indicate SD.
3.6 In vivo inhibition of prostatic carcinogenesis by dietary S. frutescens
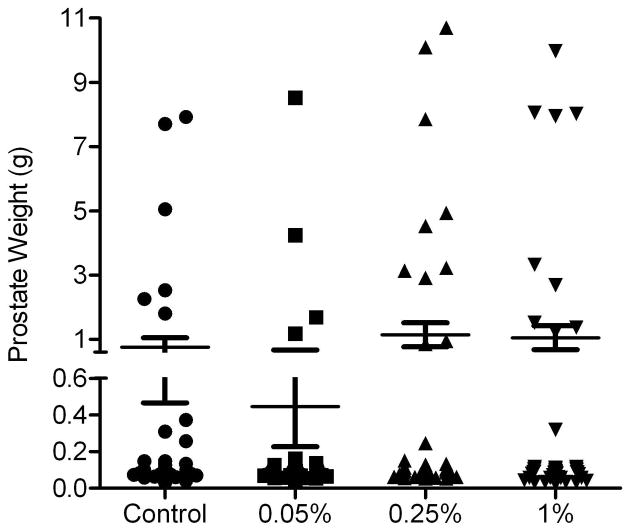
To investigate the effects of S. frutescens on prostatic tumorigenesis, diets supplemented with dry S. frutescens at three concentrations (0.05%, 0.25%, or 1% (wt/wt)) were fed to TRAMP mice that spontaneously developed autochthonous prostate tumors. Mice fed the diet containing 0.05% of S. frutescens exhibited a reduction in incidence of large poorly differentiated carcinomas (PDC) (grossly evident at necropsy) compared to the control group. The difference in large PDC incidence was matched with prostate size (Fig. 7). For the non-cancer phenotype (i.e., normal, hyperplasia, and prostatic intraepithelial neoplasia), we did not observe any significant difference between the treatment groups and control (Table 2). In addition, mice fed with 0.05% S. frutescens supplements displayed the smallest average prostate weight (Fig. 7). These data indicate that S. frutescens could suppress advanced-stage (PDC) prostate cancer growth but are less effective in non-malignant neoplasia and hyperplasia of TRAMP mice.
Figure 7. Effects of S. frutescens on the prostate weight of TRAMP mice.
S. frutescens-containing diet (0.05%) showed the fewest number of large prostates (>1g) and the lowest average prostate weight. Each symbol represents the weight for a single prostate. Error bars indicate standard error of the mean (SEM).
Table 2.
Effects of S. frutescens on the prostate tumor development in TRAMP mice.
| TRAMP Histology Phenotype | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Diet | n | Non-cancer
|
Cancer
|
|||||
| Norm | Hyp | PIN | WDC | MDC | Micro PDC | Large PDC | ||
| Control | 39 | 0 | 0 | 9(23%) | 13(33%) | 0 | 6(15%) | 11(28%)* |
| 0.05% Sutherlandia | 41 | 0 | 0 | 10(24%) | 15(37%) | 0 | 11(27%) | 5(12%)* |
| 0.25% Sutherlandia | 46 | 0 | 0 | 7(15%) | 20(43%) | 0 | 9(20%) | 10(22%) |
| 1% Sutherlandia | 37 | 0 | 0 | 9(24%) | 10(27%) | 1(3%) | 6(16%) | 11(30%) |
Chi-squared test was used to compare the large PDC incidence to the micro PDC incidence between treatment and control groups.
P<0.05.
4. Discussion
Elevated hedgehog pathway activity has been observed in advanced human prostate cancer specimens (Karhadkar et al., 2004, Sanchez et al., 2004, Sheng et al., 2004, Stecca et al., 2005). Therefore, blocking Gli/Hh signaling may be an alternative treatment for advanced prostate cancer. In this study, we found that S. frutescens extract inhibited the growth of prostate cancer cell lines and suppressed PDC formation in the prostate of TRAMP mice. S. frutescens extract also inhibited Gli/Hh signaling activity by reducing Gli1 and PTCH1 gene expression in TRAMP-C2 and PC3 cells. Of the fractions, sutherlandioside D was the most potent compound in suppressing Gli-reporter activity in Shh Light II cells. Thus, S. frutescens extract may be effective in inhibiting prostate cancer proliferation and tumorigenesis via blocking Gli/Hh signaling.
Both S. frutescens and cyclopamine can suppress the growth of prostate cancer cells in vitro and in vivo (Karhadkar et al., 2004, Sanchez et al., 2004). However, they may target different components in the Gli/Hh signaling pathway. Cyclopamine blocks Gli/Hh signaling by directly binding to Smoothened and affecting the balance of active and inactive forms (Chen et al., 2002a). In contrast, Sutherlandia extract inhibits Gli/Hh signaling by decreasing Gli1 and Patched1 expression. However, they can both suppress the Gli-reporter activity regardless of CM and SAG stimulation. Therefore, we propose that Sutherlandia extract inhibits Gli/Hh signaling by targeting the downstream compounds, whereas cyclopamine binds a site of Smoothened different from the SAG binding site.
S. frutescens extract has been demonstrated to affect cell growth and induce apoptosis in many cancer cell lines (Chinkwo, 2005, Skerman et al., 2011, Tai et al., 2004), such as prostate cancer cell lines DU-145, PC-3 and LNCaP (Chen et al., 2007, Steenkamp and Gouws, 2006). The main secondary metabolite within the leaves and stems of S. frutescens include D-pinitol, L-canavanine, γ-amino butyric acid, four cycloartenol glycosides (sutherlandiosides A-D) and four flavonoids (sutherlandins AD) (Avula et al., 2010, Van Wyk and Albrecht, 2008). The active compounds in the Sutherlandia extract have been elusive so far. Although L-canavanine has been documented to have anti-cancer effect (Van Wyk and Albrecht, 2008), it only showed weak inhibition of Gli-reporter activity in Shh Light II cells (data not shown). In contrast, sutherlandioside D had the strongest inhibition of Gli-reporter activity among the fractions (Fig. S2). It has been reported that four cycloartane glycosides, sutherlandiosides A-D, are present in commercial S. frutescens material, as is SU1, the major triterpenoid (Fu et al., 2008, Van Wyk and Albrecht, 2008). Furthermore, major triterpenoids of S. frutescens are structurally related to cycloartane-type tritepenoids which have cancer chemopreventive activity (Kikuchi et al., 2007). Since it is uncertain about which novel compounds are responsible for the many benefits of S. frutescent, the discovery of sutherlandioside D’s potent Gli/Hh inhibitory activity is a promising avenue for future research.
The Gli/Hh signaling pathway is important for the proliferation, progression and metastasis of prostate cancer, and Gli/Hh signaling is usually upregulated in prostate cancer (Chen et al., 2011a, Domenech et al., 2012, Fan et al., 2004, Karhadkar et al., 2004, Sanchez et al., 2004, Sheng et al., 2004). In this study, SLE is shown to suppress the basal and stimulated Gli-reporter activity in Shh Light II cells, whereas cyclopamine can only block stimulated Gli activity. The ability of SLE to inhibit the Gli/Hh pathway in both basal and stimulated conditions may be important for treating prostate tumors with different mechanisms of Gli/Hh pathway activation (Ruiz i Altaba et al., 2007). Therefore, SLE may not only inhibit tumors with elevated Gli/Hh signaling, but also has the potential to kill preneoplastic cells with basal Gli/Hh activity.
In the absence of Gli/Hh pathway activity, the basal Gli1 and PTCH1 mRNA levels were not significantly altered by either cyclopamine or SLE treatment in the TRAMP-C2 cells, albeit they were reduced slightly with SLE treatment (Fig. 4C and 4D). When Gli/Hh pathway was stimulated by conditioned medium in TRAMP-C2 cells, Gli1 and Patched1 gene expression was significantly suppressed by either cyclopamine or SLE. It appears that Gli/Hh pathway can be stimulated in TRAMP-C2 cells, indicating the presence of paracrine hedgehog signaling. In contrast, hedgehog signaling is not functional in PC3 cells even after stimulation with CM. Although PC3 cells express Hh signaling components, Gli1 knock-down, rather than cyclopamine treatment, is more effective for the inhibition of PC3 cell proliferation, suggesting that PC3 proliferation relies more on functional Gli1 but not activated Hh signaling (Sanchez et al., 2004). SLE can significantly inhibit the basal Gli1 and PTCH1 expression in PC3 cells, but the similar inhibition couldn’t be achieved by cyclopamine. Therefore, SLE may block the expression of Gli or target the Gli/Hh signaling components downstream of SMO in both PC3 and TRAMP-C2 cells. The PC3 cells are androgen-insensitive whereas the TRAMP-C2 cells are androgen-sensitive with their growth highly dependent on androgens. The crosstalk between Gli/Hh and androgen may contribute to the differential response of Gli and PTCH1 to conditioned medium in PC3 and TRAMP-C2 cells (Chen et al., 2011b).
TRAMP mice fed with the diet containing 0.05% of S. frutescens had fewer large PDC tumors when compared with control mice. Increasing concentration of S. frutescens didn’t show stronger inhibition of PDC tumors. This is inconsistent with the in vitro observation in TRAMP-C2 cells where there was a strong dose-dependent inhibition. The discrepancy may be attributed to the different preparations of S. frutescens and in vivo and in vitro conditions. In vitro, we treated cancer cells with a methanolic extraction of S. frutescens redissolved in ethanol, whereas in vivo we administered a crude ground mixture of the S. frutescens leaves directly into the diet. In addition, the optimal concentration for in vivo inhibition may be different from the in vitro experiments because the S. frutescens compounds must be absorbed by the gastrointestinal system to reach the prostate gland in TRAMP mice. Regarding the safety of S. frutescens, we didn’t observe any cytotoxicity until the dose was above 250 μg/mL in the tested normal prostate cells or cancer cells. Similarly, no cytotoxicity was observed when the methanolic extract of S. frutescens (400μg/mL) was administered to the cervical cancer cell line HeLaP4 (Bessong et al., 2005). In addition, S. frutescens water extract was a less potent blocker of Gli/Hh signaling pathway than the methanolic extract (Fig. S3). Consumption of 800 mg of leaf powder per day by healthy adults had no apparent side effects (Johnson et al., 2007). Thus, it appears that S. frutescens contains safe and potent compounds that can prevent and inhibit prostate cancer development possibly through interfering Gli/Hh signaling pathway.
In summary, S. frutescens extract inhibits the growth of prostate cancer cells both in vitro and in vivo, possibly through suppression of the Gli/Hh signaling pathway. Although the direct targets of SLE on the Gli/Hh pathway are unclear, they are predicted to be downstream components, such as Gli. Thus, S. frutescens extract is potentially safe and effective for the prevention and treatment of advanced prostate cancers with upregulated Gli/Hh signaling activity.
Supplementary Material
Acknowledgments
Funding
We thank all the undergraduate students for the care of the TRAMP mice; Christal Dawn Huber for the technical assistance in vivo experiment; and Dr. P. A. Beachy (Stanford University) for providing the HEK293-ShhN cell line. This study was supported by the grants from the National Center for Complementary and Alternative Medicines (NCCAM), the Office of Dietary Supplements (ODS) and the National Cancer Institute (NCI). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM, ODS, NCI, or the National Institutes of Health.
Abbreviations
- CM
Conditioned Medium
- Hh
Hedgehog
- HYP
Hyperplasia
- MDC
Moderately Differentiated Carcinoma
- PDC
Poorly Differentiated Carcinoma
- PIN
Prostatic Intraepithelial Neoplasia
- PTCH1
Patched
- Shh
Sonic hedgehog
- SLE
S. frutescens methanol extract
- SMO
Smoothened
- TRAMP
Transgenic Adenocarcinoma Mouse Prostate
- WDC
Well-differentiated Carcinoma
References
- Avula B, Wang Y-H, Smillie TJ, Fu X, Li XC, Mabusela W, Syce J, Johnson Q, Folk W, Khan IA. Quantitative determination of flavonoids and cycloartanol glycosides from aerial parts of Sutherlandia frutescens (L.) R. BR. by using LC-UV/ELSD methods and confirmation by using LC–MS method. Journal of Pharmaceutical and Biomedical Analysis. 2010;52:173–80. doi: 10.1016/j.jpba.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman JR, Watkins DN, Beachy PA. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–51. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- Bessong PO, Obi CL, Andreola ML, Rojas LB, Pouysegu L, Igumbor E, Meyer JJ, Quideau S, Litvak S. Evaluation of selected South African medicinal plants for inhibitory properties against human immunodeficiency virus type 1 reverse transcriptase and integrase. J Ethnopharmacol. 2005;99:83–91. doi: 10.1016/j.jep.2005.01.056. [DOI] [PubMed] [Google Scholar]
- Chadwick WA, Roux S, van de Venter M, Louw J, Oelofsen W. Anti-diabetic effects of Sutherlandia frutescens in Wistar rats fed a diabetogenic diet. J Ethnopharmacol. 2007;109:121–7. doi: 10.1016/j.jep.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Chen G, Goto Y, Sakamoto R, Tanaka K, Matsubara E, Nakamura M, Zheng H, Lu J, Takayanagi R, Nomura M. GLI1, a crucial mediator of sonic hedgehog signaling in prostate cancer, functions as a negative modulator for androgen receptor. Biochem Biophys Res Commun. 2011a;404:809–15. doi: 10.1016/j.bbrc.2010.12.065. [DOI] [PubMed] [Google Scholar]
- Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002a;16:2743–8. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JK, Taipale J, Young KE, Maiti T, Beachy PA. Small molecule modulation of Smoothened activity. Proc Natl Acad Sci U S A. 2002b;99:14071–6. doi: 10.1073/pnas.182542899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Carkner R, Buttyan R. The hedgehog/Gli signaling paradigm in prostate cancer. Expert review of endocrinology & metabolism. 2011b;6:453–67. doi: 10.1586/EEM.11.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC. MSc. University of Missouri-Columbia; 2007. Bioactivities of selected Sutherlandia frutescens (L.) R.Br.leaf extracts. [Google Scholar]
- Chinkwo KA. Sutherlandia frutescens extracts can induce apoptosis in cultured carcinoma cells. J Ethnopharmacol. 2005;98:163–70. doi: 10.1016/j.jep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Datta S, Datta MW. Sonic Hedgehog signaling in advanced prostate cancer. Cellular and Molecular Life Sciences CMLS. 2006;63:435–48. doi: 10.1007/s00018-005-5389-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smaele E, Ferretti E, Gulino A. Vismodegib, a small-molecule inhibitor of the hedgehog pathway for the treatment of advanced cancers. Curr Opin Investig Drugs. 2010;11:707–18. [PubMed] [Google Scholar]
- Domenech M, Bjerregaard R, Bushman W, Beebe DJ. Hedgehog signaling in myofibroblasts directly promotes prostate tumor cell growth. Integr Biol (Camb) 2012;4:142–52. doi: 10.1039/c1ib00104c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Pepicelli CV, Dibble CC, Catbagan W, Zarycki JL, Laciak R, Gipp J, Shaw A, Lamm ML, Munoz A, Lipinski R, Thrasher JB, Bushman W. Hedgehog signaling promotes prostate xenograft tumor growth. Endocrinology. 2004;145:3961–70. doi: 10.1210/en.2004-0079. [DOI] [PubMed] [Google Scholar]
- Fu X, Li XC, Smillie TJ, Carvalho P, Mabusela W, Syce J, Johnson Q, Folk W, Avery MA, Khan IA. Cycloartane glycosides from Sutherlandia frutescens. Journal of natural products. 2008;71:1749–53. doi: 10.1021/np800328r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandi M, Roselli L, Vernay M. Lessertia (Sutherlandia frutescens) et la fatigue en cancérologie*. Phytotherapie. 2005;3:110–3. [Google Scholar]
- Han Y-G, Spassky N, Romaguera-Ros M, Garcia-Verdugo J-M, Aguilar A, Schneider-Maunoury S, Alvarez-Buylla A. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008;11:277–84. doi: 10.1038/nn2059. [DOI] [PubMed] [Google Scholar]
- Jiang J, Hui CC. Hedgehog signaling in development and cancer. Developmental cell. 2008;15:801–12. doi: 10.1016/j.devcel.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson Q, Syce J, Nell H, Rudeen K, Folk WR. A randomized, double-blind, placebo-controlled trial of Lessertia frutescens in healthy adults. PLoS Clin Trials. 2007;2:e16. doi: 10.1371/journal.pctr.0020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan-Lefko PJ, Chen TM, Ittmann MM, Barrios RJ, Ayala GE, Huss WJ, Maddison LA, Foster BA, Greenberg NM. Pathobiology of autochthonous prostate cancer in a pre-clinical transgenic mouse model. Prostate. 2003;55:219–37. doi: 10.1002/pros.10215. [DOI] [PubMed] [Google Scholar]
- Karhadkar SS, Bova GS, Abdallah N, Dhara S, Gardner D, Maitra A, Isaacs JT, Berman DM, Beachy PA. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–12. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Akihisa T, Tokuda H, Ukiya M, Watanabe K, Nishino H. Cancer Chemopreventive Effects of Cycloartane-Type and Related Triterpenoids in in Vitro and in Vivo Models. Journal of natural products. 2007;70:918–22. doi: 10.1021/np068044u. [DOI] [PubMed] [Google Scholar]
- Lipinski RJ, Hutson PR, Hannam PW, Nydza RJ, Washington IM, Moore RW, Girdaukas GG, Peterson RE, Bushman W. Dose- and route-dependent teratogenicity, toxicity, and pharmacokinetic profiles of the hedgehog signaling antagonist cyclopamine in the mouse. Toxicological sciences : an official journal of the Society of Toxicology. 2008;104:189–97. doi: 10.1093/toxsci/kfn076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorusso PM, Jimeno A, Dy G, Adjei A, Berlin J, Leichman L, Low JA, Colburn D, Chang I, Cheeti S, Jin JY, Graham RA. Pharmacokinetic dose-scheduling study of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with locally advanced or metastatic solid tumors. Clin Cancer Res. 2011;17:5774–82. doi: 10.1158/1078-0432.CCR-11-0972. [DOI] [PubMed] [Google Scholar]
- Ng JM, Curran T. The Hedgehog’s tale: developing strategies for targeting cancer. Nature reviews Cancer. 2011;11:493–501. doi: 10.1038/nrc3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasca di Magliano M, Hebrok M. Hedgehog signalling in cancer formation and maintenance. Nature reviews Cancer. 2003;3:903–11. doi: 10.1038/nrc1229. [DOI] [PubMed] [Google Scholar]
- Prevoo D, Smith C, Swart P, Swart AC. The Effect of Sutherlandia frutescens on Steroidogenesis: Confirming Indigenous Wisdom. Endocrine Research. 2004;30:745–51. doi: 10.1081/erc-200044020. [DOI] [PubMed] [Google Scholar]
- Rudin CM, Hann CL, Laterra J, Yauch RL, Callahan CA, Fu L, Holcomb T, Stinson J, Gould SE, Coleman B, LoRusso PM, Von Hoff DD, de Sauvage FJ, Low JA. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. The New England journal of medicine. 2009;361:1173–8. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz i Altaba A, Mas C, Stecca B. The Gli code: an information nexus regulating cell fate, stemness and cancer. Trends in Cell Biology. 2007;17:438–47. doi: 10.1016/j.tcb.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez P, Clement V, Ruiz i Altaba A. Therapeutic targeting of the Hedgehog-GLI pathway in prostate cancer. Cancer research. 2005;65:2990–2. doi: 10.1158/0008-5472.CAN-05-0439. [DOI] [PubMed] [Google Scholar]
- Sanchez P, Hernández AM, Stecca B, Kahler AJ, DeGueme AM, Barrett A, Beyna M, Datta MW, Datta S, Ruiz i Altaba A. Inhibition of prostate cancer proliferation by interference with SONIC HEDGEHOG-GLI1 signaling. Proc Natl Acad Sci U S A. 2004;101:12561–6. doi: 10.1073/pnas.0404956101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H, Hui C, Nakafuku M, Kondoh H. A binding site for Gli proteins is essential for HNF-3beta floor plate enhancer activity in transgenics and can respond to Shh in vitro. Development. 1997;124:1313–22. doi: 10.1242/dev.124.7.1313. [DOI] [PubMed] [Google Scholar]
- Shappell SB, Thomas GV, Roberts RL, Herbert R, Ittmann MM, Rubin MA, Humphrey PA, Sundberg JP, Rozengurt N, Barrios R, Ward JM, Cardiff RD. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res. 2004;64:2270–305. doi: 10.1158/0008-5472.can-03-0946. [DOI] [PubMed] [Google Scholar]
- Sheng T, Li C, Zhang X, Chi S, He N, Chen K, McCormick F, Gatalica Z, Xie J. Activation of the hedgehog pathway in advanced prostate cancer. Mol Cancer. 2004;3:29. doi: 10.1186/1476-4598-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenouda NS, Sakla MS, Newton LG, Besch-Williford C, Greenberg NM, MacDonald RS, Lubahn DB. Phytosterol Pygeum africanum regulates prostate cancer in vitro and in vivo. Endocrine. 2007;31:72–81. doi: 10.1007/s12020-007-0014-y. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- Skerman NB, Joubert AM, Cronje MJ. The apoptosis inducing effects of Sutherlandia spp. extracts on an oesophageal cancer cell line. J Ethnopharmacol. 2011;137:1250–60. doi: 10.1016/j.jep.2011.07.054. [DOI] [PubMed] [Google Scholar]
- Slusarz A, Shenouda NS, Sakla MS, Drenkhahn SK, Narula AS, MacDonald RS, Besch-Williford CL, Lubahn DB. Common botanical compounds inhibit the hedgehog signaling pathway in prostate cancer. Cancer research. 2010;70:3382–90. doi: 10.1158/0008-5472.CAN-09-3012. [DOI] [PubMed] [Google Scholar]
- Stander A, Marais S, Stivaktas V, Vorster C, Albrecht C, Lottering ML, Joubert AM. In vitro effects of Sutherlandia frutescens water extracts on cell numbers, morphology, cell cycle progression and cell death in a tumorigenic and a non-tumorigenic epithelial breast cell line. J Ethnopharmacol. 2009;124:45–60. doi: 10.1016/j.jep.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Stecca B, Mas C, Altaba ARi. Interference with HH–GLI signaling inhibits prostate cancer. Trends in Molecular Medicine. 2005;11:199–203. doi: 10.1016/j.molmed.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Steenkamp V, Gouws MC. Cytotoxicity of six South African medicinal plant extracts used in the treatment of cancer. South African Journal of Botany. 2006;72:630–3. [Google Scholar]
- Tai J, Cheung S, Chan E, Hasman D. In vitro culture studies of Sutherlandia frutescens on human tumor cell lines. J Ethnopharmacol. 2004;93:9–19. doi: 10.1016/j.jep.2004.02.028. [DOI] [PubMed] [Google Scholar]
- Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernandez-del Castillo C, Yajnik V, Antoniu B, McMahon M, Warshaw AL, Hebrok M. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–6. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wyk B-E, Van Oudtshoorn B, Gericke N. Medicinal plants of South Africa. Pretoria: Briza Publications; 2002. [Google Scholar]
- Van Wyk BE. The potential of South African plants in the development of new medicinal products. South African Journal of Botany. 2011;77:812–29. [Google Scholar]
- Van Wyk BE, Albrecht C. A review of the taxonomy, ethnobotany, chemistry and pharmacology of Sutherlandia frutescens (Fabaceae) J Ethnopharmacol. 2008;119:620–9. doi: 10.1016/j.jep.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Von Hoff DD, LoRusso PM, Rudin CM, Reddy JC, Yauch RL, Tibes R, Weiss GJ, Borad MJ, Hann CL, Brahmer JR, Mackey HM, Lum BL, Darbonne WC, Marsters JC, Jr, de Sauvage FJ, Low JA. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. The New England journal of medicine. 2009;361:1164–72. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- Vorster C, Stander A, Joubert A. Differential signaling involved in Sutherlandia frutescens-induced cell death in MCF-7 and MCF-12A cells. Journal of ethnopharmacology. 2012;140:123–30. doi: 10.1016/j.jep.2011.12.045. [DOI] [PubMed] [Google Scholar]
- Watkins DN, Berman DM, Burkholder SG, Wang B, Beachy PA, Baylin SB. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2003;422:313–7. doi: 10.1038/nature01493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.