Abstract
Diabetes mellitus (DM) is a high risk factor for stroke and leads to more severe vascular and white-matter injury than stroke in non-DM. We tested the neurorestorative effects of delayed human umbilical cord blood cell (HUCBC) treatment of stroke in type-2 diabetes (T2DM). db/db-T2DM and db/+-non-DM mice were subjected to distal middle cerebral artery occlusion (dMCAo) and were treated 3 days after dMCAo with: 1) non-DM + PBS; 2) T2DM + PBS; 3) T2DM + naïve-HUCBC; 4) T2DM + miR-126−/−HUCBC. Functional evaluation, vascular and white-matter changes, neuroinflammation, and miR-126 effects were measured in vivo and in vitro. T2DM mice exhibited significantly decreased serum and brain tissue miR-126 expression compared with non-DM mice. T2DM+HUCBC mice exhibited increased miR-126 expression, increased tight junction protein expression, axon/myelin, vascular density and M2-macrophage polarization; However, decreased blood-brain barrier leakage, brain hemorrhage and miR-126 targeted gene VCAM-1 and MCP-1 expression in the ischemic brain as well as improved functional outcome were present in HUCBC treated T2DM mice compare with control T2DM mice. MiR-126−/−HUCBC-treatment abolished the benefits of naïve-HUCBC-treatment in T2DM stroke mice. In vitro, knock-in of miR-126 in primary cultured brain endothelial cells (BECs) or treatment of BECs with naïve-HUCBCs significantly increased capillary-like tube formation, and increased axonal outgrowth in primary cultured cortical neurons; whereas treatment of BECs or cortical neurons with miR-126−/− HUCBC attenuated HUCBC-treatment induced capillary tube formation and axonal outgrowth. Our data suggest delayed HUCBC-treatment of stroke increases vascular/white-matter remodeling and anti-inflammatory effects; MiR-126 may contribute to HUCBC-induced neurorestorative effects in T2DM mice.
Keywords: microRNA126 (miR-126), human umbilical cord blood cell (HUCBC), type-2 diabetes (T2DM), white matter (WM), Stroke
Introduction
Stroke is a major cause of death and long-term disability with unusually high accompanying social and medical costs. Diabetes mellitus (DM) is a severe health problem associated with both microvascular and macrovascular disease and leads to a 3-4 fold higher risk of experiencing ischemic stroke [1]. Hyperglycemia and diabetes instigate a cascade of events leading to vascular endothelial cell dysfunction, increased vascular permeability [2], a disequilibrium of angiogenesis (exuberant but dysfunctional neovascularization), and poor recovery after ischemic stroke [3, 4]. In addition, diabetic patients are more prone to develop white matter (WM) high-intensity lesions, and DM-mice show more severely injured WM than non-DM mice after stroke [5]. Approximately, 30% of ischemic stroke patients have diabetes. According to the Stroke Therapy Academic Industry Roundtable (STAIR) and Stem Cell Therapy as an Emerging Paradigm for Stroke (STEPS) II guidelines, it is essential to investigate the effects of cell therapy for stroke on comorbid conditions such as diabetes [6].
Treatment of stroke has historically focused on neuroprotection with treatment initiated acutely, within the first few hours after stroke. However,, except for the NINDS rtPA trial [7], this approach has yielded failed trials. Because of a short therapeutic window and the potential for hemorrhagic transformation, only 3-4% of ischemic stroke patients are treated with rtPA [8]. After decades of research focused on acute neuroprotection and the failure of clinical trials to overcome this barrier, the Stroke Progress Review Group in 2006 and in 2011 identified delayed neurorestoration after stroke as a major priority for stroke research [9]. Therefore, there is a compelling need to develop and test delayed therapeutic approaches of stroke, with treatment initiated from days after stroke.
Human umbilical cord blood cells (HUCBCs) are a rich source of hematopoietic progenitor cells [10]. HUCB-derived mononuclear cells proliferate, and secrete factors possibly beneficial for the host brain tissue in vivo [10]. Previous studies have found that HUCBC treatment of stroke in non-DM and type-1 diabetic (T1DM) stroke animals improves functional outcome and induces neurorestorative effects [11, 12]. 90% of diabetic patients are type-2 diabetes (T2DM). There is also a differential response to treatment of stroke between DM and non-DM subjects [13-15]. The effect of delayed HUCBC treatment of stroke in the T2DM population has not been investigated. In this study, we elucidate the mechanisms of action of HUCBC as a neurorestorative therapy for stroke in T2DM mice when treatment is initiated 3 days after stroke.
MicroRNAs (miRs) are small non-coding sequences of RNA that have the capacity to regulate many genes, pathways, and complex biological networks within cells, acting either alone or in concert with one another [16]. MiRs are emerging as key players in the pathogenesis of T2DM and hyperglycemia-induced vascular damage [17]. Among the miRs most consistently associated with diabetes, is miR-126 [17, 18]. miR-126 facilitates angiogenesis and regulates endothelial cell function. MiR-126 level is significantly decreased in the circulating CD34+ peripheral blood mononuclear cell (PBMCs) in DM-patients [19]. Down-regulation of miR-126 expression in CD34+ PBMCs in diabetes provides a novel pathway causing impaired proangiogenic effects [18, 20]. Diminished proangiogenic capacity can be rescued by miR-mimic-126 transfer [20]. In the present study, we tested the neurorestorative actions of delayed HUCBC treatment of stroke in T2DM and hypothesize that generation of miR-126 by HUCBCs contributes to its robust therapeutic effects in T2DM mice.
MATERIALS AND METHOODS
Animal distal middle cerebral artery occlusion (dMCAo) model
Adult male BKS.Cg-m+/+Leprdb/J (db/db, T2DM) and db/+ (non-DM) control mice (age 12-14 weeks) were purchased from Jackson Laboratory (Wilmington, MA). Mice were subjected to right extraluminal permanent dMCAo, as previously described [21]. Briefly, animals were anesthetized with 1.5%~2% isoflorane using a facemask and maintained body temperature at 37 ± 0.5°C during surgery. Animal were placed in a stereotaxic apparatus and draped with sterile drapes, eyes were protected with sterile ophthalmic ointment. A midline incision was opened between the orbit and the ear and a small burr hole was produced in the skull. The main branch of the MCA was coagulated with a small heater probe, and the vessel then transected. The wound was closed with suture. Animals were received intensive care and continuous monitoring following dMCAo until they are capable of functioning normally.
Gain or loss of function of miR-126 in mouse brain endothelial cells (BECs) or in HUCBCs
To knock-in or knockdown miR-126 expression in BECs or HUCBCs, electroporation transfection was employed [22]. Our previous studies have found that electroporation transfection has lower toxicity and more efficacy than lentiviruses or lipofectamine transfection of miR in HUCBC. To knock-in miR-126 in BECs, mouse pEGP-mmu-mir-126 Expression vector (MMU-MiR-126 for miR-126 knock-in) or pLenti-III-miR-GFP knock-in Control Vector was used; To knockdown of miR-126 in HUCBCs, mmu-miR-126-3p inhibitor (miR-126 knockdown), or negative control inhibitor (Thermo Scientific) was performed using an electroporation transfection method [22], respectively. Briefly, mouse BECs (ATCC) and HUCBCs (Saneron CCEL Therapeutics) were harvested. Cells were rinsed with 1×PBS and then resuspended in 95ul Ingenio electroporation solution (Mirus) and 5ul of 20uM miR-126 mimic or inhibitor (Dharmacon). The cell suspension was then placed in an Ingenio cuvette (Mirus) and electroporated in a Lonza Nucleofector using program T-05 for BECs and Y-01 for HUCBC were then placed in growth media, BEC (DMEM with 10% FBS and 1% antibiotic/antimycotic (Life Technologies) and HUCBC (Stemline II media, Sigma) with 40 ng/ml stem cell factor, 40 ng/ml FLT3 and 10 ng/ml thrombopoietin, all from CellGenix) for two days prior to in vitro study or for injection. On the day HUCBCs were to be injected they were harvested and counted, then diluted to the appropriate concentration with 1× PBS.
Experimental groups
Three days after dMCAo, mice were randomized to intravenous injection via tail-vein with: 1) PBS as control; 2) naïve-HUCBC (1×106) (n=9/group). 3) miR-126 knockdown HUCBCs (miR-126−/−HUCBC, n=9). Blood glucose was measured in animals before dMCAo and sacrifice by using test strips for glucose (Polymer technology System, Inc. Indianapolis, IN 46268 USA). Mice were sacrificed at 14 days after dMCAo.
Functional test
Adhesive removal test [23] and food single-pellet reaching test to characterize skilled reaching ability of the stroke-impaired left forepaw [24] were performed 3 days (before treatment) and 7, 14 days after dMCAo by an investigator blinded to the experimental groups.
Histological and immunohistochemical assessment
The brains were fixed and coronal tissue sections (6μm, bregma −1mm to +1mm) were stained with hematoxylin and eosin (H&E) for calculation of cerebral lesion volume [25]. For immunostaining, antibodies against Perls Prussian Blue (PPB) to identify iron in cerebral parenchymal as a late-stage cerebral hemmohrage marker, albumin-FITC (1:500, Abcam, Cambridge, MA) to identify blood-brain barrier (BBB) leakage, alpha-smooth muscle actin (αSMA, 1:800; Dako), a smooth muscle cell maker to identify arteries, von-Willebrand Factor (vWF, a vessels marker, 1:400; Dako, Carpenteria, CA), Occludin (a tight junction protein, 1: 100, Invitrogen, Life Technologies), SMI-31 (a marker of phospho-neurofilament, 1:1000, Covance, CA), IBA-1 (a microglia marker, 1: 1000, Wako), CD206 (1: 3000, Abcam), and CD163 (1:500, Abcam) were employed. Bielschowsky-silver and Luxol-fast blue staining were used to demonstrate axons or myelin. Control experiments consisted of staining brain coronal tissue sections as outlined above, but non-immune serum was substituted for the primary antibody.
Immunostaining quantification
For quantitative measurements of Bielschowsky-silver and Luxol-fast blue, and IBA-1, five slides from each brain, with each slide containing 4 fields from striatum of the ischemic boundary zone (IBZ) were digitized under a 20× objective (Olympus BX40) using a 3-CCD color video camera (Sony DXC-970MD) interfaced with an MCID image analysis system (Imaging Research, St. Catharines, Canada) [23]. Positive areas of Bielschowsky-silver and Luxol-fast blue were measured in the WM-bundles the IBZ. Immunoreactive-positive area of Albumin, PPB or IBA-1, CD163- and CD206-positive cell number in the IBZ, Occludin-positive area in 20 enlarged thin-wall vessels, in the IBZ was quantitated [26]. To measure the vascular density and perimeter, and arteriolar density and diameter, eight fields of view of vWF-positive vessels and αSMA-positive arterioles from the IBZ were digitized at 20×. The density/perimeter of 20 largest vWF-vessels or the density/diameters of 10 αSMA-positive arterioles (diameter > 10μm) were measured using the MCID software [27]
MiR-126 assay
For the measurement of miR-126 in the blood serum, ischemic brain tissue, and cultured BECs and HUCBCs, samples were lysed in Qiazol reagents and the total RNA was isolated using the miRNeasy Mini kit (Qiagen, Valencia, CA, http://www.qiagen.com/). By RT-PCR, we detected the miR-126 level. Briefly, miRNAs were reverse transcribed with the miRNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, http://www.lifetechnologies.com/us/en/home/brands/applied-biosystems.html) and PCR amplifcation was performed with the TaqMan miRNA assay kit (Applied Biosystems, which is specific for mature miRNA sequences, http://www.lifetechnologies.com/us/en/home/brands/applied-biosystems.html) according to the manufacturer’s protocols, with U6 snRNA as an internal control.
Primary mouse BEC culture
MiR-126 is an angiogenic-miR and is primarily expressed in endothelial cells [22]. To test whether T2DM regulates mouse brain BEC miR-126 expression, and whether HUCBC-treatment promotes BEC miR126 expression, primary mouse BECs were isolated from the IBZ of non-DM and T2DM adult (2~3 month) mice 3 days after dMCAo, as previously described [28, 29]. Mouse brain tissue was homogenized and suspended in 15% Dextran and then digested with collagenase/dispace (Roche) in RPMI1640+2% FBS (Life Technologies). The resulting suspension was centrifuged and then mixed with Percoll (Sigma) to produce a 45% Percoll solution. Then, the uppermost band was removed and washed twice with 1 × PBS. The final cell pellet was resuspended in BEC growth media (80% MCDB, Invitrogen), 0.15% NaHCO3, 10% FBS (Life Technologies), 1% ECGS (15mg/ml BD Bioscience), 1.5% Heparin, 1% antibiotics, 5% L-glutamine, 0.02% L-ascorbic acid, 1% Insulin-transferrin-selenium (Sigma) and 0.1% 2-Mercaptoethanol,. 1,000× (55 mM), and allowed to culture for 24h. The primary cultured BECs were used to measure miR-126 expression.
BEC capillary tube formation assay
Mouse BEC line was initially purchased from ATCC and cultured in DMEM with 10% FBS and 1% antibiotic/antimycotic. To test whether miR-126 regulates angiogenesis, an in vitro capillary tube formation assay was employed. BECs were transfected with pEGP-mmumir-126 Expression vector (MMU-MiR-126 for miR-126 knock-in) or pLenti-III-miR-GFP knock-in Control Vector by electroporation transfection method. To test whether HUCBC regulates angiogenesis, and whether HUCBC mediated increase of miR-126 enhances HUCBC induced angiogenesis, knockdown of miR-126 in HUCBC (mouse mmu-miR-126-3p inhibitor, miR126−/−HUCBC) and miR-126 knockdown negative control inhibitor (Thermo Scientific, miR-126−/− HUCBC-Con) was performed using electroporation transfection method.
Then an in vitro capillary tube formation in BEC, miR-126+/+Con-BECs or miR-126+/+BECs was employed. To test whether treatment with naïve-HUCBC (22500 cells/well) or +miR-126−/−HUCBC affect BEC capillary tube formation, a transwell-coculture model was employed [29]. Briefly, BECs were plated in the lower chamber of the six well plate, with or without HUCBC added in the upper chamber of a Falcon 0.4 μm cell culture insert for 24h [26], MiR-126 expression was measured in the lower chamber of BECs (n=4/group). BEC-cultures were replaced with glucose and serum free media and were placed in a hypoxia chamber (Forma Anaerobic System, Thermo Scientific) for 2h of oxygen-glucose deprivation (OGD) at 37°C. The OGD-BECs were suspended in serum free-DMEM with 37.5mM glucose and then was added into Matrigel (100 μl/well, Becton Dickinson) and cultured with serum free-DMEM in a 96 well plate (n=6). After 5h, the Matrigel wells were digitized under a 10× and tracks of BECs organized into networks of cellular cords (tubes) were counted and averaged in four randomly selected microscopic fields. Total length of capillary-tube like formation was quantitated, as in previous publications [30, 31]
Primary cortical neuron (PCN) culture and PCN-axonal outgrowth quantification by a microfluidic-chamber assay
To test whether HUCBC regulates PCN axonal outgrowth, coculture of PCN with HUCBC were performed. PCNs were harvested from pregnant embryonic day 18 Wistar rats (Charles River Laboratories). The cultures and axon-outgrowth were prepared and measured, as previously described [32]. Briefly, embryos were removed, the cerebral cortex was dissected, stripping the meninges and dissociated in Ca2+- and Mg2+-free HBSS with 0.125% trypsin to digest for 15 min, and then mechanically disassociated with a pipette (~20 strokes). The cells were filtered through a 40μm cell-strainer then counted to obtain a concentration of 3 × 107cells/ml.
To separate axons from neuronal soma, a microfluidic-chamber (Standard Neuron Device) was used [32, 33]. The small dimension of the microgrooves in the chamber allows axons to sprout from the cell-seeded compartment into the other compartment of the chamber, but prevents the passage of cell bodies [32]. The chambers were sterilized under UV light, and placed on poly-d-lysine (Sigma) coated dishes (35 mm, Corning). The PCNs were plated in two of the chambers at 6 × 105cells/well and the other two chambers were only filled with DMEM + 5% FBS. After incubation of 5h, the initial media was replaced with neurobasal growth medium (Invitrogen) with 2% B27 (Invitrogen), 2 mm GlutaMax, and 1% antibiotic-antimycotic. At day in vitro 3 (DIV 3), the PCNs are randomly grouped to (1) no-treatment control; (2) +HUCBC (22500 cells/well), (3) +miR-126−/−HUCBC using neurobasal growth media. In DIV 5-7, when axons had passed through the central channels, the cells were stained with SMI-31 (1:200, Covance) conjugated with Cy3 (1:500, Jackson Immunoresearch). The average length of axonal outgrowth was calculated under a 10× with MCID software (n=7/group).
Western blot assay
Additional set of rats were sacrificed 14d after dMCAo (n=4/group), and ischemic brain tissues were extracted from the IBZ. Protein was isolated using Trizol (Invitrogen). Anti-vascular cell adhesion molecule-1 (VCAM-1, 1:500, Santa Cruz) and monocyte chemotactic protein 1 (MCP-1, 1:1000, Abcam), and anti-β-actin (1:10000; Abcam, Cambridge, MA) antibodies were loaded for Western blot measurements.
Real-time PCR assay
Total RNA was isolated with TRIzol (Invitrogen) to make cDNA using the M-MLV (Invitrogen) standard protocol and to run a quantitative PCR using the SYBR Green real-time PCR method on a ViiA-7 PCR instrument (Applied Biosystems, Foster City, CA) using 3-stage program parameters provided. Each sample was tested in triplicate, and analysis of relative gene expression data using the 2−ΔΔCT method. The primers are: VCAM-1 (Forward) caggtggaggtctactcattcc; (Reverse) ctccagatggtcaaagggatac. MCP-1 (Forward) ctgctactcattcaccagcaag; (Reverse) ctctctcttgagcttggtgaca.
Statistical analysis
One-way ANOVA and least significant difference analysis after a Post-Hoc test were performed to assess data of lesion volume, functional outcomes, immunostaining, RT-PCR and Western blot assays in the serum and brain tissues in vivo, tube formation and axonal outgrowth in vitro. “Contract/estimate” statement was used to test the group difference. If an overall treatment group effect was detected at p<0.05, pair-wise comparisons were made. All data are presented as mean ± Standard Error (SE).
Results
T2DM stroke mice exhibit decreased miR-126 expression in blood serum, ischemic brain tissue, and primary cultured mouse BECs. Delayed HUCBC-treatment of T2DM stroke mice significantly increased miR-126 expression in blood serum, ischemic brain tissue, and primary cultured mouse BECs
T2DM-dMCAo mice exhibit significantly decreased miR-126 expression in blood serum, ischemic brain tissue in the IBZ and primary cultured BECs (Fig. 1A-C) compared to non-DM-dMCAo mice (p<0.05, n=6/group in serum/brain tissue, n=4/group in BECs). While HUCBC-treatment in T2DM-dMCAo mice or coculture T2DM-BECs with HUCBC significantly increased miR-126 expression in blood serum and ischemic brain tissue compared to T2DM-dMCAo mice or T2DM-BECs alone. These data suggest that T2DM-dMCAo decreases miR-126 expression and that HUCBCs release factors that subsequently increase miR-126 expression after T2DM-dMCAo.
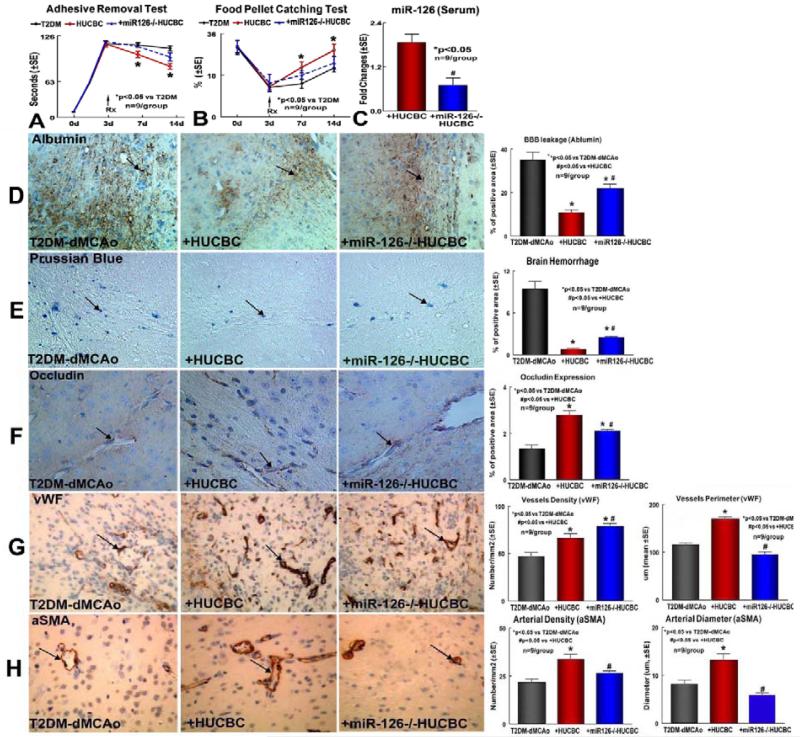
Figure 1. T2DM stroke mice exhibit decreased miR-126 expression but HUCBC treatment of T2DM stroke increases miR-126 expression in the blood serum, brain tissue and brain BEC; Konck-in miR-126 in BECs promotes capillary tube formation and miR-126 mediates HUCBC-induced angiogenesis in vitro.
A-C: The levels of miR-126 in the blood serum (A), ischemic brain tissue (B) and BECs (C) derived from non-DM or T2DM stroke mice. D: The level of miR-126 in naïve mouse BECs, miR-126 knock-in control (miR-126+/+Con) BECs and miR-126 knock-in BECs (miR-126+/+ BECs). E-G: Capillary tube formation in miR-126+/+Con (F) and in miR-126+/+ BECs (G) and quantitative data (E). H: miR-126 expression in naïve HUCBC, miR-126 knockdown control (miR-126−/−Con) HUCBC and miR-126 knockdown HUCBCs (miR-126−/−HUCBC). I-L: Capillary tube formation in BEC control (J), HUCBC treatment (K) and miR-126−/−HUCBC treatment group (L) and quantitative data (I).
Increasing miR-126 expression in BECs promotes capillary tube formation; coculture of BECs with HUCBCs increases miR-126 expression and capillary tube formation in BECs; knockdown of miR-126 in HUCBCs attenuate HUCBC-induced angiogenesis in vitro
MiR-126 knock-in BECs (miR-126+/+BECs) exhibit significantly increased miR-126 expression (Fig. 1D) and capillary tube formation (Fig. 1E-G) compared to knock-in control BECs (miR-126+/+Con-BECs) and naïve-BECs, respectively (p<0.05, n=6/group).
MiR126−/−HUCBC significantly decreased miR-126 expression in HUCBC compared to miR-126−/−Con-HUCBC and naïve-HUCBC (Fig. 1H, p<0.05, n-3/group). Total length of capillary tube formation was measured at 5h after treatment. Co-culture BEC with HUCBC or with miR-126−/−HUCBC, both significantly increase BEC capillary tube formation compared to non-treatment BEC control, while, co-culture of miR126−/−HUCBC with BEC significantly decreased capillary tube formation compared to the naïve-HUCBC treatment group (Fig. 1I-L, p<0.05, n=7/group). The data indicate that miR-126 partially mediates HUCBC induced angiogenesis.
HUCBC increases PCN axonal outgrowth. Inhibition of miR-126 attenuates HUCBC induced PCN axonal outgrowth
HUCBC treatment (Fig. 2B) signifcantly increases PCN axonal outgrowth compared to the non-treatment PCN control (Fig. 2A), while, miR-126−/−HUCBC (Fig. 2C) significantly decreased PCN axonal outgrowth compared to naïve-HUCBC treatment (Fig. 2D, p<0.05, n=7/group). The data indicate that miR-126 mediates HUCBC-induced axonal outgrowth.
Figure 2. miR-126 mediates HUCBC-induced axonal outgrowth in PCN cultures.
A-D: Axonal outgrowth in PCN control (A), HUCBC treatment (B) and miR-126−/−HUCBC treatment group (C) and quantitative data (D).
MiR-126 contributes to HUCBC treatment induced neurorestorative effects after stroke in T2DM mice; knockdown of miR-126 in HUCBC decreases HUCBC induced functional outcome and miR-126 expression in T2DM mice
HUCBC-treatment significantly improved functional outcome after stroke identified by the adhesive-removal test (Fig. 3A) and food-pellet catching test (Fig. 3B) compared to PBS treated T2DM-dMCAo control mice (p<0.05, n=9/group). However, delayed HUCBC-treatment in T2DM stroke mice did not decrease lesion volume (T2DM-dMCAo control: 14.5±3.2% vs T2DM-dMCAo+HUCBC: 12.7±3.0%) and blood glucose (T2DM-dMCAo control: day 1: 577 ±11 mg/dl, day 14: 523±52 mg/dl; T2DM-dMCAo+HUCBC: day 1: 567±14 mg/dl, day 14:517±48 mg/dl).
Figure 3. HUCBC treatment of stroke improve functional outcome in T2DM mice, HUCBC treatment significantly decreased brain hemorrhage transformation and BBB leakage, but increased tight junction protein expression and vascular remodeling in the ischemic brain in T2DM mice; miR-126 mediates HUCBC treatment induced neurorestorative effects and vascular remodeling.
A-B: Neurological function outcome measured by adhesive removal (A) and Food pellet catching (B) test; C: miR-126 level in blood serum. D-H: Albumin (D), Prussian blue (E), Occlusion (F), vWF (G) and αSMA (H) immunostaining and quantitative data.
The miR-126−/−HUCBC treatment of stroke in T2DM mice, failed to improve functional outcome compared to PBS treated T2DM-dMCAo control mice. These mice exhibited a significant decrease of miR-126 expression in blood serum (Fig. 3C), and a decrease of HUCBC induced functional outcome after stroke in T2DM mice compared to naïve-HUCBC treated T2DM-dMCAo mice (p<0.05, n=9/group). The data indicate that the restorative effect of HUCBC may be mediated through miR-126 upregulation after stroke in T2DM mice.
Delayed HUCBC treatment of stroke in T2DM mice significantly decreases brain hemorrhage and BBB leakage, and increases tight junction protein expression and vascular remodeling in the ischemic brain; knockdown of miR-126 in HUCBC decreases HUCBC induced vascular remodeling after stroke in T2DM mice
Vascular maturation and stabilization are critical for angiogenesis and the maintenance of established vasculature [34, 35]. Vascular endothelial permeability is maintained by the regulated apposition of adherent and tight junction proteins [36]. To test whether HUCBC regulates vascular normalization, brain hemorrhage transformation, vascular permeability and vascular stabilization were measured. Brain hemorrhagic transformation was identified by Prussian blue staining; Vascular permeability was measured using albumin staining; Occludin, a tight junction was measured by immunostaining in brain coronal sections. Treatment of stroke with HUCBCs or miR-126−/−HUCBC in T2DM mice, both significantly decreased brain BBB leakage (Fig. 3D) and decreased hemorrhagic transformation (Fig. 3E), and increased tight junction protein Occludin expression (Fig. 3F) in the ischemic brain in T2DM-dMCAo mice compared to PBS treated T2DM-dMCAo control. However, treatment of T2DM stroke mice with miR-126−/− HUCBC significantly reduced the decreased BBB leakage (Fig. 3D) and decreased brain hemorrhage transformation (Fig. 3E), and increased tight junction protein Occludin (Fig. 3F) expression in the ischemic brain in T2DM-dMCAo mice compared to the HUCBC treated group (p<0.05, n=9/group).
To evaluate the effects of HUCBC treatment on vascular remodeling, vascular and arterial density were measured by vWF- and αSMA-immunostaining. Treatment of T2DM stroke mice with HUCBC and miR-126−/− HUCBC both significantly increased vessel density, and HUCBC treatment significantly increased vascular perimeter (vWF-immunostaining, Fig. 3G) as well as increased arterial density and diameter (αSMA-immunostaining, Fig. 3H) in the IBZ compared to PBS treated T2DM-dMCAo controls (n=9/group, p<0.05). However, when T2DM stroke mice were treated with miR-126−/−HUCBC, significant attenuation of HUCBC-induced vascular remodeling after stroke, identified by decrease vessel perimeter, and arterial density and diameter, was evident when compared to naïve-HUCBC treated T2DM stroke mice. These data indicate that HUCBC increases vascular remodeling, and increasing miR-126 may contribute to HUCBC induced vascular remodeling effects.
Delayed HUCBC treatment of stroke in T2DM mice significantly increases WM remodeling; knockdown miR-126 in HUCBC decreases HUCBC induced WM remodeling after stroke in T2DM mice
HUCBC treatment significantly increases axonal (Fig. 4A) and myelin (Fig. 4B) density in the IBZ compared to PBS treated T2DM-dMCAo control mice (n=9/group, p<0.05). Knockdown of miR-126 in HUCBC decreases HUCBC induced WM remodeling after stroke in T2DM mice compared to the HUCBC treated group.
Figure 4. HUCBC treatment significantly increased M2 macrophage polarization in the ischemic brain in T2DM mice; miR-126 mediates HUCBC treatment induced M2 macrophage polarization.
A-E: Bielschowsky silver (A), Luxol fast blue (B), IBA-1 (C), CD206 (D) and CD163 (E) immunostaining and quantitative data.
Delayed HUCBC treatment of stroke in T2DM mice significantly increased M2 macrophage polarization; knockdown of miR-126 in HUCBC attenuates HUCBC induced M2 macrophage polarization in the ischemic brain in T2DM mice
HUCBC or miR-126−/−HUCBC treatment of stroke in T2DM mice both significantly decrease activated microglia (IBA-1) compared to PBS treated T2DM-dMCAo control mice (Fig. 4C, p<0.05). HUCBC treatment of stroke in T2DM mice also significantly increased M2 macrophage (CD206 and CD163) number compared to PBS treated T2DM-dMCAo control mice (Fig. 4D-E). Knockdown of miR-126 in HUCBC significantly decreases HUCBC increased M2 macrophage polarization in the ischemic brain, compared to naïve HUCBC treated group (Fig. 4D-E, n=9/group, p<0.05). These data indicate that HUCBC decreases neuroinflammation and promotes M2 macrophage polarization. Increasing miR-126 may play important role in HUCBC-induced M2 macrophage polarization in the ischemic brain in T2DM mice.
Delayed HUCBC treatment of stroke in T2DM mice significantly decreases miR-126 target gene VCAM-1 and MCP-1 expression
miRNA can regulate adhesion molecule expression and may affect vascular inflammation. miR-126 regulates its target genes VCAM-1 and MCP-1, which may have anti-inflammatory effects. To test whether HUCBC treatment regulates miR-126 target genes, VCAM-1 and MCP-1 expression were measured. HUCBC and miR-126−/−HUCBC treatment of stroke in T2DM mice both significantly decreased VCAM-1 and MCP-1 gene (Fig. 5A) and protein (Fig. 5B) expression in the IBZ of ischemic brain compared to PBS treatment control animals identified by real time PCR and Western blot assays p<0.05, n=4/group). However, miR-126−/−HUCBC treatment only partially attenuated HUCBC induced decreased MCP-1, but not VCAM-1 expression compared to naïve HUCBC treated animals. These data indicate that HUCBC treatment of stroke in T2DM mice decreases adhesion molecule/inflammatory factors VCAM-1 and MCP-1 expression. miR-126 may mediate HUCBC treatment induced decrease of MCP-1 expression. In addition, HUCBC induced decrease of VCAM-1 expression may not only be regulated by miR-126.
Figure 5. HUCBC treatment decreased VCAM-1 and MCP-1 gene and protein expression in the ischemic brain in T2DM mice, which is partially mediated via miR-126 in MCP-1.
A: Real time PCR assay; B: Western blot assay.
Discussion
In this study, we are the first to demonstrate that HUCBC treatment of stroke in T2DM mice increases serum, brain tissue, and BEC miR-126 expression. HUCBC treatment promotes vascular and WM remodeling and has anti-neuroinflammatory effects, as well as improves functional outcome after stroke in T2DM mice. Inhibition of miR-126 expression in HUCBC significantly attenuated HUCBC induced vascular and WM remodeling, anti-inflammatory effects and functional outcome after stroke in T2DM mice. These data suggest that increased miR-126 expression by HUCBC treatment of stroke in T2DM mice contributes to its vascular and WM remodeling and anti-inflammatory effects (Figure 6).
Figure 6. Diagram shows the mechanism of restorative effects of HUCBC treatment of stroke on vascular and WM/axon remodeling after stroke in T2DM.
A: T2DM stroke mice exhibit decreased miR-126 expression and increases miR-126 target gene VCAM-1 and MCP1 expression as well as increases inflammatory effects, vascular and WM damage and therefore decreased functional outcome after stroke in T2DM mice. B: HUCBC treatment of stroke in T2DM mice significantly increases miR-126 expression and decreases miR-126 target gene VCAM-1 and MCP1 expression as well as decreases inflammatory effects, therefore promotes vascular and WM/Axonal remodeling in the ischemic brain and improves functional outcome after stroke in T2DM mice.
HUCBC treatment induces neurorestorative effects and increases vascular and WM remodeling
We found that HUCBC treatment of stroke at 3 days after dMCAo in T2DM mice did not significantly decrease lesion volume and blood glucose level, but significantly improved functional outcome after stroke in T2DM mice. Thus, delayed HUCBC treatment of stroke induced functional outcome is not attributed to neuroprotection or decrease of blood glucose, but to HUCBC induced neurorestorative effects. Neurorestorative effects after stroke are mediated by many coupled events, including vascular remodeling, WM remodeling, neurogenesis and synaptogenesis [37]. Each factor impacts the others [38]. Vascular remodeling is a prime initiator of neurorestorative events [39]. Decreased cerebral perfusion has been observed after stroke in acutely hyperglycemic animals [40]. T2DM increases vascular damage after stroke identified by increased brain hemorrhage, BBB leakage and decreased tight junction protein expression [41]. Decreased BBB leakage, brain hemorrhage and increased tight junction protein expression may contribute to improved functional outcome after stroke [4]. HUCBC treatment of stroke in T2DM mice significantly decreases BBB leakage, brain hemorrhage and increases tight junction protein expression, as well as increases vessel and artery density compared to PBS treated T2DM control.
Diabetic subjects are more prone to develop more and earlier WM high-intensity lesions [42] T2DM stroke increases demyelination and decreases axon density in the ischemic brain [5]. Re-myelination from regenerating axons in the IBZ of cerebral infarcts is essential for long-term stroke recovery [43]. Infusion of HUCBCs induces endogenous brain gene expression that could confer oligoprotection from ischemia and protect striatum WM tracts [44]. In this study, HUCBC treatment of stroke in T2DM mice significantly promotes WM remodeling identified by reduced demyelination and increased axon density in the ischemic brain in T2DM mice. The delayed HUCBC treatment of stroke by promoting vascular and WM remodeling may contribute to HUCBC induced improvement of functional outcome in T2DM mice.
HUCBC treatment of stroke in T2DM mice increases M2 macrophage polarization
Macrophages include classically activated proinflammatory (M1) and alternatively activated anti-inflammatory (M2) macrophages. M2 macrophages produce anti- inflammatory cytokines such as interleukin (IL)-10 and transforming growth factor β [45]. Increase in M2 macrophages decreased lesion volume and promotes myelination of axons and preservation of neurons in a spinal cord injury model [46]. Immunomodulatory strategies capable of redirecting the microglia response toward a M2 phenotype in the late phase of brain ischemia may represent attractive options for stroke treatment [47]. M2 macrophage activation also improves systemic insulin sensitivity and protects against the development of cardiovascular diseases and T2DM [48]. While M1 microglia-conditioned media exacerbated OGD oligodendrocyte death, the M1 phenotypic shift may propel WM damage progression, and represents a rational target for traumatic brain injury treatment [49]. In this study, we found that HUCBC treatment increases M2 macrophage polarization as well as improves vascular and WM remodeling. The increasing M2 polarization may play an important role in HUCBC induced neurorestorative effects after stroke in T2DM mice.
HUCBC increases miR-126 expression. Increasing miR-126 expression may mediate HUCBC-induced vascular and WM remodeling
MiRNAs have been implicated in the regulation of key metabolic, inflammatory, and angiogenic pathways in T2DM [17, 50]. Among the miRNAs most consistently associated DM is miR-126 [51]. MiR-126 is one of the most abundant miRNAs in endothelial cells and plays a crucial role in regulating the function of endothelial cells, angiogenesis and vascular integrity [52, 53]. Disruption of miR-126 causes a loss of vascular integrity resulting in a phenotype containing leaky vessels and evoking hemorrhage [52]. Diabetes significantly decreases serum miR-126 level [17] and decreases endothelial progenitor cell miR-126 expression [51]. In our study, we found that T2DM substantially decreases serum and brain tissue miR-126 expression as well as increases brain hemorrhagic transformation, and BBB leakage, and decreases tight junction protein expression in T2DM mice. These mice have worse functional outcome after stroke compared to no-DM stroke mice [41]. The drastic loss of circulating miR-126 may contribute to the development of micro- and macro- vascular complications of T2DM [54]. MiR-126 deficit also reduces cardiac neovascularization and function. Overexpression of miR-126 in marrow stromal cells represents a novel and efficient therapeutic approach for ischemic angiogenesis and the improvement of cardiac function [54]. Therefore, upregulation of miR-126 expression may be a therapeutic target for T2DM-dMCAo treatment. We found that HUCBC treatment of stroke in T2DM mice significantly increases brain tissue, blood serum and BEC miR-126 expression. Increasing miR-126 promotes vascular remodeling and angiogenesis. HUCBC treatment decreases brain hemorrhage, BBB leakage and promotes vascular remodeling in the ischemic brain in T2DM mice. While knockdown of miR-126 in HUCBC treatment of stroke in T2DM mice significantly attenuates HUCBC-induced vascular remodeling in vivo and in vitro. Therefore, increasing miR-126 may contribute to HUCBC treatment induced vascular remodeling in T2DM stroke mice.
In this study, we found that HUCBC treatment of stroke increases miR-126 expression, as well as promotes WM remodeling identified by enhanced axonal and myelin density in the ischemic brain. The increased axonal density or outgrowth induced by HUCBC treatment may be regulated by direct and indirect effects of HUCBC. HUCBC co-culture with PCN could directly induce PCN axonal outgrowth; HUCBCs also increase BEC miR-126 expression. Increased miR-126 expression in BECs thereby indirectly promotes BEC crosstalk with neurons, and enhances PCN axonal outgrowth. While, knockdown-miR-126 in HUCBC decreases HUCBC induced axonal outgrowth in vitro as well as decreases WM remodeling in the ischemic brain in T2DM mice in vivo. Therefore, increasing miR-126 expression by HUCBC treatment may mediate HUCBC induced axonal remodeling after stroke in T2DM mice.
Increasing miR-126 may mediate HUCBC induced M2 macrophage polarization
miRNAs modulate monocyte formation, macrophage infiltration into tissues, and macrophage polarization [56]. miRNAs can affect monocyte infiltration via regulation of adhesion molecules presenting on the cell surface. miR-126 directly inhibits stromal cell-derived factor-1 alpha expression, and indirectly suppresses the expression of MCP-1 [57]. MiR-126 binds directly to the -untranslated region of CCL2 mRNA. Overexpression of miR-126 in a human monocyte/macrophage cell line attenuated MCP-1 production [58]. MCP-1 secretion accompanied by several adhesion molecules presenting on the cell surface also facilitates monocyte infiltration. The M1 macrophages home to injured spinal cord area also in an MCP-1 chemokine-dependent manner [59]. Increased adipocyte MCP-1 secretions may initiate adipose inflammation by attracting the migration of inflammatory cells into the tissue. In our study, HUCBC increases miR-126 expression and M2 macrophage polarization, but significantly decreases MCP-1 expression in the ischemic brain. Knockdown of miR-126 in HUCBC significantly decreases M2 macrophage polarization and increases MCP-1 expression in the ischemic brain in T2DM mice. These data indicate that increasing miR-126 by targeting MCP-1 may mediate HUCBC induced anti-inflammatory effect and promote M2 macrophage polarization. In addition, MiR-126 also targets VCAM-1 [22] Expression of VCAM-1 and its receptor are correlated with the extent of monocyte recruitment to inflamed endothelium [60]. Increasing miR-126 levels regulates decreasing of VCAM-1 expression, and thereby decreases leukocyte adherence to endothelial cells and macrophage infiltration. We found that HUCBC treatment of stroke in T2DM mice significantly decreases VCAM-1 expression in the ischemic brain and decreases monocyte recruitment into the ischemic brain. However, knockdown of mIR-126 in HUCBC did not attenuate HUCBC-induced decreased VCAM-1 expression in the ischemic brain. Therefore, HUCBC induced decreased VCAM-1 may be modulated by multiple factors, not only miR-126.
CONCLUSION
In this study, we have demonstrated that delayed HUCBC treatment of stroke in T2DM mice significantly increases miR-126 expression and promotes neurorestorative effects after stroke. Knockdown miR-126 in HUCBC significantly attenuates HUCBC induced beneficial effects after stroke in T2DM mice. Therefore, miR-126 may provide a therapeutic target in the T2DM stroke population.
Significance Statement.
In this study, we are the first to demonstrate that HUCBC treatment of stroke in type 2 diabetic mice increases serum, brain tissue, and brain endothelial cell miR-126 expression. HUCBC treatment promotes vascular and white matter remodeling, has anti-neuroinflammatory effects and improves functional outcome after stroke in T2DM mice. Inhibition of miR-126 expression in HUCBC significantly attenuated HUCBC induced vascular and WM remodeling, anti-inflammatory effects and functional outcome after stroke in T2DM mice. These data suggest that increased miR-126 expression by HUCBC treatment of stroke in T2DM mice contributes to its vascular and WM remodeling and anti-inflammatory effects.
Acknowledgments
The authors wish to thank Yisheng Cui, Cynthia Roberts, Qinge Lu and Sutapa Santra for the technical assistance. Nicole Kuzmin-Nichols, Saneron CCEL Therapeutics, Inc. Tampa, provided the HUCBC cells. This work was supported by National Institute on Aging R01 AG031811 (JC) and R01 AG 037506 (MC), National Institute of Neurological Disorders and Stroke R01 NS083078-01A1 (JC), R01 NS092917-01 (XC) and R41 NS080329-01A1 (JC), and American Heart Association grant 14GRNT20460026 (JC) and 12SDG9300009 (X.C.)
Footnotes
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1002/stem.2193
Author Contributions
J.C.: Conception and design, Data analysis and interpretation, Manuscript writing, Financial support; R.Z., A.Z., C.C., T.Y., P.V., Y.Z.: Collection and/or assembly of data; Data analysis and interpretation: X.C.: Manuscript writing and interpretation, Financial support; M.C.: Conception, interpretation, manuscript writing, financial support
Disclosure Of Potential Conflicts Of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Mast H, Thompson JL, Lee SH, et al. Hypertension and diabetes mellitus as determinants of multiple lacunar infarcts. Stroke. 1995;26:30–33. doi: 10.1161/01.str.26.1.30. [DOI] [PubMed] [Google Scholar]
- 2.Li PA, Gisselsson L, Keuker J, et al. Hyperglycemia-exaggerated ischemic brain damage following 30 min of middle cerebral artery occlusion is not due to capillary obstruction. Brain research. 1998;804:36–44. doi: 10.1016/s0006-8993(98)00651-9. [DOI] [PubMed] [Google Scholar]
- 3.Capes SE, Hunt D, Malmberg K, et al. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001;32:2426–2432. doi: 10.1161/hs1001.096194. [DOI] [PubMed] [Google Scholar]
- 4.Ye X, Chopp M, Liu X, et al. Niaspan reduces high-mobility group box 1/receptor for advanced glycation endproducts after stroke in type-1 diabetic rats. Neuroscience. 2011;190:339–345. doi: 10.1016/j.neuroscience.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Cui X, Zacharek A, et al. White matter damage and the effect of matrix metalloproteinases in type 2 diabetic mice after stroke. Stroke. 2011;42:445–452. doi: 10.1161/STROKEAHA.110.596486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Savitz SI, Chopp M, Deans R, et al. Stem Cell Therapy as an Emerging Paradigm for Stroke (STEPS) II. Stroke. 2011;42:825–829. doi: 10.1161/STROKEAHA.110.601914. [DOI] [PubMed] [Google Scholar]
- 7.Fagan SC, Morgenstern LB, Petitta A, et al. NINDS rt-PA Stroke Study Group Cost-effectiveness of tissue plasminogen activator for acute ischemic stroke. Neurology. 1998;50:883–890. doi: 10.1212/wnl.50.4.883. [DOI] [PubMed] [Google Scholar]
- 8.Armstead WM, Ganguly K, Kiessling JW, et al. Signaling, delivery and age as emerging issues in the benefit/risk ratio outcome of tPA For treatment of CNS ischemic disorders. J Neurochem. 2010;113:303–312. doi: 10.1111/j.1471-4159.2010.06613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grotta JC, Jacobs TP, Koroshetz WJ, et al. Stroke program review group: an interim report. Stroke. 2008;39:1364–1370. doi: 10.1161/STROKEAHA.107.510776. [DOI] [PubMed] [Google Scholar]
- 10.Neuhoff S, Moers J, Rieks M, et al. Proliferation, differentiation, and cytokine secretion of human umbilical cord blood-derived mononuclear cells in vitro. Experimental hematology. 2007;35:1119–1131. doi: 10.1016/j.exphem.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Sanberg PR, Li Y, et al. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- 12.Yan T, Venkat P, Ye X, et al. HUCBCs Increase Angiopoietin 1 and Induce Neurorestorative Effects after Stroke in T1DM Rats. CNS neuroscience & therapeutics. 2014 doi: 10.1111/cns.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papadopoulos SM, Chandler WF, Salamat MS, et al. Recombinant human tissue-type plasminogen activator therapy in acute thromboembolic stroke. Journal of neurosurgery. 1987;67:394–398. doi: 10.3171/jns.1987.67.3.0394. [DOI] [PubMed] [Google Scholar]
- 14.Fan X, Qiu J, Yu Z, et al. A rat model of studying tissue-type plasminogen activator thrombolysis in ischemic stroke with diabetes. Stroke. 2012;43:567–570. doi: 10.1161/STROKEAHA.111.635250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J, Ye X, Yan T, et al. Adverse effects of bone marrow stromal cell treatment of stroke in diabetic rats. Stroke. 2011;42:3551–3558. doi: 10.1161/STROKEAHA.111.627174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenzweig S, Carmichael ST. Age-dependent exacerbation of white matter stroke outcomes: a role for oxidative damage and inflammatory mediators. Stroke. 2013;44:2579–2586. doi: 10.1161/STROKEAHA.113.001796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zampetaki A, Kiechl S, Drozdov I, et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circulation research. 2010;107:810–817. doi: 10.1161/CIRCRESAHA.110.226357. [DOI] [PubMed] [Google Scholar]
- 18.Freilich RW, Woodbury ME, Ikezu T. Integrated expression profiles of mRNA and miRNA in polarized primary murine microglia. PLoS ONE. 2013;8:e79416. doi: 10.1371/journal.pone.0079416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bai Y, Cui M, Meng Z, et al. Ectopic expression of angiopoietin-1 promotes neuronal differentiation in neural progenitor cells through the Akt pathway. Biochem Biophys Res Commun. 2009;378:296–301. doi: 10.1016/j.bbrc.2008.11.052. [DOI] [PubMed] [Google Scholar]
- 20.Mocharla P, Briand S, Giannotti G, et al. AngiomiR-126 expression and secretion from circulating CD34(+) and CD14(+) PBMCs: role for proangiogenic effects and alterations in type 2 diabetics. Blood. 2013;121:226–236. doi: 10.1182/blood-2012-01-407106. [DOI] [PubMed] [Google Scholar]
- 21.Kuraoka M, Furuta T, Matsuwaki T, et al. Direct experimental occlusion of the distal middle cerebral artery induces high reproducibility of brain ischemia in mice. Experimental animals / Japanese Association for Laboratory Animal Science. 2009;58:19–29. doi: 10.1538/expanim.58.19. [DOI] [PubMed] [Google Scholar]
- 22.Harris TA, Yamakuchi M, Ferlito M, et al. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci U S A. 2008;105:1516–1521. doi: 10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui X, Chopp M, Zacharek A, et al. Deficiency of brain ATP-binding cassette transporter A-1 exacerbates blood-brain barrier and white matter damage after stroke. Stroke. 2015;46:827–834. doi: 10.1161/STROKEAHA.114.007145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farr TD, Whishaw IQ. Quantitative and qualitative impairments in skilled reaching in the mouse (Mus musculus) after a focal motor cortex stroke. Stroke. 2002;33:1869–1875. doi: 10.1161/01.str.0000020714.48349.4e. [DOI] [PubMed] [Google Scholar]
- 25.Swanson RA, Morton MT, Tsao-Wu G, et al. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 26.Zacharek A, Chen J, Cui X, et al. Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke. J Cereb Blood Flow Metab. 2007;27:1684–1691. doi: 10.1038/sj.jcbfm.9600475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zacharek A, Chen J, Cui X, et al. Simvastatin increases notch signaling activity and promotes arteriogenesis after stroke. Stroke. 2009;40:254–260. doi: 10.1161/STROKEAHA.108.524116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zacharek A, Chen J, Zhang C, et al. Nitric oxide regulates Angiopoietin1/Tie2 expression after stroke. Neuroscience letters. 2006;404:28–32. doi: 10.1016/j.neulet.2006.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teng H, Zhang ZG, Wang L, et al. Coupling of angiogenesis and neurogenesis in cultured endothelial cells and neural progenitor cells after stroke. J Cereb Blood Flow Metab. 2008;28:764–771. doi: 10.1038/sj.jcbfm.9600573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J, Cui X, Zacharek A, et al. Increasing Ang1/Tie2 expression by simvastatin treatment induces vascular stabilization and neuroblast migration after stroke. Journal of cellular and molecular medicine. 2009;13:1348–1357. doi: 10.1111/j.1582-4934.2008.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J, Cui X, Zacharek A, et al. Niaspan increases angiogenesis and improves functional recovery after stroke. Ann Neurol. 2007;62:49–58. doi: 10.1002/ana.21160. [DOI] [PubMed] [Google Scholar]
- 32.Taylor AM, Blurton-Jones M, Rhee SW, et al. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nature methods. 2005;2:599–605. doi: 10.1038/nmeth777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ueno Y, Chopp M, Zhang L, et al. Axonal outgrowth and dendritic plasticity in the cortical peri-infarct area after experimental stroke. Stroke. 2012;43:2221–2228. doi: 10.1161/STROKEAHA.111.646224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aurrand-Lions M, Johnson-Leger C, Lamagna C, et al. Junctional adhesion molecules and interendothelial junctions. Cells, tissues, organs. 2002;172:152–160. doi: 10.1159/000066967. [DOI] [PubMed] [Google Scholar]
- 35.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- 36.Alexander JS, Elrod JW. Extracellular matrix, junctional integrity and matrix metalloproteinase interactions in endothelial permeability regulation. Journal of anatomy. 2002;200:561–574. doi: 10.1046/j.1469-7580.2002.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen J, Zhang ZG, Li Y, et al. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- 38.Zhang ZG, Chopp M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet neurology. 2009;8:491–500. doi: 10.1016/S1474-4422(09)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taguchi A, Soma T, Tanaka H, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martini SR, Kent TA. Hyperglycemia in acute ischemic stroke: a vascular perspective. J Cereb Blood Flow Metab. 2007;27:435–451. doi: 10.1038/sj.jcbfm.9600355. [DOI] [PubMed] [Google Scholar]
- 41.Cui X, Chopp M, Zacharek A, et al. Angiopoietin/Tie2 pathway mediates type 2 diabetes induced vascular damage after cerebral stroke. Neurobiology of disease. 2011;43:285–292. doi: 10.1016/j.nbd.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.J SR-F, Sa-Roriz TM, Rosset I, et al. (Pre)diabetes, brain aging, and cognition. Biochim Biophys Acta. 2009;1792:432–443. doi: 10.1016/j.bbadis.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 43.Shi X, Kang Y, Hu Q, et al. A long-term observation of olfactory ensheathing cells transplantation to repair white matter and functional recovery in a focal ischemia model in rat. Brain research. 2010;1317:257–267. doi: 10.1016/j.brainres.2009.12.061. [DOI] [PubMed] [Google Scholar]
- 44.Hall AA, Guyer AG, Leonardo CC, et al. Human umbilical cord blood cells directly suppress ischemic oligodendrocyte cell death. Journal of neuroscience research. 2009;87:333–341. doi: 10.1002/jnr.21857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parsa R, Andresen P, Gillett A, et al. Adoptive transfer of immunomodulatory M2 macrophages prevents type 1 diabetes in NOD mice. Diabetes. 2012;61:2881–2892. doi: 10.2337/db11-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma SF, Chen YJ, Zhang JX, et al. Adoptive transfer of M2 macrophages promotes locomotor recovery in adult rats after spinal cord injury. Brain, behavior, and immunity. 2015;45:157–170. doi: 10.1016/j.bbi.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 47.Brifault C, Gras M, Liot D, et al. Delayed pituitary adenylate cyclase-activating polypeptide delivery after brain stroke improves functional recovery by inducing m2 microglia/macrophage polarization. Stroke. 2015;46:520–528. doi: 10.1161/STROKEAHA.114.006864. [DOI] [PubMed] [Google Scholar]
- 48.Kang K, Reilly SM, Karabacak V, et al. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell metabolism. 2008;7:485–495. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang G, Zhang J, Hu X, et al. Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. J Cereb Blood Flow Metab. 2013;33:1864–1874. doi: 10.1038/jcbfm.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sozmen EG, Kolekar A, Havton LA, et al. A white matter stroke model in the mouse: axonal damage, progenitor responses and MRI correlates. Journal of neuroscience methods. 2009;180:261–272. doi: 10.1016/j.jneumeth.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meng S, Cao JT, Zhang B, et al. Down-regulation of microRNA-126 in endothelial progenitor cells from diabetes patients, impairs their functional properties, via target gene Spred-1. J Mol Cell Cardiol. 2012;53:64–72. doi: 10.1016/j.yjmcc.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 52.Wang S, Aurora AB, Johnson BA, et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Developmental cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fish JE, Santoro MM, Morton SU, et al. miR-126 regulates angiogenic signaling and vascular integrity. Developmental cell. 2008;15:272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Regazzi R. Diabetes mellitus reveals its micro-signature. Circulation research. 2010;107:686–688. doi: 10.1161/CIRCRESAHA.110.228841. [DOI] [PubMed] [Google Scholar]
- 55.Huang F, Zhu X, Hu XQ, et al. Mesenchymal stem cells modified with miR-126 release angiogenic factors and activate Notch ligand Delta-like-4, enhancing ischemic angiogenesis and cell survival. International journal of molecular medicine. 2013;31:484–492. doi: 10.3892/ijmm.2012.1200. [DOI] [PubMed] [Google Scholar]
- 56.Liu G, Abraham E. MicroRNAs in immune response and macrophage polarization. Arterioscler Thromb Vasc Biol. 2013;33:170–177. doi: 10.1161/ATVBAHA.112.300068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Y, Yang P, Sun T, et al. miR-126 and miR-126* repress recruitment of mesenchymal stem cells and inflammatory monocytes to inhibit breast cancer metastasis. Nature cell biology. 2013;15:284–294. doi: 10.1038/ncb2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arner E, Mejhert N, Kulyte A, et al. Adipose tissue microRNAs as regulators of CCL2 production in human obesity. Diabetes. 2012;61:1986–1993. doi: 10.2337/db11-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shechter R, Miller O, Yovel G, et al. Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity. 2013;38:555–569. doi: 10.1016/j.immuni.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun C, Alkhoury K, Wang YI, et al. IRF-1 and miRNA126 modulate VCAM-1 expression in response to a high-fat meal. Circulation research. 2012;111:1054–1064. doi: 10.1161/CIRCRESAHA.112.270314. [DOI] [PMC free article] [PubMed] [Google Scholar]