Abstract
Individuals suffering from obstructive sleep apnea (OSA) are at increased risk for systemic hypertension. The importance of a healthy gut microbiota, and detriment of a dysbiotic microbiota, on host physiology is becoming increasingly evident. We tested the hypothesis that gut dysbiosis contributes to hypertension observed with OSA. OSA was modeled in rats by inflating a tracheal balloon during the sleep cycle (10 sec inflations, 60/hour). On normal chow diet, OSA had no effect on blood pressure; however, in rats fed a high fat diet, blood pressure increased 24 and 29mmHg after 7 and 14 days of OSA, respectively (p<0.05 each). Bacterial community characterization was performed on fecal pellets isolated before and after 14 days of OSA in chow and high fat fed rats. High fat diet and OSA led to significant alterations of the gut microbiota including decreases in bacterial taxa known to produce the short chain fatty acid butyrate (p<0.05). Finally, transplant of dysbiotic cecal contents from hypertensive OSA rats on high fat diet into OSA recipient rats on normal chow diet (shown to be normotensive) resulted in hypertension similar to that of the donor (increased 14 and 32mm Hg after 7 and 14 days of OSA, respectively; p<0.05). These studies demonstrate a causal relationship between gut dysbiosis and hypertension, and suggest that manipulation of the microbiota may be a viable treatment for OSA-induced, and possibly other forms of, hypertension.
Keywords: Obstructive sleep apnea, hypertension, microbiota, butyrate, dysbiosis
Introduction
Obstructive sleep apnea (OSA) is a prevalent disorder characterized by repeated collapse of the upper airway during sleep.1, 2 Individual apneic episodes lead to transient hypoxia, hypercapnia and excessive negative intrathoracic pressures as the patient attempts to breathe against a closed airway.2, 3 Additionally, sympathetic activity is increased with arousal when each apnea is resolved.3 Estimates suggest that 5-25% of the adult Western population suffer from clinically significant OSA.1 Risk factors for OSA, including obesity and aging, are on the rise, suggesting that the burden of OSA will continue to increase in the coming years.2
OSA is a significant risk factor for numerous cardiovascular diseases and in many cases increases the disease severity and progression.2, 3 The prevalence of OSA in primary hypertension is ~35%.4 In patients with drug-resistant hypertension, the prevalence of OSA climbs to ~65% to 80%.5–7 Furthermore, treatment of OSA in this population is more successful at lowering blood pressure than multiple drugs; suggesting OSA plays an integral role in hypertension.8
Commensal bacteria cover every surface of our body exposed to the environment. These bacteria, termed microbiota, outnumber our own cells 10:1, with 70% of these residing in the gastrointestinal tract.9 Under normal conditions there is a mutualistic relationship between the host and gut microbiota. The host provides nutrition and a suitable living environment for the bacteria, while the microbiota aid in maintenance of immune response, act as a barrier from invasive pathogens, and contribute nutrients to the host.10, 11 This mutualistic relationship can be compromised by shifts in the composition of the microbiota, termed dysbiosis. Proposed triggers for these bacterial shifts include the overuse of selected antibiotics, infectious agents, and diet.9 Dysbiosis in the gut has been linked to inflammatory disorders including inflammatory bowel disease, metabolic disorders including obesity and diabetes, neurologic diseases including autism spectrum disorder, and atherosclerotic heart disease.12, 13 In this study, we tested the hypothesis that gut dysbiosis contributes to the hypertension observed in OSA.
Materials and Methods
Detailed Materials and Methods are available in the online Data Supplement.
Animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals, 8th edition, published by the National Institutes of Health (NIH) and were approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine, Houston, TX. Long Evans rats were housed with a 12 hr light (6AM-6PM): 12 hr dark (6PM-6AM) cycle. At 8-9 weeks old, a sub set of rats were switched from normal chow to a high fat diet (60% calories from fat) for the remainder of the study (5 weeks total).
Endotracheal Obstruction Device Implantation
Rats, 10-11 weeks old, were implanted with an endotracheal obstruction device as described previously.14, 15 Sham rats underwent identical surgical procedures and device implantation, but endotracheal obstruction devices were never inflated. Rats that underwent periods of apnea are referred to as “OSA rats” and the sham-controls are referred to as “sham rats”.
Experimental Alterations of the Gut Microbiota
The gut microbiota were altered by antibiotics or cecal contents transplantations. Details of each protocol are outlined in the online Data Supplement.
Non-Invasive Blood Pressure Measurements
Tail cuff plethysmography (Harvard Apparatus) was used to measure systolic blood pressure in unanesthetized rats between 8 AM and noon, before, and following 7 and 14 days of OSA or sham. A minimum of 5 consecutive readings, without movement artifact, were averaged for each measurement.
Gut Microbiota Analysis
Fecal samples were collected in sterile tubes before and after 14 days of OSA or sham and stored at −80°C. Detailed methods for identification and analysis of the gut microbiota makeup, using 16S rRNA sequences, are outlined in the online Data Supplement.
Statistics
Line and bar plot data is expressed as mean ± SEM, box plots present median and quartiles. When analyzing blood pressure over several timepoints, a two-way repeated measures ANOVA was used followed by a Holm-Sidak test for individual comparisons when appropriate. Differences were considered statistically significant if p≤0.05. Statistics used for various microbiome analysis are presented in the online Data Supplement.
Results
OSA synergizes with a high fat diet to produce hypertension in Long Evans rats
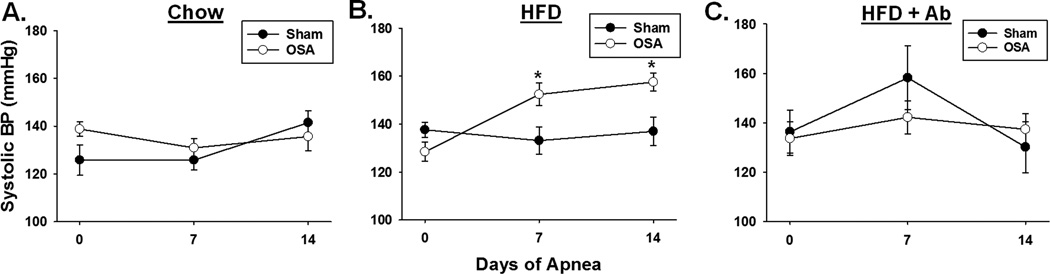
OSA (60 apneas/hr for 8 hours during the sleep cycle) for 2 weeks had no effect on blood pressure in 10 week old rats compared to sham rats (Fig. 1A). Similarly, high fat diet alone (60% total calories from fat for 5 weeks) had no significant effect on blood pressure (Figure 1B). However, when OSA was started after 3 weeks of high fat diet, blood pressure increased 24 and 29 mmHg after 1 and 2 weeks of OSA, respectively (Fig. 1B). Although body weight was greater in rats on a high fat diet (393±10g) when compared to rats on a normal chow diet (376±11g), the difference was not statistically significant. Note that neither OSA alone nor high fat diet alone produced hypertension, yet when combined the two synergized to produce hypertension.
Figure 1.
OSA-induced hypertension requires a high fat diet. A, Sham and OSA rats fed a normal chow diet had no difference in systolic blood pressures (n=4 per group). B, On high fat diet OSA rats exhibited significantly higher systolic blood pressure, as compared to sham rats, after 7 and 14 days of OSA (n=10-13 per group). C, OSA-induced hypertension was prevented by oral antibiotic treatment (n=6-9). HFD= high fat diet, Ab= antibiotic. Data are shown as the mean ± S.E.M., *p<0.05 for sham vs. OSA.
Figure 1C illustrates that administration of oral antibiotics, a tool often used to alter gut microbiota, prevented the increased blood pressure in rats on a high fat diet and undergoing OSA. In addition to altering the composition of the gut microbiota (Figure 2A), oral antibiotics dramatically decreases the gut biomass.16 These data suggested that dysbiosis may play a role in OSA-induced hypertension.
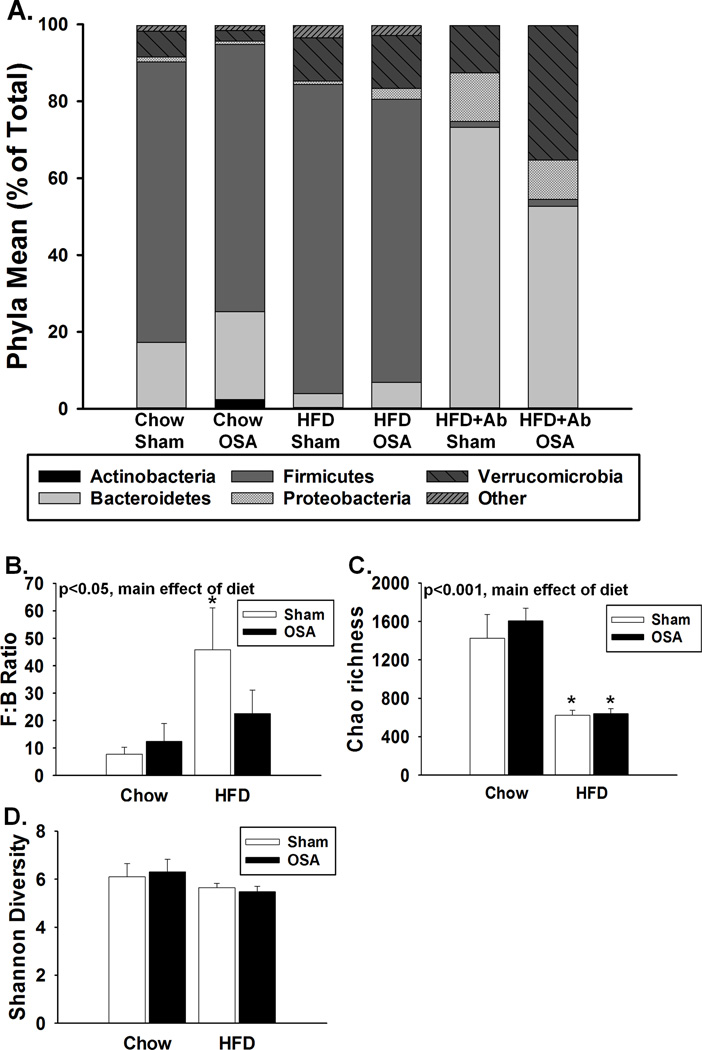
Figure 2.
Dysbiosis following changes in diet, OSA, and antibiotic treatment. A, Relative abundance of the major phyla of the gut microbiota. B, High fat diet increased the Firmicutes:Bacteroidetes (F:B) ratio. C, High fat diet decreased the estimated microbial community richness (Chao1 index). D, High fat diet or OSA did not affect the microbial community diversity (Shannon index). HFD= high fat diet, Ab= antibiotic. Data are shown as the mean ± S.E.M. n=4-9, *p<0.05 for high fat diet sham or OSA vs. respective chow groups.
OSA-induced hypertension is associated with alterations to the gut microbiota
Figure 2A demonstrates the effects of diet, apnea, and antibiotics on the major phyla of the gut microbiota. High fat diet led to a significant increase in the Firmicutes : Bacteroidetes (F:B) ratio, a well-established signature of gut dysbiosis (Fig. 2B).17 The F:B ratio of OSA rats on a high fat diet tended to be lower than rats on a high fat diet alone, however this was not statistically different (p=0.112). In addition, gut dysbiosis is generally accompanied by a decreased diversity of the bacteria present. The Chao 1 richness index, which takes into account the total number of distinct genera present, was significantly decreased following high fat diet (Fig. 2C). The Shannon index of diversity, which takes into account richness and abundance, was not statistically different between chow and high fat fed rats. OSA did not alter the Chao 1 or Shannon indices in rats compared to their corresponding sham group. It appears that a major contributor to the gut dysbiosis in our study was high fat diet.
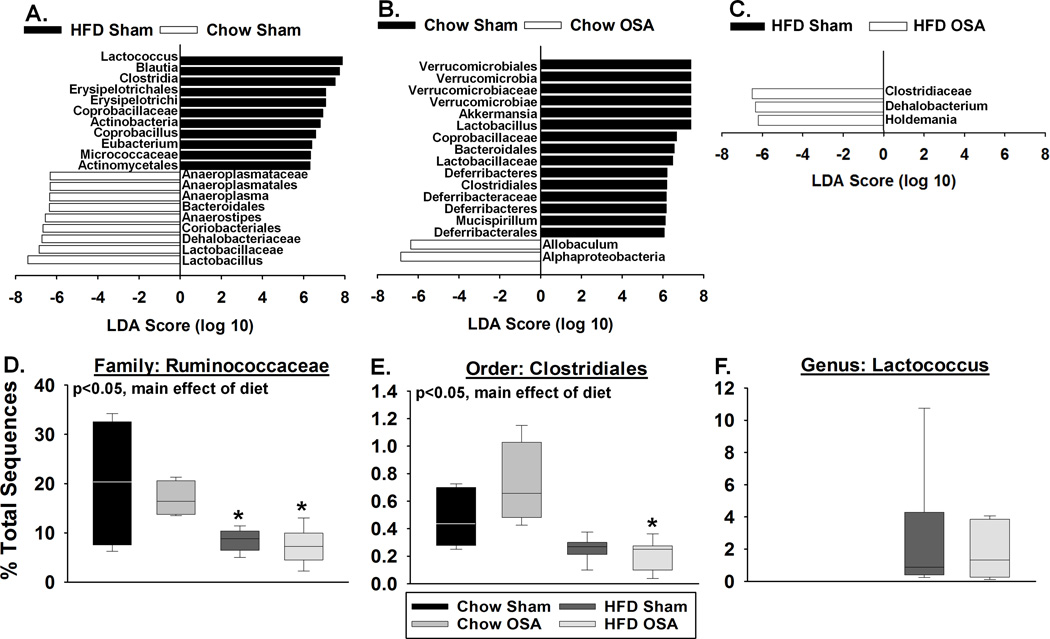
Figure 3 shows the bacterial taxa (class, order, family, and/or genus) that were altered by high fat diet and/or by OSA, according to LEfSe analysis.18 While the goal of our analysis was to classify bacteria to the genus level, the incomplete nature of available bacterial reference databases only allowed us to classify to the level of class, order, or family in some cases. When bacteria could not be classified to the genus level, we report the lowest taxonomic level achieved in that analysis. In figure 3, the fold change in relative abundance (to the log10) of statistically significant taxa are depicted on the horizontal axis. Prominent changes occurred following a high fat diet alone (no OSA) where the relative abundances of eleven taxa were increased (black in Figure 3A) and nine taxa were decreased (white) when compared to rats on a chow diet alone. OSA in rats on a normal chow diet also produced a number of changes in the microbiota taxa compared to sham rats on a chow diet (Figure 3B). Rats on a high fat diet and undergoing OSA showed decreases in the relative abundances of three taxa when compared to the microbiota from sham rats on a high fat diet (Figure 3C). However, no single taxon or group of taxa responsible for the increased blood pressure in OSA rats on a high fat diet was readily apparent at this point in our analysis.
Figure 3.
Diet and OSA alter the relative abundance of multiple bacterial taxa. LEFSe analysis was used to identify and calculate a linear discriminate analysis (LDA) score for taxa that characterize (A) high fat diet vs. chow fed sham rats, (B) OSA vs. sham chow fed rats, and (C) OSA vs. sham high fat fed rats (n=4-7). In panels A-C, positive LDA scores indicate the enrichment of taxa in condition 1 (black) relative to condition 2 (white), and negative LDA scores indicate the depletion of taxa in condition 1 relative to condition 2. Given this relationship, the negative LDA scores can also be interpreted as enrichment in condition 2 (black) relative to condition 1 (white). Effects of diet and OSA on (D) Ruminococcaceae, (E) Clostridiales, and (F) Lactococcus abundance. HFD=high fat diet. Data are shown as the median and quartiles. n=4-7, *p<0.05 for high fat diet sham or OSA vs. respective chow groups.
Potential bacterial metabolites involved with hypertension in OSA rats on a high fat diet were examined using PICRUSt analysis to predict functional gene family abundances using 16S rRNA data. When comparing the microbiota of high fat OSA compared to chow fed OSA rats, we determined that multiple steps of the butyrate (i.e., butanoate) metabolism pathway were predicted to be down-regulated. Most prominent was the predicted decrease in the relative abundance of acetate CoA transferase, the final enzymatic step in butyrate production (Supplemental Figure S1). Butyrate, a short chain fatty acid produced by bacterial fermentation of dietary fibers in the gut, has been shown to play an important role in maintaining overall gut health.19, 20
Ruminococcaceae (order Clostridiales), which are prominent producers of butyrate, decreased in relative abundance with a high fat diet (Fig. 3D). This decrease is particularly significant since Ruminococcaceae, making up ~20% of gut bacteria in chow fed OSA rats, decreased to ~10% in high fat OSA rats (Fig. 3D). Members of the order Clostridiales, other than Ruminococcaceae, also significantly decreased with high fat diet (Figure 3E).
In addition to changes in short chain fatty acid producing bacteria, LEFSe analysis demonstrated that the effects of diet and apnea were characterized by a number of bacteria involved in lactate metabolism including Lactococcus, Coprobacillus, and Holdemania (Fig. 3A and C). We observed an increase in the relative abundance of the lactate producing genus Lactococcus with high fat diet, that was completely absent on normal chow diet. This genus was unaffected by OSA (Fig. 3F).
The hypertensive phenotype of OSA rats is transferrable via the gut contents
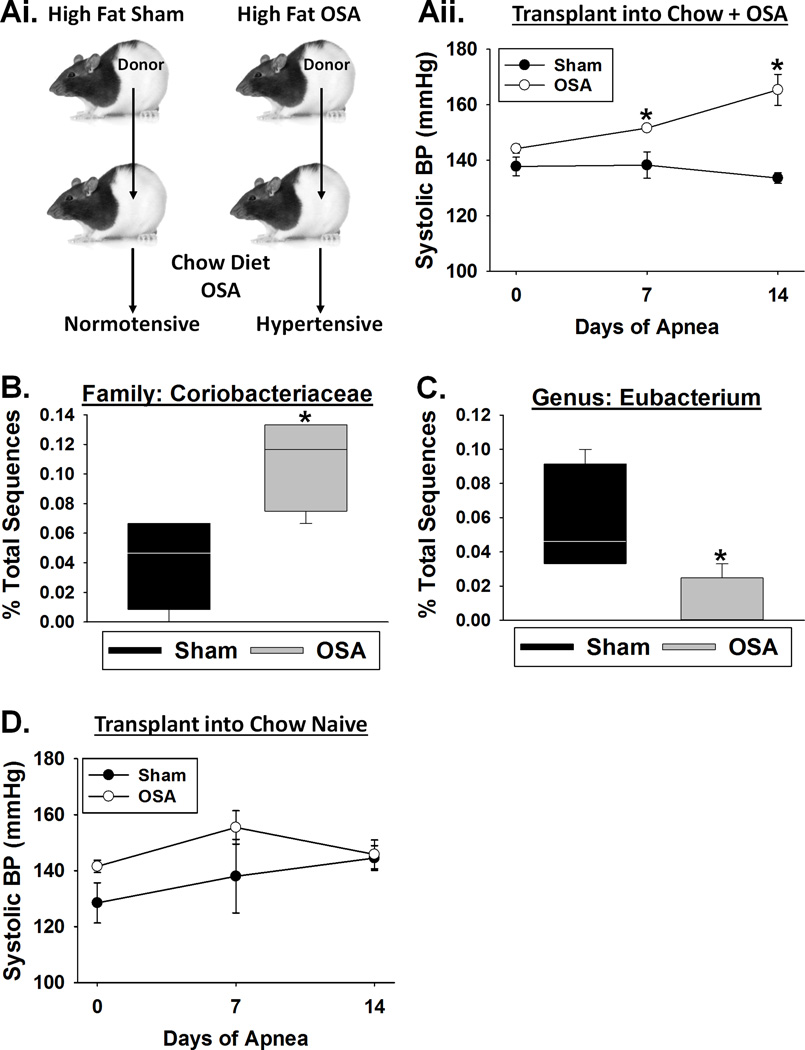
To definitively isolate the role of the gut in OSA-induced hypertension, we transplanted cecal contents from donor rats into the gastrointestinal tract of recipient rats, with the goal of establishing a gut microbiota similar to the donor.21 Recipient rats, on a normal chow diet, were gavaged with cecal contents from donor sham (normotensive) or OSA (hypertensive) rats on a high fat diet (Fig. 4A). All recipient rats remained on normal chow and underwent 2 weeks of OSA. Recall that OSA rats on normal chow diet do not develop hypertension (Fig. 1A). Recipient rats that received the cecal contents from a sham donor on high fat diet had no change in blood pressure (Fig. 4A). However, rats receiving cecal contents from an OSA donor on high fat diet exhibited a 14 and 32mmHg increase in blood pressure at 7 and 14 days of OSA, respectively (Fig. 4A).
Figure 4.
The hypertensive phenotype of OSA rats is transferrable via the gut contents. A, OSA recipient rats gavaged with cecal contents from high fat OSA donors, but not those from a high fat sham donor, exhibited significant increases in systolic blood pressure after 7 and 14 days of OSA. B, Naïve recipient rats gavaged with cecal contents from high fat sham or OSA donors exhibited no change in systolic blood pressure. Effects of high fat diet sham and OSA cecal transplants on (C) Coriobacteriaceae and (D) Eubacterium abundance in chow diet OSA recipients. Data are shown as the mean ± S.E.M. (A&B) and the median and quartiles (C&D) n=4, *p<0.05 for OSA recipient vs sham recipient.
Examination of the microbiota after 2 weeks of OSA revealed a number of differences between rats gavaged with normotensive sham versus hypertensive OSA cecal contents. Rats that received cecal transplants from OSA versus sham donors exhibited a significant increase in the relative abundance of bacteria belonging to the family Coriobacteriaceae, which contains numerous genera known to produce lactate (Fig. 4B). Additionally, there was a ~4-fold decrease in the relative abundance of the genus Eubacterium, known to convert lactate to butyrate, in OSA versus sham cecal transplant rats (Fig. 4C).22
Gavaging cecal contents into naïve rats had no effect on blood pressure regardless of whether the donor was sham (normotensive) or OSA (hypertensive) (Fig. 4D). These findings demonstrate that gut contents from a rat on high fat diet and OSA are capable but not sufficient to induce hypertension in a recipient rat, and requires OSA post-transplantation.
Discussion
We provide strong evidence that the gut microbiota plays a key role in the development of hypertension in our rat model of OSA. We report three major findings. (1) In our rat model of OSA, a complicating condition, such as that associated with high fat diet, was required to produce hypertension. (2) Gut dysbiosis, and not other factors associated with a high fat diet, was involved in the development of OSA-induced hypertension. (3) Gut dysbiosis associated with OSA-induced hypertension involved significant decreases in bacteria involved in butyrate production and increases in bacteria involved with lactate production.
We have previously demonstrated that 2, 4, or 8 weeks of OSA in young healthy Long Evans rats (10 weeks old) does not produce hypertension.(unpublished and 14, 15) However, we do note that the effect of OSA alone does appear to be strain dependent.23 In this study, we demonstrated that Long Evans rats require a complicating condition in order for OSA to produce hypertension (Fig. 1). The purpose of combining high fat diet with apneas was to mimic the human conditions where OSA is often accompanied by a complicating condition (obesity, diabetes, or aging).1–3 Neither OSA alone nor high fat diet alone altered blood pressure, yet the two synergized to produce hypertension. Of note, a possible synergy between OSA and comorbid conditions in humans is often disregarded.
While previous studies have provided a provocative correlation between gut dysbiosis and hypertension in rat models and humans, a direct cause and effect relationship had not been previously established.24, 25 Our cecal transplantation studies go beyond an association or correlation, and conclusively demonstrate the involvement of the gut in OSA-induced hypertension (Fig. 4). Furthermore, we demonstrate that changes to the gut microbiota result from both high fat diet and OSA (Figs. 3&4).
Analysis of the gut microbiota revealed decreased bacteria associated with the production of butyrate, a short chain fatty acid (SCFA), and increased bacteria associated with the production of lactate. The SCFAs, butyrate, propionate, and acetate, are produced by a number of bacterial species when dietary fiber is fermented in the colon. Each of these SCFAs is beneficial in maintaining gut health and homeostasis. Most prominently, butyrate has been shown to confer cardioprotection, reduce obesity, and improve insulin sensitivity by maintaining gut barrier function, reducing inflammation, and inhibiting histone deacetylation to alter transcriptional regulation.19 Additionally, SCFAs can be absorbed into the circulation and affect blood pressure through activation of various G protein coupled receptors in the vascular wall.16 Our analysis revealed that OSA rats on a high fat diet (hypertensive) had decreased abundance of multiple butyrate producing taxa (Fig. 3). A decrease in the production of butyrate could destabilize the gut epithelial barrier through mechanisms mentioned above or enhance vascular tone, especially in the kidney, leading to hypertension when the rat undergoes OSA.
Plasma lactate levels are associated with an increase in blood pressure.26 In our model, high fat diet led to an increase in the relative abundance of lactate producing genera Lactococcus and Coprobacillus (Fig. 3A and F). Additionally, we observed a decrease in the family Ruminococcaceae (Fig. 3D), which negatively correlates with plasma lactate levels.27 These findings demonstrate significant alterations to the gut microbiota of OSA-induced hypertensive rats; with an overall decrease in butyrate and increase in lactate producing bacteria. Similar shifts in butyrate and lactate producers were previously reported for spontaneously hypertensive and AngII rat hypertension models.24
In support of the idea that altered microbial butyrate and lactate production contributes to OSA-induced hypertension, similar microbial community shifts were observed following microbiota transplant with decreased butyrate and increased lactate producers (Fig. 4). Of note, our studies did not conclusively demonstrate that butyrate or lactate were responsible for the OSA-induced hypertension. However, our studies do provide direction for mechanistic hypotheses examining how dysbiosis synergizes with OSA to result in hypertension. Stimulation of the sympathetic nervous system in the gut compromises gut barrier function and is capable of altering the microbiota.28, 29 Importantly, OSA is a powerful stimulus of the sympathetic nervous system.1 Together the effects of high fat induced dysbiosis and OSA sympathetic activation may result in further dysbiosis, gut barrier disruption, translocation of gut bacteria or endotoxins, and systemic inflammation, which has been shown to contribute to the development of hypertension in various models.30
Supplementary Material
Perspectives.
The gut microbiota plays a critical role in modulating host metabolism, immunity, and inflammation. Studies have linked gut dysbiosis to pathologies beyond the gastrointestinal tract, including obesity, diabetes, and atherosclerosis. Previous studies have demonstrated an association between gut dysbiosis and hypertension; however a direct causal relation between the two was lacking. We demonstrate that high fat diet induced gut dysbiosis, is not only associated with, but is an underlying component of OSA-induced hypertension. Our studies demonstrate an important link between gut dysbiosis and blood pressure, and suggest manipulation of the microbiota may hold promise as a treatment of hypertension.
Novelty and Significance.
What is new?
We demonstrate a causal role for gut dysbiosis in the development of OSA-induced hypertension.
High fat diet and OSA-induced dysbiosis can be characterized by a decrease in butyrate producing bacteria.
Cecal transfer experiments demonstrate that gut dysbiosis synergizes with OSA to induce hypertension.
What is relevant?
Understanding the role of the gut microbiota in hypertension not only contributes to our understanding of the etiology of this prevalent disorder, but also offers a new potential target for therapeutics.
Summary
High fat diet synergizes with OSA resulting in a dysbiotic microbiota that is capable of producing hypertension. Methods to prevent or manipulate gut dysbiosis may be beneficial in the treatment of hypertension.
Acknowledgments
Sources of Funding
This project was funded by NINDS R01NS080531 (RMB) and by Public Health Service grant DK56338, which funds the Texas Medical Center Digestive Diseases Center (DJD).
Footnotes
Conflict(s) of Interest / Disclosure(s)
none
References
- 1.Durgan DJ, Bryan RM., Jr Cerebrovascular consequences of obstructive sleep apnea. J Am Heart Assoc. 2012;1:e000091. doi: 10.1161/JAHA.111.000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, Pickering TG, Russell R, Woo M, Young T. Sleep apnea and cardiovascular disease. Circulation. 2008;118:1080–1111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 3.Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sjostrom C, Lindberg E, Elmasry A, Hagg A, Svardsudd K, Janson C. Prevalence of sleep apnoea and snoring in hypertensive men: A population based study. Thorax. 2002;57:602–607. doi: 10.1136/thorax.57.7.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kasai T, Floras JS, Bradley TD. Sleep apnea and cardiovascular disease: A bidirectional relationship. Circulation. 2012;126:1495–1510. doi: 10.1161/CIRCULATIONAHA.111.070813. [DOI] [PubMed] [Google Scholar]
- 6.Parati G, Lombardi C, Narkiewicz K. Sleep apnea: Epidemiology, pathophysiology, and relation to cardiovascular risk. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1671–R1683. doi: 10.1152/ajpregu.00400.2007. [DOI] [PubMed] [Google Scholar]
- 7.Pedrosa RP, Drager LF, Gonzaga CC, Sousa MG, de Paula LK, Amaro AC, Amodeo C, Bortolotto LA, Krieger EM, Bradley TD, Lorenzi-Filho G. Obstructive sleep apnea: The most common secondary cause of hypertension associated with resistant hypertension. Hypertension. 2011;58:811–817. doi: 10.1161/HYPERTENSIONAHA.111.179788. [DOI] [PubMed] [Google Scholar]
- 8.Logan AG, Tkacova R, Perlikowski SM, Leung RS, Tisler A, Floras JS, Bradley TD. Refractory hypertension and sleep apnoea: Effect of CPAP on blood pressure and baroreflex. Eur Respir J. 2003;21:241–247. doi: 10.1183/09031936.03.00035402. [DOI] [PubMed] [Google Scholar]
- 9.Schippa S, Conte MP. Dysbiotic events in gut microbiota: Impact on human health. Nutrients. 2014;6:5786–5805. doi: 10.3390/nu6125786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duerkop BA, Vaishnava S, Hooper LV. Immune responses to the microbiota at the intestinal mucosal surface. Immunity. 2009;31:368–376. doi: 10.1016/j.immuni.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci U S A. 2008;105:20858–20863. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang WH, Hazen SL. The contributory role of gut microbiota in cardiovascular disease. J Clin Invest. 2014;124:4204–4211. doi: 10.1172/JCI72331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fond G, Boukouaci W, Chevalier G, Regnault A, Eberl G, Hamdani N, Dickerson F, Macgregor A, Boyer L, Dargel A, Oliveira J, Tamouza R, Leboyer M. The "psychomicrobiotic": Targeting microbiota in major psychiatric disorders: A systematic review. Pathol Biol (Paris) 2015;63:35–42. doi: 10.1016/j.patbio.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Crossland RF, Durgan DJ, Lloyd EE, Phillips SC, Reddy AK, Marrelli SP, Bryan RM., Jr A new rodent model for obstructive sleep apnea: Effects on ATP-mediated dilations in cerebral arteries. Am J Physiol Regul Integr Comp Physiol. 2013;305:R334–R342. doi: 10.1152/ajpregu.00244.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durgan DJ, Crossland RF, Lloyd EE, Phillips SC, Bryan RM. Increased cerebrovascular sensitivity to endothelin-1 in a rat model of obstructive sleep apnea: A role for endothelin receptor B. J Cereb Blood Flow Metab. 2015;35:402–411. doi: 10.1038/jcbfm.2014.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, Caplan MJ. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A. 2013;110:4410–4415. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Everard A, Cani PD. Diabetes, obesity and gut microbiota. Best Pract Res Clin Gastroenterol. 2013;27:73–83. doi: 10.1016/j.bpg.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60-2011-12-6-r60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canani RB, Costanzo MD, Leone L, Pedata M, Meli R, Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol. 2011;17:1519–1528. doi: 10.3748/wjg.v17.i12.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berni Canani R, Di Costanzo M, Leone L. The epigenetic effects of butyrate: Potential therapeutic implications for clinical practice. Clin Epigenetics. 2012;4:4-7083-4-4. doi: 10.1186/1868-7083-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruce-Keller AJ, Salbaum JM, Luo M, Blanchard E, 4th, Taylor CM, Welsh DA, Berthoud HR. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol Psychiatry. 2015;77:607–615. doi: 10.1016/j.biopsych.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duncan SH, Louis P, Flint HJ. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl Environ Microbiol. 2004;70:5810–5817. doi: 10.1128/AEM.70.10.5810-5817.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lloyd EE, Durgan DJ, Martini SR, Bryan RM. Pathological effects of obstructive apneas during the sleep cycle in an animal model of cerebral small vessel disease. Hypertension. 2015;66:913–917. doi: 10.1161/HYPERTENSIONAHA.115.05764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, Sahay B, Pepine CJ, Raizada MK, Mohamadzadeh M. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65:1331–1340. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mell B, Jala VR, Mathew AV, Byun J, Waghulde H, Zhang Y, Haribabu B, Vijay-Kumar M, Pennathur S, Joe B. Evidence for a link between gut microbiota and hypertension in the dahl rat. Physiol Genomics. 2015;47:187–197. doi: 10.1152/physiolgenomics.00136.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juraschek SP, Bower JK, Selvin E, Subash Shantha GP, Hoogeveen RC, Ballantyne CM, Young JH. Plasma lactate and incident hypertension in the atherosclerosis risk in communities study. Am J Hypertens. 2015;28:216–224. doi: 10.1093/ajh/hpu117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petriz BA, Castro AP, Almeida JA, Gomes CP, Fernandes GR, Kruger RH, Pereira RW, Franco OL. Exercise induction of gut microbiota modifications in obese, non-obese and hypertensive rats. BMC Genomics. 2014;15:511-2164-15-511. doi: 10.1186/1471-2164-15-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun Y, Fihn BM, Sjovall H, Jodal M. Enteric neurones modulate the colonic permeability response to luminal bile acids in rat colon in vivo. Gut. 2004;53:362–367. doi: 10.1136/gut.2003.015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyte M. Microbial endocrinology: Host-microbiota neuroendocrine interactions influencing brain and behavior. Gut Microbes. 2014;5:381–389. doi: 10.4161/gmic.28682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh MV, Chapleau MW, Harwani SC, Abboud FM. The immune system and hypertension. Immunol Res. 2014;59:243–253. doi: 10.1007/s12026-014-8548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.