Abstract
Vaccines are one of the greatest public health successes; yet, due to the empirical nature of vaccine design, we have an incomplete understanding of how the genes and proteins induced by vaccines contribute to the development of both protective innate and adaptive immune responses. While the advent of genomics has enabled new vaccine development and facilitated understanding of the immune response, proteomics identifies potentially new vaccine antigens with increasing speed and sensitivity. In addition, as proteomics is complementary to transcriptomic approaches, a combination of both approaches provides a more comprehensive view of the immune response after vaccination via systems vaccinology. This review details the advances that proteomic strategies have made in vaccine development and reviews how proteomics contributes to the development of a more complete understanding of human vaccines and immune responses.
Keywords: Proteomics, Systems biology, Vaccine, Vaccinology
1 Introduction
Infectious diseases are the leading cause of morbidity and mortality in the world [1]. In addition to human suffering, the yearly cost for medical treatment and lost productivity has been estimated at $120 billion in the United States alone [2]. Vaccines have significantly reduced world-wide mortality caused by infectious diseases by mimicking natural infection and producing long-term immunological memory [3]; as a result, vaccines have been one of the most cost-effective advancements in medicine. The World Health Organization (WHO) Global Vaccine Action Plan estimates 2.5 million deaths each year are prevented due to currently available vaccines [4]. While there are 25 diseases preventable by vaccines (Table 1), successful vaccines against major infectious diseases, such as HIV and malaria, are still needed (Table 2) [5, 6]. In addition, there is a growing need for the development of effective vaccines against emerging infectious pathogens. In this regard, recent technological advances may guide the development of more broadly protective vaccines, for example, a universal vaccine against all strains of influenza virus [7]. Additionally, populations who are most vulnerable to the diseases but whose immune systems do not respond well to current vaccines, including the elderly and the very young, would benefit greatly from more effective vaccines and reduced adverse effects.
Table 1.
Vaccine preventable diseases
| Bacterial | Viral |
|---|---|
| Cholera (Vibrio cholerae) | Adenovirus Type 4 and Type 7 |
| Diphtheria (Cornyebacterium diphtheriae) | Hepatitis A (Hepatitis A virus) |
| Haemophilus Influenzae Infection (Haemophilus influenzae) | Hepatitis B (Hepatitis B virus) |
| Meningococcal meningitis (Neisseria meningitidis) | Human papillomavirus (Papillomavirus family) |
| Plague (Yersinia pestis) | Influenza (Influenza virus) |
| Pneumococcus (Streptococcus pneumoniae) | Japanese encephalitis (Japanese encephalitis virus) |
| Tetanus (Clostridium tetani) | Measles (Measles Virus) |
| Tuberculosis (Mycobacterium bovis) | Mumps (Mumps Virus) |
| Typhoid fever (Salmonella typhi) | Poliomyelitis (Poliovirus) |
| Pertussis (Bordetella pertussis) | Rabies (lyssaviruses) |
| Rotavirus diarrhea (Rotavirus) | |
| Rubella (Rubella virus) | |
| Smallpox (Variola) | |
| Tick-borne encephalitis (Tick-borne encephalitis virus) | |
| Varicella (Varicella zoster) | |
| Yellow fever (Yellow fever virus) |
Table 2.
Diseases and newly emerging pathogens for which vaccines are not currently available
| Bacterial | Viral | Parasitic |
|---|---|---|
| Campylobacter infections | Cytomegalovirus infections | Leishmaniasis (Leishmania) |
| Chlamydophila (Chlamydophila trachomatis) | Ebola (Ebolavirus) | Malaria (Plasmodium) |
| MRSA (Staphylococcus aureus) | Enterovirus 68 infection | Schistosomiasis (Schistosoma) |
| Helicobacter pylori infections | Dengue Fever (Dengue virus) | |
| Shigellosis (Shigella) | Mononucleosis (Epstein-Barr virus) | |
| Streptococcus Group A and B infections (Streptococcus) | Hepatitis C (Hepatitis C virus) | |
| Tuberculosis (Mycobacterium tuberculosis) | Herpes (Herpes simplex virus) | |
| Urinary tract infections (Escherichia coli) | HIV | |
| Universal Influenza (Influenza virus) | ||
| Respiratory syncytial virus infection |
One strategy to guide vaccine development is through a more comprehensive view of protein changes that occur in response to infection and vaccination. Proteomics is the global study of the entire set of cellular proteins. It encompasses the large-scale identification, quantification, and localization of proteins, including characterization of their modifications, functions and interactions [8]. It is a powerful tool for studying pathogens for vaccine development and the host response to infection and immunization [7, 9]. As an evolving discipline, the field of proteomics is constantly changing with new technologies and methods [8]. The earliest proteomic technique, 2D gel electrophoresis, allowed hundreds to thousands of proteins to be separated, characterized and compared between different samples [10]. 2D spots initially relied on Edman degradation or immunoblotting approaches to identify proteins. With the advent of mass spectrometry techniques, microcapillary chromatography, and genome-assisted data analysis, the number, speed and sensitivity of the proteins and post-translational modifications identified in samples has increased greatly [11]. Additionally, many different quantification methods, such as spectral-counting, stable isotope labeling by amino acids in cell culture (SILAC), iTRAQ and ICAT, have allowed for both absolute and relative quantification of proteins in complex samples [12, 13]. Protein arrays have been developed to track the interactions and activities of large numbers of proteins in parallel [14, 15]. For a detailed account of these methods and techniques, we direct the readers to reviews by Schuldiner [16] and Yates [8], along with Aebersold and Mann [17]. Proteomic approaches are employed in many applications, including many aspects of vaccine development and implementation.
1.1 Types of vaccines
The majority of vaccines available today are developed through empirical methods, an approach based on observation of natural infections and immunity classically illustrated by Edward Jenner’s smallpox vaccine in 1798 [18]. Jenner used moderately harmful cowpox to immunize against the much more dangerous smallpox (Variola) disease. Jenner obtained this idea through a combination of village folklore about milkmaids not becoming infected with smallpox because of previous exposure to cowpox and a conversation in 1763 with Dr. John Fewster who speculated that cowpox might protect against smallpox [19]. When this approach proved successful, more controlled and scientific experiments were developed to lessen the pathogenicity of specific disease-causing vaccines [18]. The attenuation of infectious pathogens by passage in tissue or cell cultures was first shown by Louis Pasteur, during the development of a chicken cholera vaccine in the late 1870s that led to safer vaccines [18]. Historically, most vaccines have utilized either attenuated live pathogens or inactivated pathogens. Attenuated pathogens are passed through a foreign host to become less pathogenic while inactivated vaccines use heat or formaldehyde to kill the pathogen. However, some vaccines have been shown to be either ineffective or unsafe if they use attenuated or inactivated pathogens. For example in 1966, infants and toddlers who received a formalin-inactivated respiratory syncytial virus (RSV) vaccine still became infected [20]. Tragically, vaccinated children suffered worse symptoms during subsequent RSV infections resulting in hospitalizations and two deaths. Several studies have proposed mechanistic reasons why the formalin-fixed RSV vaccine enhanced the natural infectious disease response [21–24]. In addition to attenuated or inactivated vaccines, other types of vaccines are used today to induce immune protection. Subunit vaccines use purified proteins or carbohydrates from the pathogen to induce a protective immune response, without using the full pathogen. For example, subunit influenza vaccines use only the hemagglutinin protein for protection; however, subunit vaccines containing polysaccharides are often poorly immunogenic because polysaccharides are not processed by the antigen presenting cells that activate T cells [25]. This development has led to the development of conjugated vaccines produced by covalently attaching a poorly immunogenic antigen, typically a polysaccharide derived from the pathogen, to a carrier protein, usually from the same pathogen. By stimulating a helper T cells response, conjugated protein antigens increase the immunogenicity and long-term memory against the attached polysaccharide antigen. Today, conjugated vaccines are used for protection against pathogens such as Haemophilus influenzae, Neisseria meningitidis, and Streptococcus pneumoniae. Toxoid vaccines protect against bacterial toxins that have been inactivated by chemical or heat treatment. Toxoid vaccines have been developed to protect against diphtheria (Corynebacterium diptheriae), tetanus (Clostridium tetani), and botulism (Clostridium botulinum).
Vaccines can be either monovalent or multivalent depending on the number of antigenic targets present. Monovalent or univalent vaccines immunize against a single antigen or microorganism. Multivalent or polyvalent vaccines immunize against two or more antigens or pathogens. The MMR vaccine is a prime example of a polyvalent vaccine, as it immunizes against measles, mumps, and rubella. Another example is Merck’s Pneumovax 23 polyvalent vaccine, which protects against 23 serotypes of pneumococcal disease by including 23 different serotype polysaccharides in the vaccine [26].
In addition to the vaccine types previously described, next-generation experimental vaccines are being developed using nucleic acids. DNA vaccines contain plasmids genetically engineered to encode one or two proteins. When introduced into the host cells, the host’s own biological machinery reads the plasmid DNA and expresses the encoded proteins. Because these proteins are considered foreign, an immune response is activated [27]. Viral vectors are considered one of the best ways to illicit an immune response using DNA as vaccines [27]. These vectors can be optimized through the selection of the promoter and termination signals. In addition, N-terminal ubiquitin signals can be inserted, which target the proteins for immediate degradation and presentation on major histocompatibility complex (MHC) class I molecules, which are recognized by the host’s immune system [28]. Different DNA-vaccine delivery methods have been investigated, with the most common being hypodermic injection and gene gun delivery. Gene gun delivery introduces plasmid DNA adsorbed onto gold or tungsten microparticles into target cells using helium as an accelerant [29, 30]. While using vectors in vaccines seems promising given their ability to be customized [31] and their relative ease of production, no DNA vaccines have proved effective enough for use in humans [32].
1.2 Vaccine Adjuvants
Subunit vaccines are often poorly immunogenic as they represent a small portion of the pathogen and may require multiple vaccinations with large doses to confer protective immunity [33]. Adjuvants are added to vaccines in order to increase the stimulation of the immune system by either enhancing antigen presentation or providing co-stimulatory signals [33]. Adjuvants have been called the “dirty little secret” of vaccines in the scientific community due to how they were discovered [34]. In 1930, Glenny and colleagues noticed that after precipitating diphtheria toxoid with aluminum sulfate and injecting the “dirty” vaccine into guinea pigs, there was a much greater immune response than to the toxoid alone [35, 36]. Using an adjuvant, the amount of antigen used in the vaccine can be decreased (dose-sparing). In addition, adjuvanted vaccines can improve the immune response in populations who respond poorly to vaccines, such as elderly and immunocompromised individuals [33].
Several types of immunologic adjuvants exist depending on the mechanism used to stimulate the immune response [37]. Inorganic aluminum salts, typically aluminum phosphate and aluminum hydroxide, (termed alum) are the most widely used adjuvants in human vaccines [38]. The precise mechanism behind the action of alum adjuvants is unknown [38]. It has been hypothesized that the antigen adsorbs on the alum particles, which prolongs its availability to antigen presenting cells (APC), dendritic cells, and macrophages. However, it has also been shown that alum causes inflammation at the injection site, resulting in antigen presenting cells being recruited to the area [38].
Organic compounds have also been used to stimulate the immune response, particularly in instances when alum adjuvants have proven to be insufficient. Typically found as oil-in-water emulsions, they are thought to increase the trafficking of dendritic cells and macrophages to the lymph nodes [38]. In addition, they are thought to increase the release of cytokines and chemokines from innate cells at the injection site, thus recruiting B and T cells to the site [38]. Examples of oil-in-water adjuvants used in human vaccines are MF59 (squalene, Novartis) and AS03 (squalene + α-tocopherol, GlaxoSmithKline).
1.3 Innate and adaptive immune response to infections and vaccines
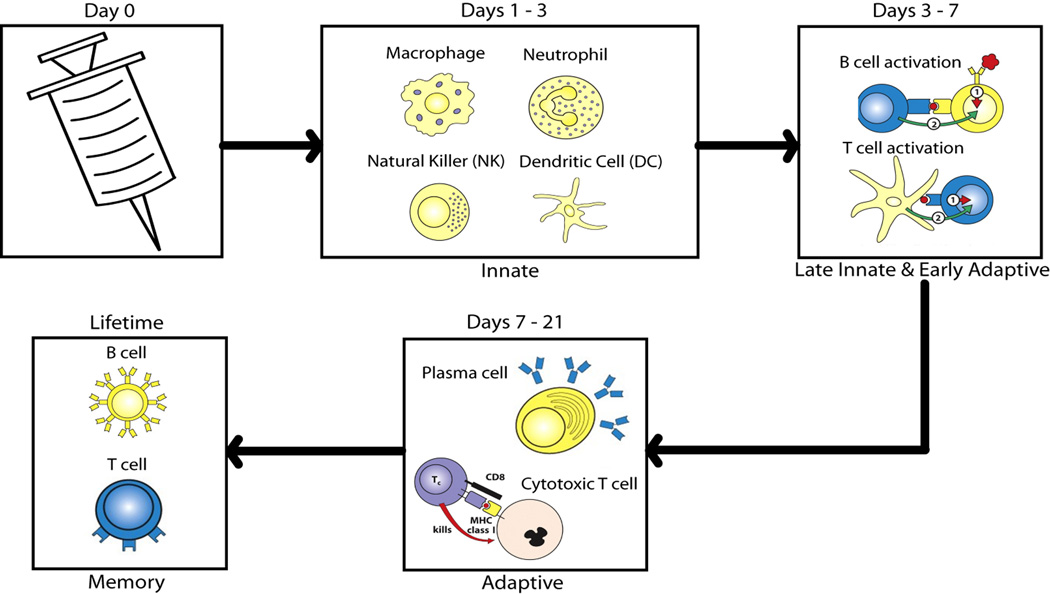
The human immune system is a complex collection of different organs, cell types and molecules that recognize, neutralize and eliminate infectious microorganisms [39]. This complex system also recognizes and responds identically to antigens in vaccines to produce immunological protection against the naturally occurring pathogenic organisms. The immune system is divided into two branches: the innate and adaptive systems. Both branches are defined by specialized immune cell types and function (Figure 1).
Figure 1.
Overview of the immune response after a vaccine is given. Vaccines mimic the natural infection. The innate immune system, comprised of macrophages, dendritic cells, natural killer cells, and neutrophils work through pattern recognition receptors on their surfaces to recognize pathogen-associated molecular patterns. These cells recruit other cells to the area and work for approximately three days to clear the antigen through endocytosis. Between days 3 and 7, antigen presenting cells help activate B and T cells by presenting antigen specific peptides. The adaptive immune response is specific to the antigen being fought. B cells secrete antibodies against the antigen, and cytotoxic T cells kill infected cells. B and T cells can become memory cells, which continuously circulate throughout the blood until reinfection. Memory immunity is the ultimate goal of vaccines. Cell icons reproduced from Janeway’s Immunobiology by Murphy, Kenneth; et al with permission of Garland Science via Copyright Clearance Center.
The innate immune system acts as the first line of defense against infectious agents. It responds rapidly, but does not lead to long lasting, protective immunity against the pathogen. Innate cells, including neutrophils, macrophages, natural killer cells (NK) and dendritic cells, rely on a limited number of receptors and secreted proteins to recognize and respond to common foreign antigenic features termed pathogen associated molecular patterns (PAMPs) [39]. Microbial products including lipopolysaccharides, peptidoglycans, bacterial RNA and DNA, and lipoteichoic acid are examples of PAMPs recognized by the host’s innate pattern recognition receptors (PRRs). When pathogens are detected, they are phagocytized and degraded by the innate cells. The activated innate cells secrete numerous cytokines and chemokines that, in turn, activate and recruit other immune cells to the site of infection [39]. Macrophages and dendritic cells display peptides from the destroyed pathogen on their cell surface using the major histocompatibility (MHC) protein complex. These antigen-presenting cells migrate to the lymph nodes where they present peptides in the context of MHC to naïve, antigen-specific T cells, thus activating them. NK cells, while innate in nature, also have ties to the adaptive immune response. A unique feature of NK cells is their ability to recognize stressed cells, without antibodies and MHC, as required for the adaptive immune response [39]. Therefore, they allow for a faster immune reaction.
Compared to innate immune responses, an adaptive immune responses takes longer to generate, but is highly specific to the particular antigen and pathogen. Critically important, the adaptive immune system provides long-lasting protection against the pathogen, which is the ultimate goal of vaccination. There are two broad classes of adaptive responses. First, B lymphocytes are responsible for the humoral antibody response. Each B cell has a unique B-cell receptor (BCR) on its surface, which binds to one particular antigen. Naïve B cells that bind antigen and receive a secondary signal from a T helper cell differentiate into either plasma cells or memory B cells. Plasma cells proliferate and secrete large amounts of antibodies that recognize the targeted pathogen, neutralizing it and marking it for destruction. Next, T lymphocytes are responsible for cell-mediated immunity, which recognizes and destroys infected host cells. Several subsets of T cells exist, each with a distinct function. T helper cells assist other cells, such as B cells, macrophages, and cytotoxic T cells, in their functions. Cytotoxic T cells destroy infected host cells by recognizing foreign antigens presented in the context of the MHC receptor on the surface of the infected cell. Finally, regulatory T cells are critical for the maintenance of tolerance by shutting down cell-mediated immunity at the end of the infection. Long-lived memory B and T cells enable the immune system to rapidly respond to and neutralize secondary infections by pathogens that have previously been encountered. The protective function of the adaptive immune response includes neutralizing the pathogens before they can enter host cells, as well as recognizing and destroying pathogen-infected cells before the pathogen can multiply. Vaccines mimic pathogenic infections by stimulating the immune system to respond and acquire immunological memory.
1.4 Vaccine development
Today, it takes an average of 10 years from the beginning of initial laboratory work to clinical trials before new vaccines are licensed in the U.S. [7]. The process involves multiple steps and numerous checks to ensure a safe and effective vaccine product for the population. The pre-investigational new drug (IND) stage involves identification of vaccine antigens, developing the vaccine manufacturing process, and preclinical testing of the vaccine candidates [40]. After this has been completed, an IND application is submitted to the Food and Drug Administration (FDA) for permission to begin clinical trials. Clinical trials are composed of three phases defined by the endpoints and sample size. Phase I trials are performed on a small group (n = 20–80) of healthy adult volunteers in order to obtain a preliminary evaluation of safety and immunogenicity. Phase II studies employ a larger study group, sometimes up to several hundred participants. These are typically randomized, placebo-controlled studies, again aimed at studying safety and immunogenicity and to determine the dose range. Phase III trials are large-scale studies to determine vaccine efficacy, while still monitoring safety. Often, there is overlap between the three phases and each phase may be repeated numerous times as new data are collected and protocols are altered. Phases II and III are also when vaccine efficacy is determined, by comparing the incidence of disease in a group that received the vaccine to a control group [41]. After successful clinical trials, a biologicals license application is submitted to the FDA, which is responsible for approval of the final vaccine in the U.S. After a vaccine is licensed, manufacturers may elect to complete Phase IV trials, or post-marketing surveillance trials, which may last another four to six years. These trials provide information on rare adverse events and can assess the duration of the immunity conferred and determine if a booster is needed. After clinical trials are deemed complete and the product is licensed in the U.S., vaccines continue to be monitored for adverse events. The Vaccine Adverse Event Reporting System (VAERS) is a U.S. vaccine safety surveillance program co-sponsored by the United States’ Centers for Disease Control and Prevention (CDC) and the FDA. The agencies collect and analyze reports of adverse events associated with all U.S. licensed vaccines. They detect unexpected patterns or changes in rates of adverse events after vaccinations [40].
In addition to the standard track to test and license vaccines, urgent health needs may require the FDA to allow an accelerated track for vaccine testing and approval. For example, the Ebola epidemic in West Africa has caused significant morbidity and mortality requiring immediate attention. As a result, trials for Ebola vaccines and drugs were given a fast track designation, which results in earlier communication between the FDA and manufacturers and accelerated approval [42].
1.5 Correlates of protection and vaccine responses
After the safety and efficacy of a vaccine is established, two important areas of vaccine research include: 1) determining the correlates of protection and 2) expression studies to measure vaccine responses. A correlate of protection is a measurable immune response that is statistically related to and responsible for protection [43]. Correlates of protection are required for every vaccine for a number of reasons. First, the efficacy of a new vaccine can be compared to a current vaccine using correlates of protection [43]. Additionally, correlates of protection evaluate consistency of production and susceptibilities of individuals post-vaccination. Antigen-specific antibody titers against the vaccine have been the gold standard for correlates of protection for most pathogens [43]. Vaccine-specific antibody levels are measured through standardized serological methods including ELISA to determine binding antibody titers, as well as neutralization and hemagglutination inhibition (HAI) assays [43]. These methods result in a single value threshold to represent the full immune response. For example, following influenza vaccination, a serum HAI antibody level greater than a 1:40 dilution against the hemagglutinin protein is considered protective [43]. However, a single value threshold may not be sufficient as a correlate of protection due to the natural variability of human biology. In addition measuring antibody response may not always be the best or only correlate of protection. T cell-mediated cytotoxicity is another form of protection that can be measured. For example, the varicella zoster virus causes chickenpox in children and shingles in older adults [18]. The zoster vaccine acts through re-stimulation of cellular responses in vaccinated adults. Therefore, CD4+ T-cell proliferation assays are used to measure the correlate of protection [44]. Similarly, the Bacillus Calmette-Guerin (BCG) vaccine against tuberculosis uses interferon levels, produced by CD4+ cells, as the correlate of protection [45]. Identifying correlates that are not based on antibodies tends to be more time consuming, expensive and difficult to perform and interpret compared to antibody assays.
In response to such limitations and difficulties to identify and measure vaccine correlates of protection, current vaccine research has seen a large push towards transcriptomic, proteomic, and systems biology studies to study global immune responses to vaccines. Currently, correlates of protection from vaccine studies are being used to predict protective immune responses [46]. A long-term goal is to predict vaccine immunogenicity and efficacy without relying on the current correlates of protection that are not necessarily the best indicators of protection [46]. It is hypothesized that changing blood transcriptomes and proteomes will show immune response events that correlate with traditional correlates of protection. Evidence supporting this hypothesis has been shown for the yellow fever virus vaccine [47, 48], trivalent inactivated influenza vaccine [49, 50] and a meningococcal conjugate vaccine [51]. In these studies, blood transcriptomes measured seven days post-vaccination showed plasmablast expansion that was proportional to antibody production measured at 28 days post-vaccination [51]. In addition, an increase in expression of translation initiation factor EIF2AK4 was observed to predict cellular immunity responses to the yellow fever vaccine [47]. These events could potentially be classified as correlates of protection after validation. These types of studies, termed systems vaccinology, have offered insights into how protective immunity is generated by investigating multiple immune system components, including mRNA transcripts, antibody responses, and proteins, to obtain a comprehensive assessment of the response to vaccines [46, 52].
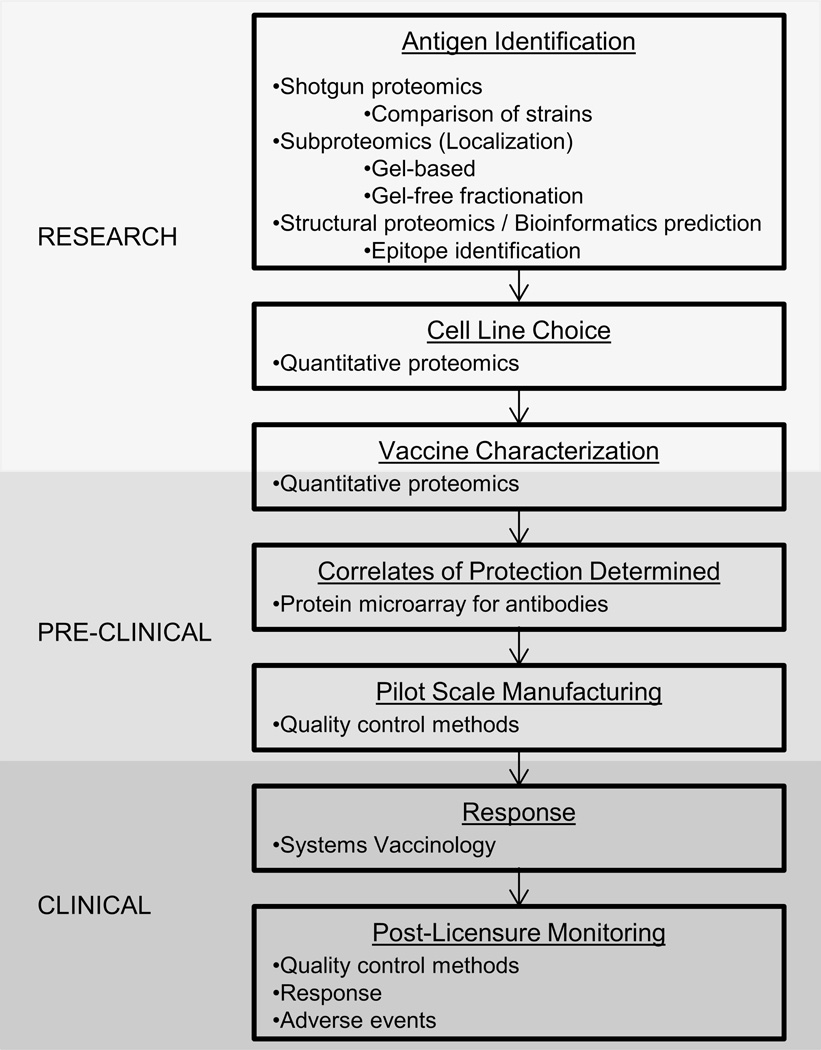
While transcriptomic studies are currently expanding our knowledge base about the response to vaccines, they offer little in the way of vaccine discovery and development. In addition, current studies using transcriptomics to assess the immune system response have largely ignored the proteomic piece of the puzzle. Transcriptomics and proteomics are closely related in that they measure dynamic expression patterns in response to stimuli. However, multiple studies demonstrate that mRNA and protein expression do not correlate at a given time or even over time [53–55]. By excluding proteomic data, important information about the immune response and possible correlates of protection are potentially missed. The contributions of proteomics in antigen discovery and vaccine development, vaccine characterization, and understanding the human immune response to vaccines are discussed in the following sections (Figure 2).
Figure 2.
General outline of the vaccine development and production process highlighting areas where proteomics is used.
2 Antigen identification for vaccine development
The idea of stimulating the immune system artificially in order to prevent future disease has been applied for centuries. The first vaccines, utilizing live, attenuated bacteria or viruses, were highly protective against smallpox and chicken cholera [18]. However, several live attenuated or killed bacterial and viral vaccines, including the first killed whole-cell pertussis vaccine, caused severe adverse reactions and therefore were removed from public use [18]. In response, vaccines containing purified bacterial proteins and/or polysaccharides as the vaccine antigen(s) were developed [18]. More recently, recombinant DNA and reverse vaccinology have shown great promise in the development of new and more effective vaccines [7]. Reverse vaccinology uses bioinformatics tools to predict immunogenic genes after bacterial and viral genomes have been sequenced [56]. This has led to the identification of a large number of vaccine candidate antigens. However, those candidate antigens need to be narrowed. Proteomics has proven to be a powerful method that can identify candidate proteins that are expressed most abundantly and react positively with the sera of immunized subjects [9].
The ability of proteomics to quickly identify a large number of proteins at high sensitivity has aided vaccine development for both bacteria and viruses [11, 57]. It is currently aiding research towards a number of vaccines. The differences between the structure and mode of action of bacteria, viruses, fungi, and parasites have caused a need for a variety of proteomic methods to be employed for vaccine antigen identification.
2.1 Role of proteomics in bacterial vaccine development
One of the first steps in most proteomic vaccine studies is to obtain a global view of the pathogen proteome and host’s immune response after infection. Historically, this has been done using 2D gel electrophoresis of the pathogen’s proteome followed by a Western blot using antibodies found in the serum of individuals infected with the pathogen [58]. The antibodies react with proteins in the blot, and those proteins are then either purified or digested for mass spectrometry analysis. The widespread use of mass spectrometry has allowed proteins in the spots to be quickly and rapidly identified at high sensitivity. For example, 2D gels and mass spectrometry were used to discover that urease is a dominant immunogenic protein in the bacterial pathogen Helicobacter pylori [58]. McAtee et al. digested 2D protein spots that reacted with sera from infected patients and analyzed them with MALDI-TOF-MS. They identified twenty proteins, including urease as seropositive candidates. Clinical investigators have moved forward with urease-based vaccines in a clinical trial [59].
In addition, comparative proteomics provides an approach to improve existing vaccines and potentially generate universal vaccines against microbes with multiple infectious strains. Comparative studies identified differences between the proteomes of Mycobacterium tuberculosis and Mycobacterium bovis, a cow tuberculosis strain used in the only available tuberculosis vaccine [60, 61]. Unique proteins were identified for each strain. The dissimilarities at the protein level included differences that were not predicted from the genomic comparisons [61]. Similarly, a comparison of spore proteins of the Bacillus cereus group, including virulent and avirulent strains of B. anthracis, has identified 15 highly immunogenic anthrax vaccine candidates [62]. A comparison of two Bordetella pertussis strains identified 15 proteins not represented in the current anti-pertussis vaccine [63].
While these approaches have generated a number of potential vaccine candidates, a more specific approach can be used to reduce the initial candidate list. Unlike tuberculosis, which is able to invade host cells and avoid detection using a variety of methods, most bacteria do not enter host cells [64]. Instead, they act by invading tissues and releasing toxins [65]. Bacteria have well-developed cell walls and are classified into two groups based on cell wall composition. First, gram-positive bacteria have a thick peptidoglycan layer outside of their cell membrane [39]. This layer is linked to the cell membrane through teichoic acid and lipoteichoic acid. Second, gram-negative bacteria have a much thinner peptidoglycan layer which is protected by an outer lipid membrane that contains proteins and lipopolysaccharides [39]. Antibacterial vaccines that target the cell walls of gram-positive and -negative bacteria is a very promising area of research, albeit with many challenges [66]. Isolating membrane and surface proteins is difficult due to the hydrophobic nature of these proteins. Membrane proteins are underrepresented in classical proteomic strategies where proteins are separated on a 2-DE gel and identified using MALDI-TOF-MS due to precipitation under standard IEF conditions [66]. In addition, the differences between a gram-positive and gram-negative cell walls increases the complexity and do not allow for a standardized procedure for each sample. Several methods have been developed to combat this issue, such as low pH elution, which was used to identify the surface protein ACE393 in Campylobacter jejuni as a potential vaccine antigen [67]. ACE393 has further gone on to a vaccination challenge study (NCT00859716). Outer-membrane protein extraction identified ETAE_0245 and OmpA as vaccine candidates against Edwardsiella tarda which causes disease in fish [68]. However, the development of LC/MS/MS and multidimensional protein separation methods allows for analysis of an increasing number of these proteins. An enzymatic-shaving technique, which uses proteases to cleave membrane proteins off living cells, allows more thorough targeting of surface proteins [69]. This technique has permitted the surface of Brucella abortus [70], Streptococcus pneumoniae [9, 66], and Neisseria meningitidis [71] to be analyzed for potential vaccine candidates.
Outer membrane vesicles (OMV) have also been targeted as potential antigens for subunit vaccines due to their implied involvement in the pathogenesis of gram-negative bacteria [72]. OMVs are spherical-bilayer structures containing cellular enzymes that are discharged from the surface of bacteria [72]. Analyzing OMVs has been notoriously difficult, as they contain both membrane proteins and periplasmic components [72]. A number of studies performed on N. meningitidis have implicated the OMV component OpcA as a protective antigen through the combined use of the chaotrope urea and in-gel rehydration [71, 73, 74]. The OMV vaccine against serogroup B meningococcus (Bexsero®, GSK) has been licensed for use in Canada, Europe, and Australia [7, 75]. This vaccine was also approved for use as an investigational new drug in the U.S. during the 2013-14 meningitis outbreaks at Princeton University and University of California, Santa Barbara [76]. While gram-negative bacteria produce OMVs, it has also been discovered that gram-positive bacteria produce corresponding extracellular vesicles (EVs) [77]. Proteomic analysis of S. pneumoniae EVs revealed that they contain membrane-associated proteins that also reside in the bacterial membrane and are not generated from the dead host cells [77]. Surprisingly, the EV also contained unique proteins not found in the bacterial membrane that have been previously reported as immunogenic [77].
2.2 Role of proteomics in vaccine development against viral pathogens
In contrast to bacteria, viruses are infectious agents that rely on the infected host cell machinery for replication [78]. Viruses are cloaked in a protein or lipid coat to protect their DNA or RNA genome. Because enveloped viruses replicate inside and then exit host cells usually through budding, host cell proteins have been identified in newly formed virions [79]. Therefore, antiviral vaccines have to be carefully designed to avoid targeting host proteins, which may cause adverse reactions. Additionally, viruses are known to alter their protein sequences via mutations in order to escape the immune response, with the most notable example being the influenza and human immunodeficiency viruses. Proteins of many viruses have been thoroughly analyzed through gel methods, mass spectrometry, and sequence analysis and only a few amino acid sequences that are immunogenic have been identified due to the selective pressure put on them from the host’s immune system [7]. For hepatitis C and herpes simplex virus, peptide vaccines based on such selected epitopes have been developed and subsequently tested in phase 1 trials [7].
Because most viruses do not have complex proteomes like prokaryotic or eukaryotic cells, most proteomic studies for vaccine development against viruses have included studying viral pathogenesis to identify which proteins are active during infection. For example, no licensed vaccine for dengue virus (DENV) currently exists. The virus is thought to target cells of the innate immune system: dendritic cells, macrophages, and monocytes [79]. One proteomic study using K562, a human myeloid leukemia cell line infected in vitro with dengue virus, showed up-regulation of the GRP78 protein [80]. Another study of infected THP1 monocytes showed up-regulation of PDIA3 and hnRNP-H proteins [81]. However, another study using ex vivo endothelial cells infected with dengue virus identified 38 host proteins that were up-regulated after infection, including proteins involved in endocytosis, the oxidative stress response, mRNA transcription and translation, cytoskeleton assembly, protein degradation, cell growth regulation, apoptosis and the antiviral response [82].
In addition to observing host protein responses to natural infection, isolating antibodies that neutralize DENV is another strategy being employed [83]. Blood from naturally infected individuals was collected, and peripheral blood mononuclear cells (PBMC) were isolated and transformed. The transformed B cells were then exposed to live DENV. A flow cytometry-based neutralization assay was then used to identify and isolate the B cells producing strongly neutralizing antibodies. These reactive cells were then fused with myeloma cells to form hybridoma cells secreting human monoclonal antibodies against DENV. These antibodies were characterized and their epitopes mapped. Smith et al. suggest that future DENV vaccines should include some of these epitopes, as they induce a strong antibody response [83].
Similar approaches have been taken in the development of an anti-HIV vaccine, and this work has been reviewed previously [84–86]. This quest for an anti-HIV vaccine is considered one of the most important areas of research in global health. Current research is involved in identifying broadly neutralizing monoclonal antibodies from infected individuals and testing to see if they can be used as a vaccine or as prophylactic therapy [87]. While the identified antibodies tend to fall into one of four categories based on their epitope position on the Env surface glycoprotein, Huang et al. identified a novel HIV-specific monoclonal antibody 35O22 [87]. B cells from infected donors were isolated and screened for neutralizing activity. A competition assay between 35O22 and other known antibodies was performed and showed that 35O22 was not affected by the other antibodies, suggesting it bound glycans, but had a different specificity. Huang et al. speculate that the 35O22 antibody could be used with other antibodies in prophylaxis. In addition, the novel epitope bound by 35O22 is a new potential target of future HIV vaccines [87].
Influenza vaccines are also benefitting from host proteomic response studies. As a common infectious virus, these studies are frequently done with the hope of producing a more effective vaccine. Numerous reviews have summarized this work [88–91]. Unlike the DENV and HIV examples above, studies of the host proteomic response to influenza have focused on T cells [92, 93]. Attenuated and inactivated influenza vaccines induce both humoral and cellular immune responses [94], however cellular immunity is not induced by subunit influenza vaccines [91]. Proteomic studies of natural influenza infections have shown that CD4+, CD8+, and regulatory T cells are all induced [91]. While CD8+ T cells have been shown not to be required, except in severe influenza infections [95], a T-cell response is beneficial as it is specific for conserved proteins and thus can lead to cross-reactivity [91]. Based on this idea, Berthoud et al. designed a Modified Vaccinia virus Ankara (MVA) vector encoding internal influenza nucleoprotein and matrix protein 1 (MVA-NP+M1) to boost cellular immunity in adults with some success [93].
2.2.1 MHC epitopes as vaccines
The major histocompatibility complex (MHC) is group of cell surface proteins that controls a large portion of the immune system in all vertebrates. The MHC binds pathogen-derived peptide fragments and displays them on the host’s cell surface for T cells to recognize. As previously mentioned, viruses must enter host cells in order to replicate and, in turn, infect other cells. In a healthy cell, the proteasome degrades intracellular proteins into short peptides for recycling and building of new proteins. Some of these peptides are transported to the endoplasmic reticulum, where they are associated with MHC class I molecules and presented on the cell’s surface in the MHC complex, which indicates that the cell is healthy [96]. Cells infected with viruses display peptides derived from viral proteins on the cell surface in the MHC class I receptor, which alerts the immune system that the cell is infected. Interaction of the CD8+ cytotoxic T cell receptor and the viral peptides in the context of MHC class I complex, along with additional receptors and signals, results in the activation of T cells and the destruction of the infected host cell [97]. Because these peptides directly target cell-mediated immunity, the identification of MHC peptide epitopes has been a growing field for the development of vaccines [98, 99]. MHC class II receptors display peptides derived from extracellular proteins that have been phagocytosed and digested in the lysosomes of the host cell. MHC class II complexes interact with CD4+ helper T cells, which then can trigger an immune response [39].
Traditionally, the MHC peptide epitopes have been identified via predictive algorithms and tested using synthetic peptides. MHCs are highly polymorphic, which can make prediction of MHC binding challenging [97]. As first described by Engelhart and Hunt’s group, tandem mass spectrometry have allowed direct analysis and sequencing of MHC complexes to identify the associated peptides [100]. This allows for the analysis of peptides that are actually processed and displayed on the cell surface, not just the ones predicted. This approach, focusing on MHC class I and II molecules, has been used extensively for the vaccinia virus, with the hope that a new vaccine can be developed that does not have severe side effects [7, 101]. Using a combination of T cell screening, fractionation, and LC-MS/MS, Strug et al. identified peptides originating from three vaccinia virus proteins: the telomere binding protein I6L; the early transcription factor VETF-1; and the major core protein A10L [102]. Enzyme-linked immunospot (ELISPOT) analysis showed these peptides were recognized by CD4+ T cells as evidenced by the IFN-γ response of T cells from an immunized donor treated ex-vivo with purified peptides. Because these peptides are conserved in the poxvirus family, their potential inclusion in a subunit epitope vaccine could potentially allow for a more universal protective response [102]. Other MHC epitope vaccines are also currently being investigated for vaccines against respiratory syncytial virus [103], HIV [96] and Chlamydophila [104].
2.2.2 Vaccination against influenza virus
Each year, seasonal influenza outbreaks cause 3–5 million infections and 250,000–500,000 deaths worldwide [91]. This is largely due to the mutagenic nature of the virus [105]. Influenza is an enveloped virus with eight negative-sense ssRNA segments in its genome. Because of random point mutations in the antibody-binding sites of the two surface glycoproteins, hemagglutinin (HA) and neuraminidase (NA), caused by antigenic drift, and unpredictable genetic reassortments, termed antigenic shift, the virus is able to evade the immune system. In contrast to seasonal influenza vaccines typically have a 70–80% effectiveness, pandemic vaccines are not particularly effective for unknown reasons [106]. In addition, seasonal influenza vaccines have to be reformulated each year to represent the predicted circulating strains based on worldwide surveillance of antigenic changes [105]. One promising area of study for influenza vaccines has resulted in attempts at making a universal vaccine, which would protect against all seasonal and pandemic strains [106]. Regions of the HA and NA proteins are conserved across all influenza strains and protection could be attained by prompting the immune system to recognize and respond to those specific areas [106]. The majority of these studies start with in silico proteomic studies to identify the conserved amino acid regions, and then proceed with experimental testing and proteomic studies to show protection. The main focus has been the HA2 stalk domain of the HA protein (Figure 3) [107, 108]. In addition, a universal therapeutic vaccine using the FI6 antibody has been proposed, due to its ability to target hemagglutinin on all 16 subtypes of influenza A viruses, but again there is the concern that the virus would mutate to escape this antibody as well [109].
Figure 3.
Ribbon model of a monomer of the influenza hemagglutinin protein. Blue represents the HA1 portion, and the green is the HA2 portion. HA1 contains the receptor-binding domain and is the main target of the immune responses. HA2 is the anchor of the virus and is responsible for the fusion of the envelope and cellular endosomal membranes.
2.3 Investigating parasitic vaccines using proteomics
Parasitic diseases, such as hookworm infections, schistosomaisis, and Chagas disease, affect more than 1 billion people annually and tend to inflict a chronic burden [110]. Currently, vector control and drug-treatment plans are the only methods to control this problem. Even with continuous research, there are still no safe and effective vaccines against human parasitic diseases due to their complexity as immunologic targets and their ability to adapt under immunologic pressure by actively altering their surface antigenicity. Parasitic diseases tend to be chronic, which implies an ability of the parasites to avoid immune system detection. Two vaccines have used whole sporozoites to protect against P. falciparum malaria and Leishmania major leishmaniasis in humans [111]. However, both have major limitations that make widespread use of the vaccines nearly impossible. The whole-sporozoite malaria vaccine required over 600,000 sporozoites [112] and the L. major vaccine induced severe primary lesions [113]. Since then, development of subunit vaccines has been the main focus for parasitic vaccines. Proteomics offers insight into the identity of potential antigens through analyzing the sera of naturally exposed subjects and sporozoite-immunized volunteers [114, 115]. However, a monovalent blood-stage malaria vaccine using the apical membrane antigen 1 (AMA1) protein has shown to be ineffective in a Phase II clinical trial [116]. The cumulative results of various studies and clinical trials have been previously reviewed for malaria and leishmaniasis, with the general conclusion being that a vaccine containing an antigen cocktail is likely to be the best option to combat the diversity of parasite species [114–116].
Other parasitic diseases have not been as thoroughly studied for antigen identification and therefore require a broader approach. A shotgun proteomic analysis of trypomastigote forms of Trypanosoma cruzi, the etiologic agent of Chagas disease, identified 6154 individual proteins of T. cruzi, increasing the proteome coverage for the parasite and identifying proteins from the TS/gp85 superfamily, which is considered essential for parasite virulence [117]. Nakayasu et al. further performed epitope analysis to identify 296 peptide candidates for MHC-I and 45 for MHC-II presentation to be further tested and validated. Similarly, potential vaccine candidates for a schistosomiasis vaccine have been identified through proteomic approaches [118]. Based on the results of previous studies, Tian et al. analyzed antigens identified by soluble worm antigen-specific IgG2 antibodies. Unmatched spots on 2-DE gels between the control and other groups were considered uniquely expressed and were submitted for identification by mass spectrometry analysis. Of the 116 spots analyzed by MALDI-TOF MS, 113 were identified as proteins from Schistosoma japonicum and were suggested for further validation and efficacy studies.
3 Characterizing vaccines with quantitative proteomics
While the main focus of vaccine development is the identification of antigens, other aspects of vaccine development are also crucial to producing a safe and effective vaccine product. Choosing a proper cell culture line and monitoring the batch-to-batch variation in the product are key aspects to be considered when mass-producing vaccines. This information can lead to new information, a better understanding, and improvement of current vaccines. In addition, characterizing currently available vaccines and especially comparing different vaccines for the same disease, allows for the most efficient vaccine to be chosen and made available worldwide.
A variety of quantitative proteomic methods aids in these vaccine characterization tasks. Quantitative proteomics involves measuring the abundances of observed proteins and making comparisons between the different conditions or samples being studied. Many methods have been developed for protein quantitation, including label-free methods, isobaric tagging methods, and chemical labeling methods [119–121]. Previous reviews have discussed the quantitative proteomics approaches [8, 10, 12]. Which quantitative method used for any given experiment is determined based on the question being investigated, the proteins being observed, and occasionally the preference of the researcher. This section will discuss examples of protein quantitation methods used to characterize vaccines and how these discoveries aid the fight for better vaccines.
3.1 Cell line selection using proteomics
Mass production of vaccines requires large amounts of bacterial or viral material. While bacteria can be grown in the laboratory by culture in media supplemented with the necessary combination of nutrients, viruses require living cells in order to reproduce. Historically, viral vaccines have been developed using viruses from animal hosts. However, this has increased the variability in the final product and tragically, has occasionally resulted in severe reactions such as limb paralysis caused by the 1930s polio vaccine developed using monkey spinal cords [18]. Moving from live animal hosts to cell cultures for the production of vaccines allows for more control over the vaccine product and the majority of vaccines are produced this way today (www.cdc.com); however, the vast majority of influenza vaccines are still produced using embryonated chicken eggs, a method with many drawbacks. Each egg can produce about 15 µg of a monovalent influenza strain to be used for the seasonal flu vaccine [122]. The standard dose flu vaccine contains about 15 µg of each strain, and the high dose vaccine requires 60 µg per strain [123]. As the seasonal flu vaccine contains either 3 or 4 different influenza strains each year, hundreds of millions of eggs and a significant amount of time are required to produce one vaccine [124]. In addition, this manufacturing method produces a product that contains egg components, preventing individuals with egg allergies from being vaccinated and leaving them unprotected.
Research towards a continuous cell line production system is ongoing, particularly with mammalian cells. A cell-culture based system would increase reproducibility, reduce risk of animal-derived protein products in the vaccine, and allow individuals with egg allergies to be vaccinated. Two cell lines are the main focus of research: African green monkey kidney cells (Vero) and Madin-Darby canine kidney cells (MDCK). A comparison study of these two cell lines infected with a human influenza virus identified 55 proteins and 32 proteins respectively, which were shown to be differentially expressed through 2D DIGE coupled with nLC-ESI-MS/MS [125]. 2D DIGE allowed for relative quantitation of the protein spots. The proteins that were identified showed different host cell responses to the virus, which may affect vaccine production. For example, the Vero cells showed a stronger stress response to the virus, which may increase cell apoptosis early before enough product is generated. More studies need to be performed with these cells to confirm this; however, MDCK cells did not show stress responses, and in 2012 the FDA approved the Flucelvax (Novartis) vaccine, which uses MDCK cells for manufacture. Additionally, in 2013 the FDA approved Flublok (Protein Sciences Corporation), the second influenza vaccine based on cell technology [126]. However, this vaccine utilizes an insect cell line and recombinant DNA technology, resulting in production of purified viral HA proteins rather than an entire live or killed virus for use as the virus antigen (www.fda.gov). These vaccines are currently only licensed for adults with egg allergies. More research and optimization needs to be performed before these techniques can be used to produce all influenza vaccines.
3.2 Analyzing vaccine composition using proteomics
Even though vaccines are highly regulated before and after they are licensed, minute details about vaccine composition are not necessarily known, especially with complex vaccines such as outer membrane vesicle (OMV) vaccines, which contain lipids, outer membrane proteins, endotoxins, and periplasmic proteins [127]. Thus, defining the composition of vaccines is essential in monitoring the quality of vaccine production and determining how they work. One extreme example involves an Actinobacillus pleuropneumoniae subunit vaccine that was developed from bacterial cultures grown under iron restriction followed by a protein detergent wash and shown to be protective against serotype 1, 2, and 5 with cross-protection against serotype 9 [128]. Goethe et al. hypothesized as to the composition of the vaccine; however the exact protein composition was not determined until a decade later [129]. Buettner et al. cultured serotype 1, 2, and 5 A. pleuropneumoniae bacteria and performed a detergent wash similar to the vaccine manufacturing procedure. These serotypes, along with a sample of vaccine, were analyzed through 2D DIGE for relative quantitation followed by MS for identification and compared. This approach led to identification of 47, 43, and 36 proteins for serotypes 1, 2, and 5 respectively [129]. Fourteen proteins identified by at least 10 peptide matches in at least one serotype, were described as “abundant protein fraction.” Eight of these fourteen proteins were outer membrane proteins, three were extracellular Apx toxins, two were cytosolic proteins, and one was a periplasmic protein. Additionally, Q-TOF MS/MS analysis of single spots from 2-D gels was performed and identified 16, 16, and 20 proteins respectively from the three serotypes [129]. Between the MS and the gel approach, 75 different proteins were identified in the vaccine. The proteins likely responsible for the cross-protection were identified as secreted and outer membrane proteins [129]. Similarly, quantitative gel methods have been used to determine the protein composition of two tuberculosis strains used in BCG vaccines: BCG Moreau, used in the Brazilian BCG vaccine, and BCG Pasteur, the reference strain obtained in the 1920s [130]. Using a combination of 2DE and MALDI-TOF/TOF, the BCG Moreau strain showed increased expression of immunogenic proteins and lower expression of heat shock proteins compared to the standard BCG Pasteur vaccine strain, even though both vaccines are considered protective [130]. These differences potentially explain why the BCG Moreau vaccine is considered one of the most immunogenic among currently available vaccine preparations.
As mentioned, outer membrane vesicle (OMV) vaccines are some of the most complex vaccines due to the inclusion of a phospholipid bilayer, membrane proteins, and periplasmic proteins. Three versions of OMV vaccines have been developed based on the manufacturing methods: detergent-extraction OMV (DOMV), detergent-free extraction with a chelating agent (NOMV), and no extraction (SOMV) [127]. Initial gel analysis has shown that there are slight protein variations in composition between the three OMV vaccines. Using data-dependent LC-MS/MS quantitative proteomics employing duplex dimethyl labeling combined with N-terminal peptide enrichment, it was shown that DOMV vaccines contain significantly more bacterial cytoplasmic proteins than NOMV or SOMV, while SOMV vaccines are free of cytoplasmic contamination [127]. The dimethyl labeling strategy allows for relative quantitation by comparing each light-labeled sample with a heavy-labeled common reference sample. N-terminal peptide enrichment through a phospho-tag approach allows for the sample complexity to be reduced, as internal peptides are depleted and fractionated by strong cation exchange (SCX). In addition, data-dependent acquisition can be utilized to further reduce the complexity of the sample. Using these methods, it was concluded that detergent-free vaccines have the preferred protein composition [127]. Furthermore, this method serves as a starting point for analysis of other complex vaccines.
3.3 Using proteomics to monitor vaccine quality
As previously discussed, having vaccines that can be reproducibly made is of the utmost importance for the health and safety of everyone. While the current continuous cell-line methods of vaccine production has increased reproducibility of the final product, errors and contamination could occur. Therefore, vaccine products are closely monitored. In the U.S., vaccines are regarded as biologicals and are regulated by the Food and Drug Administration’s (FDA) Center for Biologics Evaluation and Research (CBER). While many regulations are in place to test the vaccine during production, the final material must be tested through assays specific for each product contained in the vaccine. This is typically performed using antibodies specific for each main antigen and a number of other assays for all other components, such as visual examination for color and colorimetric assays for moisture level [40]. While this is the gold standard for quality control, new methods are being proposed to increase the throughput of such tests and to have methods where multiple vaccine aspects could be analyzed at one time.
Quantitative proteomic methods are being suggested for quality control of protein-based vaccines, as complex mixtures of proteins can be analyzed at one time. An example of this type of analysis was performed on the Novartis meningitis B vaccine, Bexsero® [131]. As an OMV vaccine, Bexsero® is routinely tested using SDS-PAGE; however this method does not provide a comprehensive picture of all of the components. Tani et al. sought to use a Hi3 label-free quantitation approach. Hi3 operates on the observation that the average MS signal response for the three most intense tryptic peptides per mole of protein is constant. By using an internal standard, absolute quantitation can be performed. While analyzing six independent lots of the Bexsero® vaccine, no significant differences were detected at a 95% confidence level [131]. In addition, it was determined that the current SDS-PAGE method and the Hi3 method showed good correlation with the 13 bands identified by SDS-PAGE; however, the Hi3 method is superior due to its ability to measure lower-abundance proteins. This approach has also been shown to work for influenza vaccines [132, 133]. The process to completely validate proteomic approaches for vaccine quality control will be time intensive; however, these results show that this approach is a valid method for future development and use.
Another quantitative approach proposed for assessing the quality of vaccines is multiple reaction monitoring (MRM). MRM is already used for quantifying small molecules in the pharmaceutical industry [134]. MRM utilizes ion-monitoring techniques in which the MS analysis is focused on ions of specific masses while excluding other ions. Specifically, MRM uses pre-selected precursor and product pairs (transitions) for quantitation of known analytes [135]. MRM is especially useful when antibodies are not available. Therefore, this technique could make initial quality control of vaccines quicker, as antibodies would not need to be generated. In addition, specific MRM methods would only need to be developed once for each vaccine being tested by determining the specific precursor/fragment ion pair masses; the same transitions could then be used for each subsequent batch. Further, MRM allows for multiplexing the analysis, as 50–100 precursor/fragment ion pair masses can be set during one run, so fewer analysis runs are required in order to obtain results for all the pertinent components [135, 136].
4 Analyzing the human proteomic response to vaccination
All aspects of vaccine development are necessary; however, the most important is the human response after vaccination. Although vaccines are tested in animal models and mammalian cell cultures, the human immune response is significantly more complex and precise mechanisms have not been elucidated for most responses [137]. For example, while it is known that infants and the elderly have a lower level of protection from vaccines, the exact mechanism leading to this observation has not been fully elucidated [138]. Similarly, the mechanism underlying severe adverse reactions to vaccines would be beneficial to understand. Being able to quickly determine vaccine efficacy based on specific protein changes would aid in instances of epidemics and pandemics when speed is the critical factor.
Systems biology studies the human response to vaccines and has typically utilized transcriptomic studies. However, a strong increase in studies utilizing proteomic approaches to achieve the same endpoints has occurred in the past two years. Initial proteomic studies took an almost identical approach to RNA studies and used protein and peptide microarrays. Davies et al. printed the entire proteome of vaccinia virus on microarray chips and then probed the chips with serum from vaccinated individuals [139]. Antibodies in the serum bound to proteins on the chip and were observed using fluorescent secondary antibodies to obtain a quantitative measurement. This showed that half of the antibodies were specific to non-envelope proteins such as core proteins A10, L4, and I1 as well as the A-type inclusion protein WR148, suggesting that a large portion of the antibody response is not involved in virus neutralization. However it was difficult to identify the antigens recognized by all subjects, indicating a large heterogeneity in individual antibody profiles [139]. Price et al. found similar results using both whole protein and peptide microarrays for influenza. In addition, peptide-array reactivity significantly correlated with age and neutralization titer, a correlate of protection [140]. Using a time course study after a tetanus booster, several specific clonotypes were found to be highly expanded on day 7, and >40% of the detectable serum antibody response after 9 months was traceable to three antibody clonotypes [141]. Surprisingly, it was noted that the post-booster antibody repertoire was largely identical to the pre-vaccination serum repertoire.
While these studies have investigated the immune response post-vaccination, it has also been proposed that certain biomarkers present prior to vaccination can also predict the vaccine response. Using peptide microarrays, four viral influenza hemagglutinin peptides were identified with expression levels that correlated to the pre-vaccination HAI titer [142]. This allowed for a model that successfully identified “good” and “poor” responders to influenza vaccination based on their baseline antibody repertoire. This type of finding is potentially useful during pandemics, as individuals could be tested before being vaccinated to determine how well they might respond to the vaccine. From those results, a second dose of vaccine could be given to those individuals who are predicted to be poor responders.
5 Using proteomics to develop cancer vaccines
Vaccines can be either prophylactic or therapeutic. Prophylactic vaccines are used to prevent or reduce the effects of infection by natural pathogens, while therapeutic vaccines aid in the treatment of disease [143]. Therapeutic vaccines are being developed to target an adaptive immune response against cancer, and in particular, tumors. Similar to vaccines targeting viral components, vaccines containing specific host MHC epitopes have been a large focus of cancer vaccines to directly target the adaptive response to cancer cells [7, 144]. Much like other epitope vaccines, the majority of the research to identify epitopes has been using genetic and bioinformatic methods [144, 145]. However, LC-MS/MS was used to verify a melanoma-specific nine-residue peptide, RTKQLYPEW, recognized by cytotoxic T cells from a melanoma patient [146]. These vaccines contain cancer cell-specific antigens; however, the vaccines must also take into consideration the individual chemotherapy treatment plan, as the cells are changed because of treatment [147]. This has greatly increased the difficulty for developing such vaccines as they are practically personalized. Continued research in this area of personalized cancer vaccines will identify the appropriate targets for these vaccines and determine how to use them in practice.
Similarly, identification of neoantigens for cancer immunotherapy has been of interest. Neoantigens are created by tumor-specific DNA alterations that result in novel protein sequences [148]. These are completely absent from the normal human proteome, and thus act as non-self proteins and can stimulate the immune response. Two recent preclinical studies in mice tumor cell lines have identified potential MHC class I bound neoantigens, using a combination of whole-exome sequencing, transcriptomics, and mass spectrometry [149, 150]. Yadav et al. then modeled their identified neoantigens in the context of the MHC receptor and identified seven that had good potential to be immunogenic. After injecting these into mice and monitoring the response, they identified T cells responding specifically to Reps1, Adpgk, and Dpag1 for further validation [149]. Currently, their method is too complicated for a clinical application, but they have hopes of creating a model to better identify these immunogenic neoantigens.
To date, only one cancer-specific therapeutic vaccine has been approved by the FDA: a dendritic-cell-based vaccine for castration-resistant prostate cancer [151]. This vaccine consists of a prostate antigen, prostatic acid phosphatase (PA2024) fused to a granulocyte-macrophage colony-stimulating factor. However, the vaccine has not been shown to induce prostate cancer remission; rather, it only prolongs survival by an average of four months. While these results are provocative, they also motivate researchers to identify the reasons these vaccines have been largely ineffective.
6 Concluding remarks
Recent improvements in proteomic technology have helped aid our understanding of vaccine development and how the immune system responds when subjected to a vaccine. However, there is still a large gap in our understanding for the majority of bacteria and viruses. The WHO expects at least one new emerging or one reemerging pathogen per year, meaning there will always be a need to develop new vaccines and to improve old vaccines. The most rapidly evolving area of vaccine research is in systems vaccinology (Figure 4). While many studies presented here utilize the concepts of systems biology, they fall short on integrating all the different types of data available. Post-translational modifications are not identified, as most systems vaccinology studies have focused solely on transcriptomics. These types of modifications are only detectable through proteomic methods. While transcriptomic data and proteomic data are related, each offers a different perspective. Through a combination of the techniques, a more complete picture of the system can be obtained [152]. While current systems vaccinology papers tend to focus only on one of these techniques, the future of this field will rely on a combination of both transcriptomics and proteomics.
Figure 4.

Overview of systems vaccinology methods. Systems vaccinology aims to collect and integrate data from all components of the immune response. A combination of standardized immune serologic assays with the high-throughput transcriptomic, proteomic, and metabolomic measurements will provide the opportunity to predict immunologic protection from vaccines. [153]. Reprinted from Seminars in Immunology, 25, Li, S. et al., Systems biological approaches to measure and understand vaccine immunity in humans, 209–218, 2013, with permission from Elsevier.
Acknowledgment
This work was funded by the Immunobiology of Blood and Vascular Systems training grant 5T32HL069765-12, RO1 grant GM64779, and Vanderbilt University School of Medicine IDEAS Program grant 1-04-066-9530, and in part with Federal funds from the National Institutes of Allergy and Infectious Disease, National Institutes of Health, Department of Health and Human Services, under Contract No. 272200800007C and the Vanderbilt Clinical and Translational Science Award grant NIH RR024975. AG was supported by grant 5T32HL069765-12 and AL was supported by RO1 grant GM64779. We sincerely thank Parimal Samir, Dr. Kristen Hoek, Dr. Elizabeth M. Link, and Dr. Clarence Creech for their thoughtful comments in the preparation of this manuscript.
Abbreviations
- APC
Antigen Presenting Cell
- BCG
Bacillus Calmette-Guerin
- BCR
B-Cell Receptor
- CBER
Center for Biologics Evaluation and Research
- CDC
Centers for Disease Control and Prevention
- DENV
Dengue Virus
- DOMV
Detergent-extraction Outer Membrane Vesicle
- ELISPOT
Enzyme-Linked ImmunoSpot
- EV
Extracellular Vesicle
- FDA
Food and Drug Administration
- HA
Hemagglutinin
- HAI
Hemagglutination Inhibition
- IND
Investigational New Drug
- MDCK
Madin-Darby Canine Kidney cells
- MHC
Major Histocompatibility Complex
- MMR
Measles Mumps Rubella
- NA
Neuraminidase
- NK
Natural Killer
- OMV
Outer Membrane Vesicle
- PAMP
Pathogen-Associated Molecular Pattern
- PBMC
Peripheral Blood Mononuclear Cell
- PRR
Pattern Recognition Receptor
- SCX
Strong Cation Exchange
- SILAC
Stable Isotope Labeling by Amino acids in Cell culture
- VAERS
Vaccine Adverse Event Reporting System
- WHO
World Health Organization
Footnotes
Conflict of Interest:
The authors have declared no conflict of interest.
References
- 1.World Health Organization. The Global Burden of Disease 2004 Update. Geneva: Switzerland; 2008. [Google Scholar]
- 2.National Institute of Allergy and Infectious Diseases. NIAID: Planning for the 21st Century. 2000 [Google Scholar]
- 3.Germain RN. Vaccines and the Future of Human Immunology. Immunity. 2010;33:441–450. doi: 10.1016/j.immuni.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Global Vaccine Action Plan 2011–2020. Geneva: Switzerland; 2013. [Google Scholar]
- 5.World Health Organization. Antigenic and genetic characteristics of zoonotic influenza viruses and development of candidate vaccine viruses for pandemic preparedness. 2013 [PubMed]
- 6.Koff WC, Burton DR, Johnson PR, Walker BD, et al. Accelerating Next-Generation Vaccine Development for Global Disease Prevention. Science. 2013;340:1232910. doi: 10.1126/science.1232910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adamczyk-Poplawska M, Markowicz S, Jagusztyn-Krynicka EK. Proteomics for development of vaccine. J Proteomics. 2011;74:2596–2616. doi: 10.1016/j.jprot.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Yates JR, Ruse CI, Nakorchevsky A. Proteomics by Mass Spectrometry: Approaches, Advances, and Applications. Annu Rev Biomed Eng. 2009;11:49–79. doi: 10.1146/annurev-bioeng-061008-124934. [DOI] [PubMed] [Google Scholar]
- 9.Jagusztyn-Krynicka EK, Dadlez M, Grabowska A, Roszczenko P. Proteomic technology in the design of new effective antibacterial vaccines. Expert Rev Proteomics. 2009;6:315–330. doi: 10.1586/epr.09.47. [DOI] [PubMed] [Google Scholar]
- 10.Oudenhove LV, Devreese B. A review on recent developments in mass spectrometry instrumentation and quantitative tools advancing bacterial proteomics. Appl Microbio Biotechnol. 2013;97:4749–4762. doi: 10.1007/s00253-013-4897-7. [DOI] [PubMed] [Google Scholar]
- 11.Yarmush ML, Jayaraman A. Advances in proteomic technologies. Annu Rev Biomed Eng. 2002;4:349–373. doi: 10.1146/annurev.bioeng.4.020702.153443. [DOI] [PubMed] [Google Scholar]
- 12.Nikolov M, Schmidt C, Urlaub H. In: Quantitative Methods in Proteomics. Marcus K, editor. New York: Humana Press; 2012. pp. 85–100. [Google Scholar]
- 13.Gant-Branum RL, Kerr TJ, McLean JA. Labeling strategies in mass spectrometry-based protein quantitation. Analyst. 2009;134:1525. doi: 10.1039/b904643g. [DOI] [PubMed] [Google Scholar]
- 14.Lee JR, Magee DM, Gaster RS, LaBaer J, Wang SX. Emerging protein array technologies for proteomics. Expert Rev Proteomics. 2013;10:65–75. doi: 10.1586/epr.12.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall DA, Ptacek J, Snyder M. Protein microarray technology. Mechanisms of ageing and development. 2007;128:161–167. doi: 10.1016/j.mad.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breker M, Schuldiner M. The emergence of proteome-wide technologies: systematic analysis of proteins comes of age. Nature reviews. Molecular cell biology. 2014;15:453–464. doi: 10.1038/nrm3821. [DOI] [PubMed] [Google Scholar]
- 17.Aebersold R, Mann M. Mass spectrometry-based proteomics. Nature. 2003;422:198–207. doi: 10.1038/nature01511. [DOI] [PubMed] [Google Scholar]
- 18.Plotkin SL, Plotkin SA. In: Vaccines. Plotkin SA, Orenstein WA, Offit PA, editors. China: Elsevier; 2008. pp. 1–16. [Google Scholar]
- 19.Jesty R, Williams G. Who invented vaccination? Malta Medical Journal. 2011;23:29–32. [Google Scholar]
- 20.Blanco JCG, Boukhvalova MS, Shirey KA, Prince GA, Vogel SN. New insights for development of a safe and protective RSV vaccine. Hum Vaccin. 2014;6:482–492. doi: 10.4161/hv.6.6.11562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moghaddam A, Olszewska W, Wang B, Tregoning JS, et al. A potential molecular mechanism for hypersensitivity caused by formalin-inactivated vaccines. Nature medicine. 2006;12:905–907. doi: 10.1038/nm1456. [DOI] [PubMed] [Google Scholar]
- 22.Delgado MF, Coviello S, Monsalvo AC, Melendi GA, et al. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nature medicine. 2009;15:34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knudson CJ, Hartwig SM, Meyerholz DK, Varga SM. RSV vaccine-enhanced disease is orchestrated by the combined actions of distinct CD4 T cell subsets. PLoS Pathog. 2015;11:e1004757. doi: 10.1371/journal.ppat.1004757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loebbermann J, Durant L, Thornton H, Johansson C, Openshaw PJ. Defective immunoregulation in RSV vaccine-augmented viral lung disease restored by selective chemoattraction of regulatory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:2987–2992. doi: 10.1073/pnas.1217580110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldblatt D. Conjugate Vaccines. Clin Exp Immunol. 2000;119:1–3. doi: 10.1046/j.1365-2249.2000.01109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Artz AS, Ershler WB, Longo DL. Pneumococcal Vaccination and Revaccination of Older Adults. Clin Microbiol Rev. 2003;16:308–318. doi: 10.1128/CMR.16.2.308-318.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson HL, Pertmer TM. DNA vaccines for viral infections: basic studies and applications. Adv Virus Res. 2000;55:1–74. doi: 10.1016/s0065-3527(00)55001-5. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez F, Zhang J, Whitton JL. DNA Immunization: ubiquitination of a viral protein enhances cytotoxic T-lymphocyte induction and antiviral protection but abrogates antibody induction. J Virol. 1997;71:8497–8503. doi: 10.1128/jvi.71.11.8497-8503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis PJ, Babluk LA. DNA vaccines: a review. Adv Virus Res. 1999;54:129–188. doi: 10.1016/s0065-3527(08)60367-x. [DOI] [PubMed] [Google Scholar]
- 30.O’Hagan DT, Singh M, Ulmer JB. Microparticle-based technologies for vaccines. Methods. 2006;40:10–19. doi: 10.1016/j.ymeth.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 31.Ferraro B, Morrow MP, Hutnick NA, Shin TH, et al. Clinical applications of DNA vaccines: current progress. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;53:296–302. doi: 10.1093/cid/cir334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiner DB, Nabel GJ. In: Vaccies, Expert Consult. Plotkin SA, Orenstein WA, Offit PA, editors. China: Elsevier; 2013. pp. 1232–1242. [Google Scholar]
- 33.Coffman RL, Sher A, Seder RA. Vaccine Adjuvants: Putting Innate Immunity to Work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Travis K. Deciphering Immunology’s Dirty Secret. The Scientist. 2007 [Google Scholar]
- 35.Glenny A. Insoluble precipitates in diptheria and tetanus immunization. Br Med J. 1930;2:244–245. doi: 10.1136/bmj.2.3632.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Veer M. New developments in vaccine research -- unveiling the secret of vaccine adjuvants. Discov Med. 2011;12:195–204. [PubMed] [Google Scholar]
- 37.Sayers S, Ulysse G, Xiang Z, He Y. Vaxjo: a web-based vaccine adjuvant database and its application for analysis of vaccine adjuvants and their uses in vaccine development. Journal of biomedicine & biotechnology. 2012;2012:831486. doi: 10.1155/2012/831486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ribeiro CMS, Schijns VEJC. In: Vaccine Adjuvants. Davies G, editor. New York: Humana Press; 2010. pp. 1–14. [Google Scholar]
- 39.Murphy K. Janeway’s Immunobiology. London: Garland Science; 2012. [Google Scholar]
- 40.Baylor NW, Midthun K. Vaccines. China: 2008. pp. 1611–1627. [Google Scholar]
- 41.Weinberg GA, Szilagyi PG. Vaccine epidemiology: efficacy, effectiveness, and the translational research roadmap. The Journal of infectious diseases. 2010;201:1607–1610. doi: 10.1086/652404. [DOI] [PubMed] [Google Scholar]
- 42.Food and Drug Administration. Guidance for industry: expedited programs for serious conditions - drugs and biologics. Silver Spring, MD: 2014. [Google Scholar]
- 43.Plotkin SA. Correlates of Protection Induced by Vaccination. Clin Vaccine Immunol. 2010;17:1055–1065. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weinberg A, Zhang JH, Oxman MN, Johnson GR, et al. Varicella-Zoster Virus-Specific Immune Responses to Herpes Zoster in Elderly Participants in a Trial of a Clinically Effective Zoster Vaccine. The Journal of infectious diseases. 2009;200:1068–1077. doi: 10.1086/605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fletcher H. Correlates of Immune Protection from Tuberculosis. Curr Mold Med. 2007;7:319–325. doi: 10.2174/156652407780598520. [DOI] [PubMed] [Google Scholar]
- 46.Pulendran B, Li S, Nakaya HI. Systems Vaccinology. Immunity. 2010;33:516–529. doi: 10.1016/j.immuni.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Querec TD, Akondy RS, Lee EK, Cao W, et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2009;10:116–125. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gaucher D, Therrien R, Kettaf N, Angermann BR, et al. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J Exp Med. 2008;205:3119–3131. doi: 10.1084/jem.20082292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakaya HI, Wrammert J, Lee EK, Racioppi L, et al. Systems biology of vaccination for seasonal influenza in humans. Nat Immunol. 2011;12:786–795. doi: 10.1038/ni.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bucasas KL, Franco LM, Shaw CA, Bray MS, et al. Early Patterns of Gene Expression Correlate With the Humoral Immune Response to Influenza Vaccination in Humans. The Journal of infectious diseases. 2011;203:921–929. doi: 10.1093/infdis/jiq156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li S, Rouphael N, Duraisingham S, Romero-Steiner S, et al. Molecular signatures of antibody responses derived from a systems biology study of five human vaccines. Nat Immunol. 2014;15:195–204. doi: 10.1038/ni.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.D’Argenio DA, Wilson CB. A Decade of Vaccines: Integrating Immunology and Vaccinology for Rational Vaccine Design. Immunity. 2010;33:437–440. doi: 10.1016/j.immuni.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 53.Yeung ES. Genome-wide Correlation between mRNA and Protein in a Single Cell. Angew Chem Int Ed Engl. 2011;50:583–585. doi: 10.1002/anie.201005969. [DOI] [PubMed] [Google Scholar]
- 54.Anderson L, Silhamer J. A comparison of selected mRNA and protein abundances in human liver. Electrophoresis. 1997;18:533–537. doi: 10.1002/elps.1150180333. [DOI] [PubMed] [Google Scholar]
- 55.Greenbaum D, Colangelo C, Williams K, Mark G. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol. 2003;4:117.111–117.118. doi: 10.1186/gb-2003-4-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rappuoli R. Reverse Vaccinology. Curr Opin Microbiol. 2000;3:445–450. doi: 10.1016/s1369-5274(00)00119-3. [DOI] [PubMed] [Google Scholar]
- 57.Link AJ, Eng J, Schieltz DM, Carmack E, et al. Direct analysis of protein complexes using mass spectrometry. Nat Biotechnol. 1999;17:676–682. doi: 10.1038/10890. [DOI] [PubMed] [Google Scholar]
- 58.McAtee CP, Fry KE, Berg DE. Identification of potential diagnostic and vaccine candidates of Helicobacter pylori by “proteome” technologies. Helicobacter. 1998;3:163–169. [PubMed] [Google Scholar]
- 59.Velin D, Michetti P. Advances in vaccination against Helicobacter pylori. Expert Rev Gastroenterol Hepatol. 2010;4:157–166. doi: 10.1586/egh.10.6. [DOI] [PubMed] [Google Scholar]
- 60.Jagusztyn-Krynicka EK, Roszczenko P, Grabowska A. Impact of proteomics on anti-Mycobacterium tuberculosis (MTB) vaccine development. Pol J Microbiol. 2009;58:281–287. [PubMed] [Google Scholar]
- 61.Gunawardena HP, Feltcher ME, Wrobel JA, Gu S, et al. Comparison of the Membrane Proteome of Virulent Mycobacterium tuberculosis and the Attenuated Mycobacterium bovis BCG Vaccine Strain by Label-Free Quantitative Proteomics. J Proteome Res. 2013;12:5463–5474. doi: 10.1021/pr400334k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Delvecchio VG, Connolly JP, Alefantis TG, Walz A, et al. Proteomic profiling and identification of immunodominant spore antigens of Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis. Appl Environ Microbiol. 2006;72:6355–6363. doi: 10.1128/AEM.00455-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gouw Dd, Jonge MId, Hermans PWM, Wessels HJCT, et al. Proteomics-Identified Bvg-Activated Autotransporters Protect against Bordetella pertussis in a Mouse Model. PLoS One. 2014;9:e105011. doi: 10.1371/journal.pone.0105011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Flynn JL, Chan J. Immune evasion by Mycobacterium tuberculosis: living with the enemy. Curr Opin Immunol. 2003;15:450–455. doi: 10.1016/s0952-7915(03)00075-x. [DOI] [PubMed] [Google Scholar]
- 65.Peterson JW. In: Medical Microbiology. Baron S, editor. Galveston, TX: University of Texas Medical Branch at Galveston; 1996. [PubMed] [Google Scholar]
- 66.Olaya-Abril A, Gómez-Gascón L, Jiménez-Munguía I, Obando I, Rodríguez-Ortega MJ. Another turn of the screw in shaving Gram-positive bacteria: Optimization of proteomics surface protein identification in Streptococcus pneumoniae. J Proteomics. 2012;75:3733–3746. doi: 10.1016/j.jprot.2012.04.037. [DOI] [PubMed] [Google Scholar]
- 67.Schrotz-King P, Prokhorova TA, Nielsen PN, Crawford JS, Morsczeck C. Campylobacter jejuni proteomics for new travellers’ diarrhoea vaccines. Travel Med Infect Dis. 2007;5:106–109. doi: 10.1016/j.tmaid.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 68.Wang C, Liu Y, Li H, Xu W-j, et al. Identification of plasma-responsive outer membrane proteins and their vaccine potential in Edwardsiella tarda using proteomic approach. J Proteomics. 2012;75:1263–1275. doi: 10.1016/j.jprot.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 69.Rodríguez-Ortega MJ, Norais N, Bensi G, Liberatori S, et al. Characterization and identification of vaccine candidate proteins through analysis of the group A Streptococcus surface proteome. Nat Biotechnol. 2006;24:191–197. doi: 10.1038/nbt1179. [DOI] [PubMed] [Google Scholar]
- 70.Connolly JP, Comerci D, Alefantis TG, Walz A, et al. Proteomic analysis of Brucella abortus cell envelope and identification of immunogenic candidate proteins for vaccine development. Proteomics. 2006;6:3767–3780. doi: 10.1002/pmic.200500730. [DOI] [PubMed] [Google Scholar]
- 71.Tsolakos N, Brookes C, Taylor S, Gorringe A, et al. Identification of vaccine antigens using integrated proteomic analyses of surface immunogens from serogroup B Neisseria meningitidis. J Proteomics. 2014;101:63–76. doi: 10.1016/j.jprot.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 72.Pierson T, Matrakas D, Taylor YU, Manyam G, et al. Proteomic Characterization and Functional Analysis of Outer Membrane Vesicles of Francisella novicida Suggests Possible Role in Virulence and Use as a Vaccine. J Proteome Res. 2011;10:954–967. doi: 10.1021/pr1009756. [DOI] [PubMed] [Google Scholar]
- 73.Vipond C, Suker J, Jones C, Tang C, et al. Proteomic analysis of a meningococcal outer membrane vesicle vaccine prepared from the group B strain NZ98/254. Proteomics. 2006;6:3400–3413. doi: 10.1002/pmic.200500821. [DOI] [PubMed] [Google Scholar]
- 74.Rosenqvist E, Hoiby EA, Wedege E, Bryn K, et al. Human antibody responses to meningococcal outer membrane antigens after three doses of the Norwegian Group B Meningococcal vaccine. Infect Immun. 1995;63:4642–4652. doi: 10.1128/iai.63.12.4642-4652.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nøkleby H, Aavitsland P, O’Hallahan J, Feiring B, et al. Safety review: two outer membrane vesicle (OMV) vaccines against systemic Neisseria meningitidis serogroup B disease. Vaccine. 2007;25:3080–3084. doi: 10.1016/j.vaccine.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 76.Novartis . In: Novartis, editor. Switzerland: Basel; 2013. pp. 1–3. [Google Scholar]
- 77.Olaya-Abril A, Prados-Rosales R, McConnell MJ, Martín-Peña R, et al. Characterization of protective extracellular membrane-derived vesicles produced by Streptococcus pneumoniae. J Proteomics. 2014;106:46–60. doi: 10.1016/j.jprot.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 78.Smith AE, Helenius A. How Viruses Enter Animal Cells. Science. 2004;304:237–242. doi: 10.1126/science.1094823. [DOI] [PubMed] [Google Scholar]
- 79.Salazar MI, del Angel RM, Lanz-Mendoza H, Ludert JE, Pando-Robles V. The role of cell proteins in dengue virus infection. J Proteomics. 2014;111:6–15. doi: 10.1016/j.jprot.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 80.Wati S, Soo ML, Zilm P, Li P, et al. Dengue virus infection induces upregulation of GRP78, which acts to chaperone viral antigen production. J Virol. 2009;83:12871–12880. doi: 10.1128/JVI.01419-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mishra KP, Shweta Diwaker D, Ganju L. Dengue virus infection induces upregulation of hn RNP-H and PDIA3 for its multiplication in the host cell. Virus Res. 2012;163:573–579. doi: 10.1016/j.virusres.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 82.Kanlaya R, Pattanakitsakul S-n, Sinchaikul S, Chen S-T, Thongboonkerd V. The Ubiquitin−Proteasome Pathway Is Important for Dengue Virus Infection in Primary Human Endothelial Cells. J Proteome Res. 2010;9:4960–4971. doi: 10.1021/pr100219y. [DOI] [PubMed] [Google Scholar]
- 83.Smith SA, de Alwis AR, Kose N, Jadi RS, et al. Isolation of dengue virus-specific memory B cells with live virus antigen from human subjects following natural infection reveals the presence of diverse novel functional groups of antibody clones. J Virol. 2014;88:12233–12241. doi: 10.1128/JVI.00247-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Palermo RE, Fuller DH. In: Systems Biology. Katze MG, editor. Heidelberg: Springer Berlin Heidelberg; 2013. pp. 87–116. [Google Scholar]
- 85.Navare AT, Sova P, Purdy DE, Weiss JM, et al. Quantitative proteomic analysis of HIV-1 infected CD4+ T cells reveals an early host response in important biological pathways: Protein synthesis, cell proliferation, and T-cell activation. Virology. 2012;429:37–46. doi: 10.1016/j.virol.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stein DR, Burgener A, Ball TB. Proteomics as a novel HIV immune monitoring tool. Curr Opin HIV AIDS. 2013;8:140–146. doi: 10.1097/COH.0b013e32835d3271. [DOI] [PubMed] [Google Scholar]
- 87.Huang J, Kang BH, Pancera M, Lee JH, et al. Broad and potent HIV-1 neutralization by a human antibody that binds the gp41-gp120 interface. Nature. 2014;515:138–142. doi: 10.1038/nature13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mintern JD, Guillonneau C, Turner SJ, Doherty PC. In: Influenza Vaccines for the Future. Del Giudice G, Rappuoli R, editors. Berlin: Springer; 2010. pp. 173–197. [Google Scholar]
- 89.Teijaro JR. The role of cytokine responses during influenza virus pathogenesis and potential therapeutic options. Current topics in microbiology and immunology. 2015;386:3–22. doi: 10.1007/82_2014_411. [DOI] [PubMed] [Google Scholar]
- 90.Iwasaki A, Pillai PS. Innate immunity to influenza virus infection. Nat Rev Immunol. 2014;14:315–328. doi: 10.1038/nri3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kreijtz JHCM, Fouchier RAM, Rimmelzwaan GF. Immune responses to influenza virus infection. Virus Res. 2011;162:19–30. doi: 10.1016/j.virusres.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 92.Ulmer JB. Influenza DNA vaccines. Vaccine. 2002;20:S74–S76. doi: 10.1016/s0264-410x(02)00136-6. [DOI] [PubMed] [Google Scholar]
- 93.Berthoud TK, Hamill M, Lillie PJ, Hwenda L, et al. Potent CD8+ T-cell immunogenicity in humans of a novel heterosubtypic influenza A vaccine, MVA-NP+M1. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;52:1–7. doi: 10.1093/cid/ciq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cox R, Brokstad K, Zuckerman M, Wood J, et al. An early humoral immune response in peripheral blood following parenteral inactivated influenza vaccination. Vaccine. 1994;12:993–999. doi: 10.1016/0264-410x(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 95.Bender BS, Croghan T, Zhang L, Small PAJ. Transgenic mice lacking class I major histocompatability complext-restricted T cells have delayed viral clearance and increased mortality after influenza virus challenge. J Exp Med. 1992;175:1143–1145. doi: 10.1084/jem.175.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shoshan SH, Admon A. MHC-bound antigens and proteomics for novel target discovery. Future Med. 2004;5:845–859. doi: 10.1517/14622416.5.7.845. [DOI] [PubMed] [Google Scholar]
- 97.Patronov A, Doytchinova I. T-cell epitope vaccine design by immunoinformatics. Biol Open. 2013;3:120139. doi: 10.1098/rsob.120139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hurtgen BJ, Hung CY, Ostroff GR, Levitz SM, Cole GT. Construction and evaluation of a novel recombinant T cell epitope-based vaccine against Coccidioidomycosis. Infect Immun. 2012;80:3960–3974. doi: 10.1128/IAI.00566-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gilchuk P, Hill TM, Wilson JT, Joyce S. Discovering protective CD8 T cell epitopes-no single immunologic property predicts it! Curr Opin Immunol. 2015;34:43–51. doi: 10.1016/j.coi.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hunt DF, Henderson RA, Shabanowitz J, Sakaguchi K, et al. Characterization of peptides bound to the Class I MHC molecule HLA-A2.1 by mass spectrometry. Science. 1992;255:1261–1263. doi: 10.1126/science.1546328. [DOI] [PubMed] [Google Scholar]
- 101.Gilchuk P, Spencer CT, Conant SB, Hill T, et al. Discovering naturally processed antigenic determinants that confer protective T cell immunity. The Journal of clinical investigation. 2013;123:1976–1987. doi: 10.1172/JCI67388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Strug I, Calvo-Valle JM, Green KM, Cruz J, et al. Vaccinia peptides eluted from HLA-DR1 isolated from virus-infected cells are recognized by CD4+ T cells from a vaccinated donor. J Proteome Res. 2008;7:2703–2711. doi: 10.1021/pr700780x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Johnstone C, Lorente E, Barriga A, Barnea E, et al. The viral transcription group determines the HLA class I cellular immune response against Human Respiratory Syncytial Virus. Molecular & cellular proteomics : MCP. 2015 doi: 10.1074/mcp.M114.045401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Karunakaran KP, Yu H, Jiang X, Chan Q, et al. Outer membrane proteins preferentially load MHC class II peptides: Implications for a Chlamydia trachomatis T cell vaccine. Vaccine. 2015 doi: 10.1016/j.vaccine.2015.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bridges CB, Katz JM, Levandrowski RA, Cox NJ. Vaccines, Expert Consult. China: Elsevier, Inc; 2008. pp. 259–290. [Google Scholar]
- 106.Gottlieb T, Ben-Yedidia T. Epitope-based approaches to a universal influenza vaccine. J Autoimmun. 2014 doi: 10.1016/j.jaut.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 107.Uranowska K, Tyborowska J, Jurek A, Szewczyk B, Gromadzka B. Hemagglutinin stalk domain from H5N1 strain as a potentially universal antigen. Acta Biochim Pol. 2014;61:541–550. [PubMed] [Google Scholar]
- 108.Kesik-Brodacka M, Plucienniczak G. A universal flu vaccine. Acta Biochim Pol. 2014;61:523–530. [PubMed] [Google Scholar]
- 109.Lu Y, Welsh JP, Swartz JR. Production and stabilization of the trimeric influenza hemagglutinin stem domain for potentially broadly protective influenza vaccines. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:125–130. doi: 10.1073/pnas.1308701110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hotez PJ, Bethony JM. In: Vaccines. Plotkin SA, Orenstein WA, Offit PA, editors. China: Elsevier Saunders; 2013. pp. 1154–1160. [Google Scholar]
- 111.Sacks DL. Vaccines against tropical parasitic diseases: a persisting answer to a persisting problem. Nat Immunol. 2014;15:403–405. doi: 10.1038/ni.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Seder RA, Chang L-J, Enama ME, Zephir KL, et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science. 2013;341:1359–1365. doi: 10.1126/science.1241800. [DOI] [PubMed] [Google Scholar]
- 113.Khamesipour A, Dowlati Y, Asilian A, Hashemi-Fesharki R, et al. Leishmanization: use of an old method for evaluation of candidate vaccines against leishmaniasis. Vaccine. 2005;23:3642–3648. doi: 10.1016/j.vaccine.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 114.Bautista JM, Marín-García P, Diez A, Azcárate IG, Puyet A. Malaria proteomics: Insights into the parasite-host interactions in the pathogenic space. J Proteomics. 2014;97:107–125. doi: 10.1016/j.jprot.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 115.Dumonteil E. Vaccine development against Trypanosoma cruzi and Leishmania species in the post-genomic era. Infect Genet Evol. 2009;9:1075–1082. doi: 10.1016/j.meegid.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 116.Thera MA, Doumbo OK, Coulibaly D, Laurens MB, et al. A field trial to assess a blood-stage malaria vaccine. N Engl J Med. 2011;365:1004–1013. doi: 10.1056/NEJMoa1008115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nakayasu ES, Sobreira TJP, Torres R, Ganiko L, et al. Improved Proteomic Approach for the Discovery of Potential Vaccine Targets in Trypanosoma cruzi. J Proteome Res. 2012;11:237–246. doi: 10.1021/pr200806s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tian F, Hou M, Chen L, Gao Y, et al. Proteomic analysis of schistosomiasis japonica vaccine candidate antigens recognized by UV-attenuated cercariae-immunized porcine serum IgG2. Parasitol Res. 2013;112:2791–2803. doi: 10.1007/s00436-013-3447-7. [DOI] [PubMed] [Google Scholar]
- 119.Gygi SP, Rist B, Gerber SA, Turecek F, et al. Quantitative analysis of complex protein mixtures using isotype-coded affinity tags. Nat Biotechnol. 1999;17:994–999. doi: 10.1038/13690. [DOI] [PubMed] [Google Scholar]
- 120.Ong SE, Mann M. Mass spectrometry-based proteomics turns quantitative. Nature chemical biology. 2005;1:252–262. doi: 10.1038/nchembio736. [DOI] [PubMed] [Google Scholar]
- 121.Ross PL, Huang YN, Marchese JN, Williamson B, et al. Multiplexed Protein Quantitation in Saccharomyces cerevisiae Using Amine-reactive Isobaric Tagging Reagents. Molecular & cellular proteomics : MCP. 2004;3:1154–1169. doi: 10.1074/mcp.M400129-MCP200. [DOI] [PubMed] [Google Scholar]
- 122.Racaniello V. Influenza virus growth in eggs. New York City: 2009. [Google Scholar]
- 123.CDC, editor. Centers for Disease Control and Prevention. Seasonal influenza vaccine supply for the U.S. 2014–2015 influenza season. 2014. [Google Scholar]
- 124.Govorkova EA, Murti G, Meignier B, Taisne Cd, Webster RG. African green monkey kidney (Vero) cells provide an alternative host cell system for influenza A and B viruses. J Virol. 1996;70:5519–5524. doi: 10.1128/jvi.70.8.5519-5524.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vester D, Rapp E, Kluge S, Genzel Y, Reichl U. Virus-host cell interactions in vaccine production cell lines infected with different human influenza A virus variants: A proteomic approach. J Proteomics. 2010;73:1656–1669. doi: 10.1016/j.jprot.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 126.McPherson CE. Development of a novel recombinant influenza vaccine in insect cells. Biologicals : journal of the International Association of Biological Standardization. 2008;36:350–353. doi: 10.1016/j.biologicals.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 127.van de Waterbeemd B, Mommen GPM, Pennings JLA, Eppink MH, et al. Quantitative Proteomics Reveals Distinct Differences in the Protein Content of Outer Membrane Vesicle Vaccines. J Proteome Res. 2013;12:1898–1908. doi: 10.1021/pr301208g. [DOI] [PubMed] [Google Scholar]
- 128.Goethe R, Gonzáles OF, Lindner T, Gerlach GF. A novel strategy for protective Actinobacillus pleuropneumoniae subunit vaccines: detergent extraction of cultures induced by iron restriction. Vaccine. 2000;19:966–975. doi: 10.1016/s0264-410x(00)00212-7. [DOI] [PubMed] [Google Scholar]
- 129.Buettner FF, Konze SA, Maas A, Gerlach GF. Proteomic and immunoproteomic characterization of a DIVA subunit vaccine against Actinobacillus pleuropneumoniae. Proteome Sci. 2011;9:23. doi: 10.1186/1477-5956-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Berredo-Pinho M, Kalume DE, Correa PR, Gomes LH, et al. Proteomic profile of culture filtrate from the Brazilian vaccine strain Mycobacterium bovis BCG Moreau compared to M. bovis BCG Pasteur. BMC Microbiol. 2011;11:80. doi: 10.1186/1471-2180-11-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tani C, Stella M, Donnarumma D, Biagini M, et al. Quantification by LC-MSE of outer membrane vesicle proteins of the Bexsero® vaccine. Vaccine. 2014;32:1273–1279. doi: 10.1016/j.vaccine.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 132.Getie-Kebtie M, Sultana I, Eichelberger M, Alterman M. Label-free mass spectrometry-based quantification of hemagglutinin and neuraminidase in influenza virus preparations and vaccines. Influenza Other Respir Viruses. 2013;7:521–530. doi: 10.1111/irv.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Creskey MC, Li C, Wang J, Girard M, et al. Simultaneous quantification of the viral antigens hemagglutinin and neuraminidase in influenza vaccines by LC-MSE. Vaccine. 2012;30:4762–4770. doi: 10.1016/j.vaccine.2012.05.036. [DOI] [PubMed] [Google Scholar]
- 134.Eichmeyer MA, Roof MB, Schaeffer ML, Vaughn EM, et al. Boehringer Ingelheim Vetmedica, Inc; 2014. [Google Scholar]
- 135.Kitteringham NR, Jenkins RE, Lane CS, Elliott VL, Park BK. Multiple reaction monitoring for quantitative biomarker analysis in proteomics and metabolomics. J Chromatogr B Biomed Sci Appl. 2009;877:1229–1239. doi: 10.1016/j.jchromb.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 136.Picotti P, Aebersold R. Selected reaction monitoring-based proteomics: workflows, potential, pitfalls and future directions. Nat Methods. 2012;9:555–566. doi: 10.1038/nmeth.2015. [DOI] [PubMed] [Google Scholar]
- 137.Mestas J, Hughes CCW. Of Mice and Not Men: Differences between Mouse and Human Immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 138.Siegrist C. Vaccines, Expert Consult. China: Elsevier, Inc; 2008. pp. 17–36. [Google Scholar]
- 139.Davies DH, Molina DM, Wrammert J, Miller J, et al. Proteome-wide analysis of the serological response to vaccinia and smallpox. Proteomics. 2007;7:1678–1686. doi: 10.1002/pmic.200600926. [DOI] [PubMed] [Google Scholar]
- 140.Price JV, Jarrell JA, Furman D, Kattah NH, et al. Characterization of Influenza Vaccine Immunogenicity Using Influenza Antigen Microarrays. PLoS One. 2013;8:e64555. doi: 10.1371/journal.pone.0064555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lavinder JJ, Wine Y, Giesecke C, Ippolito GC, et al. Identification and characterization of the constituent human serum antibodies elicited by vaccination. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:2259–2264. doi: 10.1073/pnas.1317793111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Furman D, Jojic V, Kidd B, Shen-Orr S, et al. Apoptosis and other immune biomarkers predict influenza vaccine responsiveness. Mol Syst Biol. 2013;9 doi: 10.1038/msb.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Six A, Bellier B, Thomas-Vaslin V, Klatzmann D. Systems biology in vaccine design. Microb Biotechnol. 2012;5:295–304. doi: 10.1111/j.1751-7915.2011.00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vergati M, Intrivici C, Huen NY, Schlom J, Tsang KY. Strategies for cancer vaccine development. Journal of biomedicine & biotechnology. 2010:2010. doi: 10.1155/2010/596432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Arens R, van Hall T, van der Burg SH, Ossendorp F, Melief CJ. Prospects of combinatorial synthetic peptide vaccine-based immunotherapy against cancer. Semin Immunol. 2013;25:182–190. doi: 10.1016/j.smim.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 146.Vigneron N, Stroobant V, Chapiro J, Ooms A, et al. An antigenic peptide produced by peptide splicing in the proteasome. Science. 2004;304:587–590. doi: 10.1126/science.1095522. [DOI] [PubMed] [Google Scholar]
- 147.Balashova EE, Dashtiev MI, Lokhov PG. Proteomic Footprinting of Drug-Treated Cancer Cells as a Measure of Cellular Vaccine Efficacy for the Prevention of Cancer Recurrence. Molecular & cellular proteomics : MCP. 2012;11 doi: 10.1074/mcp.M111.014480. M111.014480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schumacher TN. Neoantigens in cancer immunogherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 149.Yadav M, Jhunjhunwala S, Phung QT, Lupardus P, et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. 2014;515:572–576. doi: 10.1038/nature14001. [DOI] [PubMed] [Google Scholar]
- 150.Gubin MM, Zhang X, Schuster H, Caron E, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kantoff PW, Higano CS, Shore ND, Berger ER, et al. Sipuleucel-T Immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 152.Hoek KL, Samir P, Howard LM, Niu X, et al. A cell-based systems biology assessment of human blood to monitor immune responses after influenza vaccination. PLoS One. 2015;10 doi: 10.1371/journal.pone.0118528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li S, Nakaya HI, Kazmin DA, Oh JZ, Pulendran B. Systems biological approaches to measure and understand vaccine immunity in humans. Semin Immunol. 2013;25:209–218. doi: 10.1016/j.smim.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]