Abstract
An expanded computational model of surface induced thrombin generation was developed that includes hemodynamic effects, 22 biochemical reactions and 44 distinct chemical species. Surface binding of factors V, VIII, IX, and X was included in order to more accurately simulate the formation of the surface complexes tenase and prothrombinase. In order to model these reactions, the non-activated, activated and inactivated forms were all considered. This model was used to investigate the impact of surface bound heparin on thrombin generation with and without the additive effects of thrombomodulin (TM). In total, 104 heparin/TM pairings were evaluated (52 under venous conditions, 52 under arterial conditions), the results demonstrating the synergistic ability of heparin and TM to reduce thrombin generation. Additionally, the role of circulating tissue factor (TFp) was investigated and compared to that of surface-bound tissue factor (TFs). The numerical results suggest that circulating TF has the power to amplify thrombin generation once the coagulation cascade is already initiated by surface-bound TF. TFp concentrations as low as 0.01 nM were found to have a significant impact on total thrombin generation.
Keywords: Surface induced thrombin generation, Antithrombogenic surface, Numerical model, Circulating tissue factor
INTRODUCTION
Despite a growing understanding of the coagulation system, individual thrombotic risk is difficult to estimate. Moreover, the potential efficacy of novel anticoagulants or new strategies to limit the risk of device related thrombosis is difficult to establish without extensive preclinical and clinical investigations. The coagulation cascade is complex, particularly when coupled with a unique, organ specific, hemodynamic environment, thereby creating a system that is sensitive to a variety of parameters, in addition to coagulation factors. Clotting factors participate in a non-linear cascade, predominantly initiated by surface bound tissue factor (TF) via the extrinsic pathway, producing thrombin, a serine protease. Thrombin, in turn, serves to activate many components of the cascade, as well as the conversion of fibrinogen to fibrin, the major component of the hemostatic plug. Coagulation pathway inhibitors, such as antithrombin III (ATIII), tissue factor pathway inhibitor (TFPI) and activated protein C (APC) impede cascade reactions reducing the rate of thrombin production and, ultimately, clot formation. In addition to reaction pathway kinetics, blood flow plays an important role in modulating clot formation by delivering substrates to surface bound TF, while removing activated clotting factors and inhibitors.
Due to the complex nature of the coagulation cascade, numerical models have been developed in an effort to elucidate the relationship between clotting factors, thrombin generation, and thrombus formation. These models have analyzed the impact of the geometry of TF regions,20–23,41 the complex chemical mechanism of coagulation,8,16,17 diffusion and spatial effects,2,49,50 as well platelets in the presence of hemodynamics.13,26,27 These models have provided important insights in the identification of dominant coagulation reactions and those factors, which have the largest contribution toward clotting disorders. Obtaining these insights experimentally would have been prohibitive and nearly impossible to analyze without numerical methods.
Devices designed for blood contacting applications have often been modified with surface bound heparin to locally inactivate thrombin.15,40,47 In this regard, the selection among various heparin types, such as unfractionated heparin, low molecular weight heparin, or a heparin pentasaccharide, as well as the optimal choice of heparin surface concentration has been largely empirical. Such an approach, as related to the incorporation of surface bound heparin or for other thrombin inhibitors, dictates a design strategy that is time intensive and costly. Moreover, the ability to fully define failure modes under specific implant conditions or to predict the effect, if any, of design changes to the implantable blood-contacting device on subsequent performance may be limited. The effect of surface-bound heparin in limiting surface induced thrombin generation has yet to be investigated in a numerical model of coagulation. Of particular interest is the degree to which heparin reduces the local concentration of thrombin as a function of heparin type and the extent of this effect as compared to other surface bound inhibitors of thrombin generation, either alone or in combination. For example, heparin serves as an anticoagulant by binding ATIII, thereby enhancing the capacity of ATIII to inactivate thrombin and activated factor X. In contrast, in binding thrombin, thrombomodulin (TM), which may also be used to modify blood contacting devices,35,36,43,45 reduces the local concentration of thrombin and alters its substrate specificity so that it preferentially activates protein C.
Previous computational models of coagulation at an interface have considered surface bound TF, but have not accounted for the potential effects of circulating TF. Recent studies have suggested that circulating TF may play an important role in either the initiation or propagation of the coagulation cascade. Indeed, a number of reports have demonstrated a correlation between circulating TF and venous thromboembolism (VTE).12,29,51 Nonetheless, the clinical relevance of circulating TF levels remains poorly defined, particularly, as a potentiating factor for thrombin formation in the presence of locally expressed, surface bound TF.
We have previously reported the formulation of a computational model of surface-induced thrombin generation in a flow field, where subendothelial surface reactions are considered, as well as the effects of coagulation inhibitors, including ATIII, TFPI, and APC.18 This model was subsequently used to assess individual patient risk for VTE in the presence or absence of a history of oral contraceptive usage.19 We report, herein, an extended model, which we believe more accurately describes the formation of surface-bound prothrombinase and tenase, by incorporating the phenomenon of direct surface adsorption of non-activated, activated, and inactivated species. In addition, the model has also been enhanced to account for additional surface-bound inhibitory species, specifically, heparin, as well as circulating plasma TF. This expanded model has allowed us to evaluate the interactive effects of surface bound heparin and TM, as well as the effect of circulating TF under a variety of flow and surface conditions..3
METHODS
Thrombin generation initiated by surface-bound TF under specified flow conditions is modeled using the finite element method (FEM) implemented in COMSOL Multiphysics 4.3a. The degree of thrombin formation is controlled by reaction kinetics, diffusion, and convection due to simulated blood flow. Transport of fluid phase chemicals is governed by 2-D, unsteady convection–diffusion equations. Surface species are governed by transient differential equations. Fluid phase reactions are included in species-specific partial differential equations, while surface reactions are implemented as boundary conditions. The lower wall, defined by discrete regions of surface bound TF, TM and heparin, is a reactive surface with kinetically described boundary conditions. The upper wall is far removed from any diffusing species and, therefore, is treated as an inert surface with a constant flux boundary condition for all species. All equations and physical parameters are provided in the Supporting Information.
Coagulation Reaction Scheme
Initial Conditions
The initial concentration of all activated compounds are set to zero with the exception of factor VIIa, which is set to 1% of the non-activated concentration.33 Mean physiological levels were employed for initial concentrations of all plasma species, excluding plasma tissue factor (TFp) (see Supporting Information). Activated, non-activated, and inactivated forms of factors V, VIII, IX, and X are able to adsorb and desorb to and from the lower surface wall, given a fixed number of specific surface sites (see Supporting Information). For surface-bound species, including TM, tissue factor (TFs), and heparin, the total amount of surface species (free and complexed) remains constant at the specified initial level. Initial total levels of TFp vary from 0 to 100 nM. Complexed factor VIIa and TFp are initially set to equilibrium values.
General
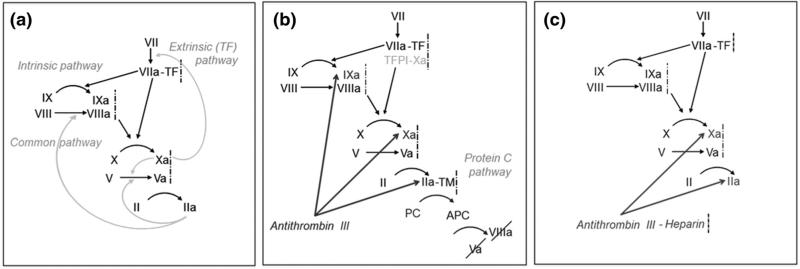
The coagulation pathway is initiated when factor VIIa binds reversibly to surface-bound TF, forming extrinsic tenase (Fig. 1). Extrinsic tenase activates factors X and IX, leading to factors Xa and IXa, respectively. Subsequently, surface-bound Xas activates factors VII and V, affording factors VIIa and Va. Prothrombinase is formed when surface-bound Vas or Xas complexes with Xa or Va, respectively. Prothrombinase activates factor VII, as well as factor II, producing thrombin and establishing a positive feedback loop with the activation of factors VIII and V, generating VIIIa and Va. Intrinsic tenase is formed when surface-bound VIIIas or IXas forms a complex with IXa or VIIIa, respectively. Intrinsic tenase activates factor X, which further amplifies the cascade.
FIGURE 1.
Thrombin generation and inhibition pathways. (a) Thrombin (IIa) generation via the extrinsic, intrinsic, and common pathways. (b) Thrombin inhibition via tissue factor pathway inhibitor (TFPI), antithrombin III, and activated protein C (APC). (c) Heparin catalyzes thrombin inhibition by potentiating the activity of ATIII.
Three thrombin inhibitors, including APC, ATIII, and TFPI are included. Surface-bound TM, which binds and sequesters thrombin, follows an upstream region of surface-bound TF. TM-thrombin surface complexes generate APC, which inactivates Vas and VIIIas. ATIII binds to thrombin, IXa/IXas, and Xa/Xas, inactivating these compounds. TFPI binds to fluid phase Xa, which can bind to surface-bound extrinsic tenase, eliminating its ability to activate other factors.
The chemical mechanism used herein is based on a previously described mechanism,18 with several modifications. The predominant model expansion is the inclusion of surface sites for factors V, VIII, IX, and X, which interact with their non-activated, activated, and inactivated forms. Factors V, VIII, IX, and X bind to these surface sites and once bound, remained fixed in position, unable to move due to convective flux or diffusion, thereby, altering their availability to react with other species. By including surface sites, many surface reactions, including the formation of prothrombinase and tenase, are more accurately simulated. In addition to including surface site behavior, the reaction equations for the inactivation of FVa and FVIIIa by APC42 and the activation of FV by FXa32 were revised to account for surface behavior in order to better match experimental results (see Supporting Information). In the original model, activation of FVII by FXa was treated as a bulk reaction occurring in the plasma, but has been revised as a surface reaction, as experimental evidence suggests that this reaction occurs on a lipid surface.5 Finally, the activation of FVII by thrombin was replaced by its activation via prothrombinase since this reaction rate is several orders higher than activation by thrombin.5 Chemical transport is described by convection–diffusion equations with boundary conditions determined by the chemical kinetics at each region. Where rate constants have not been measured due to very fast reaction conditions, diffusion limited rate constants were used. It is assumed that enzyme reactions are at equilibrium and follow Michaelis–Menten kinetics. Specific rate constants are summarized in the Supporting Information.
Surface-Bound Heparin
Where indicated, surface-bound heparin was incorporated as an additional thrombin inhibitor. Heparin forms a complex with ATIII potentiating its ability to bind and inactivate thrombin and factor Xa. Three different forms of surface bound heparin, including a pentasaccharide, a low molecular weight heparin (26 saccharides), and an unfractionated version of heparin (70 saccharides), each with different, literature-defined rate constants for complexing ATIII, were considered in this analysis.38
Circulating Plasma Tissue Factor
Circulating TFp was modeled as a free species capable of interacting with factor VIIa, forming circulating extrinsic tenase that could initiate or propagate coagulation. The diffusivity of microparticle-bound TFp was estimated using the Stokes–Einstein equation based -on an average microparticle radius of 500 nm. The impact of surrounding erythrocytes on the diffusivity of these microparticles was taken into consideration by determining an effective diffusivity, assuming a hemocrit of 40%, an effective erythrocyte radius of 2.75 μm, and a Brownian diffusivity of 10−9 m2/s (8.6 × 10−8 cm2/s for venous shear and 6.6 × 10−7 cm2/s for arterial shear).48
Vascular Compartment
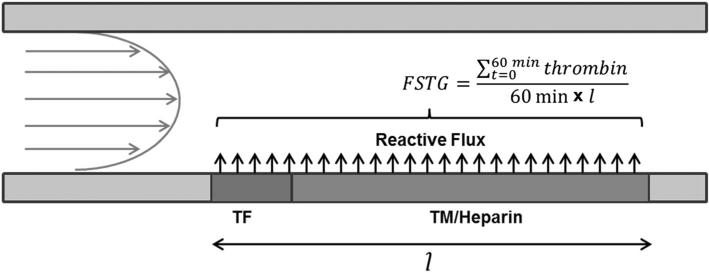
The vascular compartment is modeled as a 10 mm wide, 2-D channel (Fig. 2). Plasma flows from left to right, passing over a 1 mm region of surface-bound tissue factor (TFs), followed by a 10 mm downstream region containing surface-bound TM, representative of an intact endothelium. In selected simulations, both surface-bound heparin and TM are present in the downstream region.
FIGURE 2.
Model schematic of surface bound tissue factor initiating thrombin generation in a flow field. Fluid enters a 10 mm diameter blood vessel from the left, with a parabolic flow profile. The fluid, carrying coagulation proteins, passes over a 1 mm region of surface bound tissue factor (TF), which then activates the coagulation cascade. Immediately downstream is a 10 mm region of surface bound thrombomodulin (TM), which may also contain surface bound heparin. These surface bound moieties inhibit coagulation and reduce thrombin formation. Flow simulated thrombin generation, FSTG, is determined by averaging the thrombin concentration along the TF/TM region over length (11 mm) and time (60 min).
Flow profiles were represented by incompressible Navier–Stokes equations with inlet profiles based on either a simulated venous shear rate of 50 s−1 or an arterial shear rate of 500 s−1. Fluid flow was assumed to be unidirectional and laminar. Consistent with the Navier–Stokes equation, flow was parabolic with peak fluid velocity located at the center of the vessel.
Model Outputs of Thrombin Generation
The simulations yielded transient, 2-D concentration profiles for each reactant and product species. Flow-simulated thrombin generation (FSTG), as first defined by Jordan and Chaikof,18 was used to quantify the amount of thrombin generated throughout the simulation. FSTG was defined as the integral over a 60 min simulation time of thrombin generated along the entire reactive surface, including both TF and TM regions, normalized by time and reactive surface length. FSTG facilitated a comparative global analysis of multiple simulation conditions.
FEM Mesh
While the distance between upper and lower walls was 10 mm, the chemical diffusive boundary layer thickness was approximately 5 or 10 μm at arterial and venous shear rates, respectively. In order to capture transport within this boundary layer, the mesh used in the finite element solver was refined significantly in the region adjacent to the reactive surface containing surface-bound TF or TM. Along the reactive surface the mesh was specified to have 150 elements along the 1 mm TFs region and 500 elements along the region containing surface-bound TM. Additionally, the mesh was refined at the junction of TFs/TM and at the end of the downstream TM region. Enhanced mesh refinement was extended out 50 and 100 μm to include 16,447 and 19,052 elements for simulated arterial and venous shear rates, respectively.
RESULTS
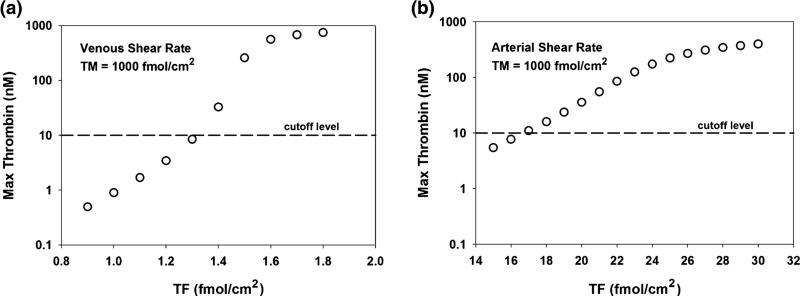
TF Surface Density
The coagulation cascade modeled in these simulations is initiated by surface-bound TF. If the initial surface density of TF is too low, the coagulation cascade does not propagate, irrespective of other model parameters. However, if the TF surface density exceeds a defined level, the cascade will propagate under nearly all conditions and the impact of negative feedback systems is negligible. This threshold-like behavior is illustrated in Fig. 3. In the presence of simulated venous and arterial shear rates, the maximum thrombin level at the interface of TF and TM containing regions was measured as a function of varying TF surface densities with TM surface density held constant at 1000 fmol/cm2. Venous conditions were associated with a larger chemical boundary layer, which allows the products of the initiation reaction to remain near the reactive surface for longer periods of time, leading to higher levels of thrombin than under similar conditions at arterial shear rates.
FIGURE 3.
The effect of tissue factor surface density on peak thrombin concentration at the TF/TM interface. Maximum thrombin concentrations (nM) at the TF/TM interface were measured under varying TF surface densities under (a) venous (50 s−1) and (b) arterial (500 s−1) shear rates. TM levels were held constant at 1000 fmol/cm2. A thrombin concentration of 10 nM (dashed line) was used to indicate a surface threshold of bound TF that would lead to significant thrombin generation and platelet activation. This “cutoff level” occurs just beyond a TF surface density of 1.3 and 16 fmol/cm2 under venous and arterial shear rates, respectively.
Prior studies have demonstrated that below a critical TF threshold, thrombin levels were less than 10 nM. Above this TF threshold, where explosive thrombin generation was noted, thrombin concentrations rapidly exceeded 10 nM.18 Therefore, a thrombin concentration of 10 nM, which surpasses the level required to induce significant platelet activation,3 was selected as indicative of a locally prothrombotic microenvironment enabling propagation of the coagulation cascade. Under simulated venous and arterial shear rates, this threshold occurred at TF surface densities of 1.3 and 16 fmol/cm2, respectively.
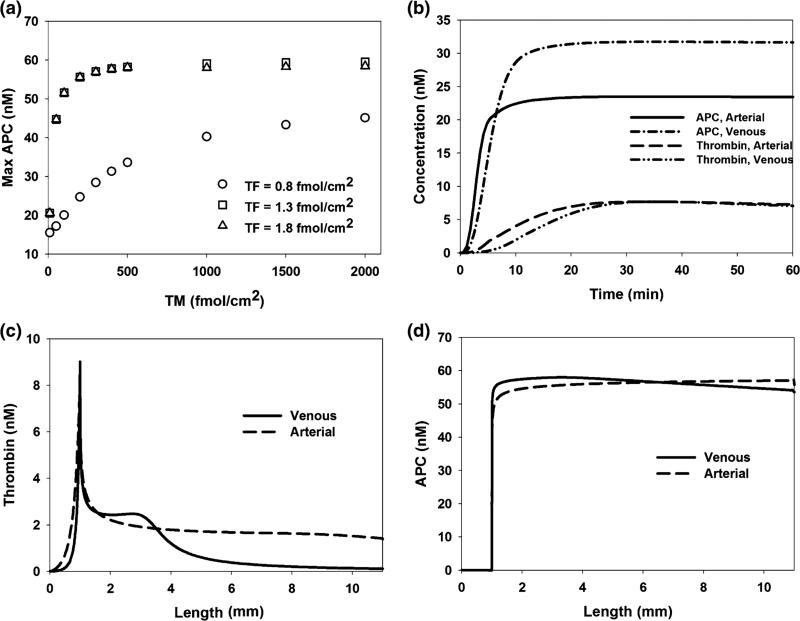
TM Surface Density
TM initially inhibits thrombin generation by forming a complex with thrombin, which alters its procoagulant catalytic activity to that which catalyzes the formation of APC. At a TM surface density of 1000 fmol/cm2, APC generation is transport limited, such that increasing TM surface density does not promote further enhancement of local APC concentration (Fig. 4a). Unless otherwise specified, TM surface density was set at this level in all model simulations.
FIGURE 4.
Defining thrombomodulin, region length, and reaction time as model parameters. (a) Maximum activated protein C (APC) concentration is measured under venous shear conditions in response to varying TM densities at TF surface densities below, at, and above the surface bound TF “cutoff level”. When TM exceeds 1000 fmol/cm2, APC is transport limited and further increases in TM have a negligible effect on APC generation. (b) Thrombin and APC concentrations at the TF/TM interface were measured over 60 min under venous and arterial shear rates. TF surface densities were set to the corresponding cutoff levels and TM was set to 1000 fmol/cm2. After 60 min all key chemical behavior is captured. (c, d) Thrombin and APC are measured, after 60 min, along the reactive surface (TF and TM) under venous and arterial shear rates with TF densities set to the corresponding cutoff levels and TM set to 1000 fmol/cm2. Length of 0 mm signifies the upstream boundary of the TF region. All significant changes are visible along the 11 mm length.
Reaction Scheme Time and Length
Thrombin generation is initiated at surface-bound TF and propagates forward in space and time, moving downstream past the initial site of surface bound TF. Within the first 60 min after initiation, maximum levels of thrombin have been achieved at the TF/TM interface (Fig. 4b). Additionally, maximum levels of APC have been achieved after 60 min, indicating that all key chemical behavior has been captured within this time window. The reactive surface is 11 mm long, with 1 mm of surface-bound TF and 10 mm of surface-bound TM. After 60 min, all major changes in thrombin and APC have occurred within the reactive region, indicating that the region is of sufficient length to model the cascade behavior under both arterial and venous flow conditions (Figs. 4c and 4d).
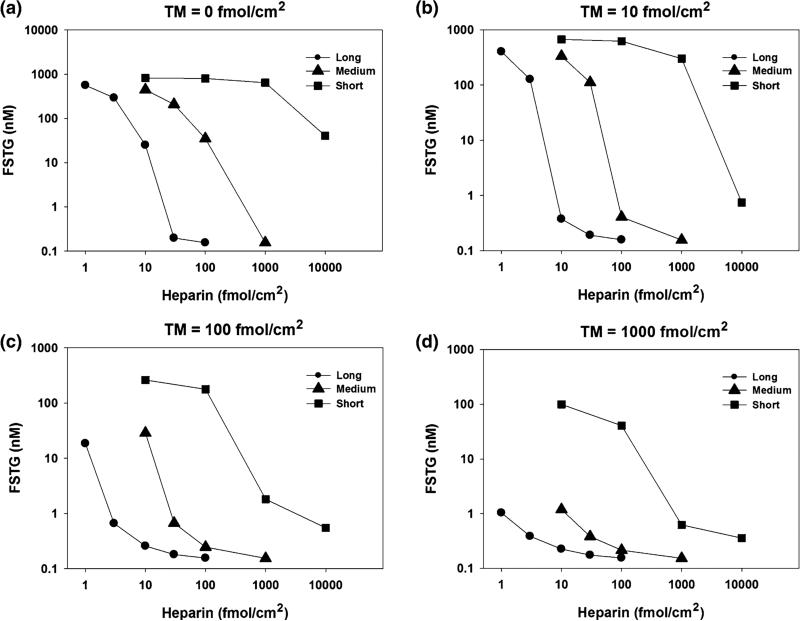
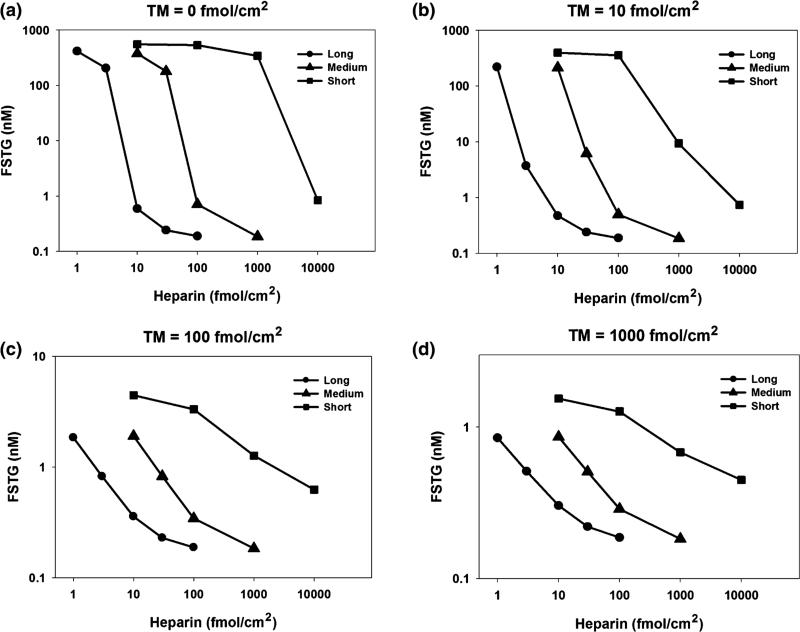
Surface-Bound Heparin
Surface-bound heparin forms a complex with ATIII that serves to inactivate thrombin and factor Xa. The relative impact of heparin and TM on FSTG was evaluated under simulated venous and arterial conditions (Figs. 5 and 6). Each of the three different forms of surface-bound heparin, representing a commercially available heparin pentasaccharide, low molecular weight heparin (LMWH), and unfractionated heparin; each displaying distinct reaction kinetics, as reported, with reaction rates increasing with heparin chain length.38 As a consequence, simulations revealed that high molecular weight heparin was most effective at reducing FSTG, by as much as three orders of magnitude, as compared to the heparin pentasaccharide and LMWH.
FIGURE 5.
The effect of distinct surface bound heparin species and thrombomodulin under venous shear conditions. FSTG was measured in response to varying TM and heparin surface densities, with surface bound tissue factor density set just above the cutoff level to 1.4 fmol/cm2 and at a shear rate 50 s−1. Three different heparin chain lengths were used: short (5 saccharides), medium (26 saccharides), and long (70 saccharides). While high levels of surface bound TM and heparin alone inhibit thrombin generation, these results suggest that a combination of moderate levels of TM and heparin work in concert to inhibit the coagulation cascade. For instance, thrombin formation is fully inhibited (FSTG < 10 nM) at a TM surface density of 100 fmol/cm2 and at a surface density of long chain heparin of 3 fmol/cm2. Surface bound TM or heparin alone would require tenfold higher levels to achieve similar levels of inhibition. Additionally, long chain heparin was significantly more effective at inhibiting thrombin generation as compared to short chain heparin.
FIGURE 6.
The effect of distinct surface bound heparin species and thrombomodulin under arterial shear conditions. FSTG was measured in response to varying TM and heparin surface densities, with surface bound tissue factor density set just above the cutoff level to 17 fmol/cm2 and at a shear rate 500 s−1. Three different heparin chain lengths were used: short (5 saccharides), medium (26 saccharides), and long (70 saccharides). While high levels of surface bound TM and heparin alone inhibit thrombin generation, these results suggest that a combination of moderate levels of TM and heparin work in concert to inhibit the coagulation cascade. For instance, thrombin formation is fully inhibited at a TM surface density of 10 fmol/cm2 and at a surface density of long chain heparin of 3 fmol/cm2. Surface bound TM or heparin alone would require tenfold or threefold higher levels, respectively, to achieve similar levels of inhibition. Long chain heparin was significantly more effective at inhibiting thrombin generation as compared to short chain heparin. Inhibition was more successful under arterial conditions due to a higher shear rate, which reduced the time available for thrombin generation before inhibition of the coagulation cascade.
While high levels of TM and heparin successfully inhibited thrombin generation, simulation studies confirmed that a combination of moderate levels of both TM and heparin were synergistic in their ability to limit thrombin concentration. For instance, under venous conditions, thrombin formation was fully inhibited (FSTG < 10 nM) at a TM surface density of 100 fmol/cm2 when combined with a density of surface bound unfractionated heparin of 3 fmol/cm2. If used as the sole inhibitory agent, a tenfold higher density of surface bound TM or heparin would be required to obtain similar levels of inhibition. Similarly, under arterial conditions, thrombin formation was fully inhibited when a TM surface density of 10 fmol/cm2 was combined with a surface concentration of unfractionated heparin of 3 fmol/cm2. Similarly, tenfold or threefold greater levels of TM or heparin, respectively, would be necessary to achieve comparable levels of inhibition, if either agent was used alone. In general, the extent of thrombin inhibition was greater, as represented by a lower FSTG, under arterial conditions. Presumably, this effect is due to the higher shear rate, which limits the time interval available for thrombin generation within the reactive region.
Plasma TF Model
Circulating plasma phase TF, TFp, provided an additional mechanism for initiation and propagation of the coagulation cascade. The impact of TFp on FSTG was characterized under both venous and arterial conditions (Table 1). Simulations were conducted in the presence of surface bound TF (TFs), at or slightly above the critical surface threshold level, as well as in the absence of surface bound TF. Under venous conditions the impact of TFs is rapid near the cutoff point (1.3 fmol/cm2, Fig. 3). However, beginning at TFp = 0.01 nM, FSTG is enhanced in the presence of TFp with significant increases over that found with TFs alone. Similar results were observed under arterial conditions, albeit at higher levels of TFp. Under arterial conditions, the thickness of the chemical boundary layer is reduced. Thus, products of the initiation reactions are more rapidly removed and higher levels of both plasma or surface-bound TF are required to achieve thrombin generation levels similar to those found under venous conditions. As a result, the levels of FSTG under venous and arterial conditions can be several orders of magnitude different, particularly near any TF threshold.
TABLE 1.
Flow simulated thrombin generation (FSTG) in response to varying concentrations of circulating tissue factor (TFp) and surface-bound tissue factor (TFs) under venous and arterial shear rates.
| FSTG venous | |||
|---|---|---|---|
| TFs (fmol/cm2) |
|||
| TFp (nM) | 0 | 1.3 | 1.4 |
| 0 | – | 0.3 | 107.6 |
| 0.001 | 2.9 × 10–7 | 0.4 | 113.0 |
| 0.01 | 3.1 × 10–5 | 0.5 | 164.8 |
| 0.1 | 2.9 × 10–3 | 348.2 | 518.1 |
| 1 | 687.5 | 851.4 | 856.8 |
| 10 | 954.6 | 960.5 | 960.7 |
| 100 | 981.2 | 982.1 | 983.5 |
| FSTG arterial | |||
|---|---|---|---|
| TFs (fmol/cm2) |
|||
| TFp (nM) | 0 | 16 | 17 |
| 0 | – | 0.9 | 1.6 |
| 0.001 | 3.5 × 10–8 | 0.9 | 1.6 |
| 0.01 | 3.6 × 10–6 | 0.9 | 1.6 |
| 0.1 | 3.8 × 10–4 | 1.1 | 2.0 |
| 1 | 2.4 × 10–2 | 19.7 | 59.5 |
| 10 | 571.5 | 707.5 | 711.3 |
| 100 | 857.0 | 857.9 | 858.0 |
TM surface density was set to 1000 fmol/cm2 and coagulation factors and inhibitors were set to mean physiological levels. TFs levels above and below the cutoff level were considered, as well as the case in which surface bound tissue factor is absent. Under venous conditions the impact of TFs is rapid near the cutoff point (see Fig. 3), with significant FSTG occurring with only TFs (TFp = 0). However, at TFp = 0.01 nM, FSTG is enhanced with significant increases over that found with TFs alone. Similar results are achieved under arterial conditions, albeit at higher levels of TFp. Under both shear rates, the presence of circulating tissue factor potentiates thrombin generation, as indicated by FSTG, even when TFs is below a critical cutoff level. Thus, TFp reduces the threshold necessary to initiate the coagulation cascade.
TFp demonstrated the greatest effect when TFs exceeds the critical threshold; requiring the least amount of TFp to noticeably enhance FSTG (0.01 and 1 nM for venous and arterial conditions, respectively). However, even when there is an absence of surface bound TF, TFp is able to initiate and propagate the coagulation cascade at 1 nm and 10 nM, for venous and arterial conditions, respectively.
DISCUSSION
A Biochemical Framework for Computational Modeling of Surface Induced Thrombin Generation in a Flow Field
The chemical model used herein extends a prior mathematical formulation initially developed by Jordan and Chaikof.18 The current model accounts for surface absorption of coagulation factors V, VIII, IX, and X and, therefore, we believe more accurately accounts for the capacity of the activated forms of these factors to form surface bound tenase and prothrombinase, as has been noted in a variety of experimental studies.25,31,37,39 In the original model, rates of tenase and prothrombinase formation were approximated, based solely on the plasma concentration of the activated factors adjacent to the reactive surface. In extending this model, surface-binding sites for factors V, VIII, IX, and X were included to more accurately reflect the kinetics of surface bound tenase and prothrombinase formation. Specifically, while the total number of surface sites was assumed constant, the rate of surface adsorption and desorption of coagulation factors was considered proportional to the available density of surface binding sites. The constant number of surface sites, bound or unbound, places an additional limit on the formation of surface species, such as tenase and prothrombinase. This constraint limits thrombin generation, which is largely driven by such surface-bound entities. We accounted for adsorption and desorption reactions for non-activated, activated, and inactivated forms of factors V, VIII, IX, and X resulting in 12 new chemical reactions, greatly expanding the complexity of the mathematical model. All told, we believe these changes allow for a more accurate biochemical representation of the formation of surface tenase and prothrombinase complexes, consistent with the current experimental understanding of the coagulation cascade.
A Combined Model of Surface Bound Heparin and Thrombomodulin
While many studies have investigated heparin as a systemic and surface bound anticoagulant, few studies, either experimental or computational, have defined the capacity of surface bound heparin to limit local thrombin formation in the presence of complex flow fields or in response to variable local thrombogenic triggers, such as TF. Few numerical models have been published that incorporate heparin reaction kinetics. Byun et al.6 used a simple numerical model consisting of 5 proteins in a parabolic flow field to determine that ATIII binding to surface-bound heparin does not depend on flow rates. However, this study did not investigate the role of surface-bound heparin in limiting local thrombin concentrations or determine threshold values for thrombin generation. Several in vitro studies have sought to determine optimal heparin surface concentrations under flow conditions. Whole blood experiments using a Chandler device demonstrated improved blood compatibility by increasing heparin surface density from 6 to 12 pmol ATIII/cm2.1 Parallel-plate experiments have observed maximum thrombin inactivation rates at heparin (MW ~14 kD) surface densities greater than 4.4 pM/cm2.44 While these studies consider the impact of a hemodynamic environment, they do not include local thrombin generation, such as that initiated by surface bound TF. Since heparinized surfaces reduce thrombin generation, at least in part, due to neutralizing local thrombin production, we believe it important to include local triggers in any such model.24,28
A more comprehensive representation of the ability of heparin or other surface bound thrombin inhibitors to limit local thrombin levels in response to initiators of coagulation and in the context of clinically relevant hemodynamic conditions would provide a very useful tool to characterize potential failure modes for such systems. For example, Tseng et al.45 demonstrated the additive impact of surface bound heparin and TM under flow conditions, suggesting that lower surface concentrations of each factor would be sufficient to inhibit thrombin generation, if both factors were incorporated onto the surface.45 However, due the complexity of the experimental system, only a single set of surface bound heparin and TM substrates could be experimentally evaluated under restrictive set of flow conditions. The computational model used in this report facilitated the evaluation of 104 heparin/TM pairings; 52 under venous flow conditions and 52 under arterial conditions. This large parameter space provides an approach to both focus experimental studies and assist in assessing proposed designs of blood contacting surfaces and devices in a more cost- and time-effective manner.
The Amplification Effect of Circulating Tissue Factor
The clinical significance of circulating TF is an area of active investigation.10,14,52 While there is evidence of correlation between circulating TF and thromboembolism, the mechanistic role of circulating TF remains incompletely defined.12,30,51 In vivo studies using a photochemical carotid artery injury model and inferior vena cava ligation suggest that TF-bearing leukocytes have little impact on thrombus formation, implying that surface-bound TF along the vessel wall drives thrombosis.11 Alternatively, laser induced injury models provide evidence that both blood-borne and surface-bound TF are involved in initiating and propagating thrombosis.7,9 However, results from a mouse DVT model involving restricted blood flow, without associated endothelial injury, revealed that only hematopoietic TF, and not vessel wall TF, initiated the coagulation cascade.46 It remains unclear if circulating TF promotes the initiation and propagation of coagulation in a manner similar to that of surface-bound TF.
Likewise, the relationship between the plasma concentration of TF and the initiation of the coagulation cascade under clinically relevant flow regimes has not been thoroughly studied. Whole blood experiments under rocking conditions considered a range of circulating TF concentrations and showed that 100 fM of relipidated TF reduced clotting time by approximately 50%.4 However this threshold-like value was obtained without considering the presence of surface-bound TF or distinct hemodynamic flow regimes. When surface-bound TF is included, parallel-plate experiments demonstrated that under flow conditions 100 fM of circulating TF increases fibrin deposition more than twofold.34 These in vitro experiments suggest that 100 fM of circulating TF is sufficient to impact the coagulation cascade. However, simulations involving TFp, modeled as behaving in a chemically identical manner to TFs, suggest higher concentration thresholds for TFp in order to significantly increase thrombin production. At a concentration of TFs below which propagation of the coagulation cascade is not observed, the calculated threshold for TFp to trigger increased thrombin generation was 100 pM and 1 nM for venous and arterial conditions, respectively. Above the threshold value of TFs (1.3 fmol/cm2 for venous conditions and 16 fmol/cm2 for arterial conditions), the threshold value for TFp to trigger increased thrombin generation was reduced to 10 pM under venous conditions, while remaining unchanged at 1 nM for arterial conditions. According to these simulated results, TFp amplifies thrombin generation once the cascade is already initiated by TFs. Significantly, under venous shear rates, TFp serves to promote the initiation of the coagulation cascade, even when the surface density of TFs is insufficient. Clinical studies involving circulating TF are difficult to compare with these results as the method for reporting TF activity is often expressed in inconsistent units. However, a study investigating VTE in cancer patients found higher median levels of circulating TF in patients with pancreatic cancer (12.67 pM, range 0.05–112.04 pM) than in those with other cancers (2.01 pM, range 0.05–43.92 pM).12 Of note, these experimental values are within the range of the calculated threshold pro-thrombotic concentrations for circulating TF estimated by our numerical model under simulated venous conditions.
Model Limitations
The computational model presented herein simulates the kinetics of thrombin generation initiated by a constant level of surface-bound TF within a uniform, homogeneous liquid exhibiting simple laminar flow. This model does not include the behavior of platelets and, therefore, does not consider how platelets may physically limit the availability of surface-bound TF. Furthermore, the lack of platelet interactions prevents accounting for the exposure of additional phospholipid surfaces and the resulting reactions that take place on the surface of activated platelets. Similarly, the model does not include the impact of fibrinogen cleavage and the formation of thrombus, which would also physically limit the exposure of surface-bound species. Likewise, while the investigated shear rates are based on well-characterized venous and arterial conditions, flow was considered to be non-disturbed and unidirectional. Substantially different results were observed under venous and arterial conditions. Therefore, it is likely that complex hemodynamic regimes, as noted for heart valves, venous valves, aneurysms, or for a variety of blood contacting artificial organs, will significantly influence thrombotic failure modes.
CONCLUSIONS
The surface complexes tenase and prothrombinase are critical to the formation of surface-initiated thrombin. These surface complexes form when one species is already adsorbed to the surface and then binds to another species carried in the plasma. Previous models have not included adsorbed coagulation factors and, therefore, have potentially generated inaccurate results. This model's focus on surface species allowed it to be applied to two cases that are highly dependent on surface reactions.
The efficacy of surface-bound heparin was compared and combined with that of surface-bound TM. The simulated results suggest that by using both of these surface moieties, lower total surface concentrations are needed to achieve similar results to that when only one species is present. This observation may impact the development of implantable devices, where consistently obtaining and sustaining high surface densities of covalently bound species may be challenging.
While the significance of circulating TF is an ongoing area of research, it has been shown that clinically measured levels of TFp are large enough to generate a thrombotic response when based on the currently accepted mechanism of thrombin generation. Simulated results indicate that concentrations as low as 10 pM are able to noticeably increase thrombin production. If it is observed that even lower levels of TFp are capable of shifting the clinically relevant threshold for initiation the clotting cascade, this would suggest that the model may be incomplete and further study required to determine how TFp is able to have such an impact. In this case, the power of the computational model is not in predicting clinical outcomes but in highlighting the potential for gaps in our mechanistic description of thrombin formation.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by Grants from the National Institutes of Health (HL106018 and HL56819).
Footnotes
Author Contributions: E.V.D. and E.L.C. jointly conceived the project. Computational modeling was carried out by E.V.D. All authors analyzed the data and contributed to the preparation of the manuscript.
ELECTRONIC SUPPLEMENTARY MATERIAL
The online version of this article (doi:10.1007/s10439-015-1377-5) contains supplementary material, which is available to authorized users.
REFERENCES
- 1.Andersson J, Sanchez J, Ekdahl KN, Elgue G, Nilsson B, Larsson R. Optimal heparin surface concentration and antithrombin binding capacity as evaluated with human non-anticoagulated blood in vitro. J. Biomed. Mater. Res. A. 2003;67:458–466. doi: 10.1002/jbm.a.10104. [DOI] [PubMed] [Google Scholar]
- 2.Ataullakhanov F, Zarnitsina V, Pokhilko A, Lobanov A, Morozova O. Spatio-temporal dynamics of blood coagulation and pattern formation A theoretical approach. Intern. J. Bifurc. Chaos Appl. Sci. Eng. 2002;12:1985–2002. [Google Scholar]
- 3.Brummel KE, Paradis SG, Butenas S, Mann KG. Thrombin functions during tissue factor—induced blood coagulation. Blood. 2002;100:148–152. doi: 10.1182/blood.v100.1.148. [DOI] [PubMed] [Google Scholar]
- 4.Butenas S, Bouchard BA, Brummel-Ziedins KE, Parhami-Seren B, Mann KG. Tissue factor activity in whole blood. Blood. 2005;105:2764–2770. doi: 10.1182/blood-2004-09-3567. [DOI] [PubMed] [Google Scholar]
- 5.Butenas S, Mann KG. Kinetics of human factor VII activation. Biochemistry. 1996;35:1904–1910. doi: 10.1021/bi951768c. [DOI] [PubMed] [Google Scholar]
- 6.Byun Y, Jacobs HA, Kim SW. Binding of antithrombin III and thrombin to immobilized heparin under flow conditions. Biotechnol. Prog. 1996;12:217–225. doi: 10.1021/bp950076c. [DOI] [PubMed] [Google Scholar]
- 7.Chou J, Mackman N, Merrill-Skoloff G, Pedersen B, Furie BC, Furie B. Hematopoietic cell-derived microparticle tissue factor contributes to fibrin formation during thrombus propagation. Blood. 2004;104:3190–3197. doi: 10.1182/blood-2004-03-0935. [DOI] [PubMed] [Google Scholar]
- 8.Danforth CM, Orfeo T, Everse SJ, Mann KG, Brummel-Ziedins KE. Defining the boundaries of normal thrombin generation: investigations into hemostasis. PLoS One. 2012;7:e30385. doi: 10.1371/journal.pone.0030385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darbousset R, Thomas GM, Mezouar S, Frere C, Bonier R, Mackman N, Renne T, Dignat-George F, Dubois C, Panicot-Dubois L. Tissue factor-positive neutrophils bind to injured endothelial wall and initiate thrombus formation. Blood. 2012;120:2133–2143. doi: 10.1182/blood-2012-06-437772. [DOI] [PubMed] [Google Scholar]
- 10.Date K, Hall J, Greenman J, Maraveyas A, Madden LA. Tumour and microparticle tissue factor expression and cancer thrombosis. Thromb. Res. 2013;131:109–115. doi: 10.1016/j.thromres.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Day SM, Reeve JL, Pedersen B, Farris DM, Myers DD, Im M, Wakefield TW, Mackman N, Fay WP. Macrovascular thrombosis is driven by tissue factor derived primarily from the blood vessel wall. Blood. 2005;105:192–198. doi: 10.1182/blood-2004-06-2225. [DOI] [PubMed] [Google Scholar]
- 12.Delluc A, Rousseau A, Delluc C, Le Moigne E, Le Gal G, Mottier D, Van Dreden P, Lacut K. Venous thromboembolism in patients with pancreatic cancer: implications of circulating tissue factor. Blood Coagul. Fibrinolysis. 2011;22:295–300. doi: 10.1097/MBC.0b013e32834512f4. [DOI] [PubMed] [Google Scholar]
- 13.Fogelson AL, Tania N. Coagulation under flow: the influence of flow-mediated transport on the initiation and inhibition of coagulation. Pathophysiol. Haemost. Thrombosis. 2005;34:91–108. doi: 10.1159/000089930. [DOI] [PubMed] [Google Scholar]
- 14.Geddings JE, Mackman N. Tumor-derived tissue factor-positive microparticles and venous thrombosis in cancer patients. Blood. 2013;122:1873–1880. doi: 10.1182/blood-2013-04-460139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta V, Aravamuthan BR, Baskerville S, Smith SK, Gupta V, Lauer MA, Fischell TA. Reduction of subacute stent thrombosis (SAT) using heparin-coated stents in a large-scale, real world registry. J. Invasive Cardiol. 2004;16:304–310. [PubMed] [Google Scholar]
- 16.Hockin MF, Jones KC, Everse SJ, Mann KG. A model for the stoichiometric regulation of blood coagulation. J. Biol. Chem. 2002;277:18322–18333. doi: 10.1074/jbc.M201173200. [DOI] [PubMed] [Google Scholar]
- 17.Jones KC, Mann KG. A model for the tissue factor pathway to thrombin. II. A mathematical simulation. J. Biol. Chem. 1994;269:23367–23373. [PubMed] [Google Scholar]
- 18.Jordan S, Chaikof E. Simulated surface-induced thrombin generation in a flow field. Biophys. J. 2011;101:276. doi: 10.1016/j.bpj.2011.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jordan S, Corriere M, Vossen C, Rosendaal F, Chaikof E. Flow-simulated thrombin generation profiles as a predictor of thrombotic risk among pre-menopausal women. Thromb. Haemost. 2012;108:258–265. doi: 10.1160/TH12-02-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kastrup CJ, Boedicker JQ, Pomerantsev AP, Moayeri M, Bian Y, Pompano RR, Kline TR, Sylvestre P, Shen F, Leppla SH, Tang WJ, Ismagilov RF. Spatial localization of bacteria controls coagulation of human blood by ‘quorum acting’. Nat. Chem. Biol. 2008;4:742–750. doi: 10.1038/nchembio.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kastrup CJ, Runyon MK, Shen F, Ismagilov RF. Modular chemical mechanism predicts spatiotemporal dynamics of initiation in the complex network of hemostasis. Proc. Natl. Acad. Sci. USA. 2006;103:15747–15752. doi: 10.1073/pnas.0605560103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kastrup CJ, Shen F, Ismagilov RF. Response to shape emerges in a complex biochemical network and its simple chemical analogue. Angew. Chem. Int. Ed. Engl. 2007;46:3660–3662. doi: 10.1002/anie.200604995. [DOI] [PubMed] [Google Scholar]
- 23.Kastrup CJ, Shen F, Runyon MK, Ismagilov RF. Characterization of the threshold response of initiation of blood clotting to stimulus patch size. Biophys. J. 2007;93:2969–2977. doi: 10.1529/biophysj.107.109009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keuren JF, Wielders SJ, Willems GM, Morra M, Cahalan L, Cahalan P, Lindhout T. Thrombogenicity of polysaccharide-coated surfaces. Biomaterials. 2003;24:1917–1924. doi: 10.1016/s0142-9612(02)00620-8. [DOI] [PubMed] [Google Scholar]
- 25.Krishnaswamy S, Jones KC, Mann KG. Prothrombinase complex assembly. Kinetic mechanism of enzyme assembly on phospholipid vesicles. J. Biol. Chem. 1988;263:3823–3834. [PubMed] [Google Scholar]
- 26.Kuharsky AL, Fogelson AL. Surface-mediated control of blood coagulation: the role of binding site densities and platelet deposition. Biophys. J. 2001;80:1050–1074. doi: 10.1016/S0006-3495(01)76085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leiderman K, Fogelson AL. Grow with the flow: a spatial–temporal model of platelet deposition and blood coagulation under flow. Math. Med. Biol. 2011;28:47–84. doi: 10.1093/imammb/dqq005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindhout T, Blezer R, Schoen P, Willems GM, Fouache B, Verhoeven M, Hendriks M, Cahalan L, Cahalan PT. Antithrombin activity of surface-bound heparin studied under flow conditions. J. Biomed. Mater. Res. 1995;29:1255–1266. doi: 10.1002/jbm.820291013. [DOI] [PubMed] [Google Scholar]
- 29.Manly DA, Boles J, Mackman N. Role of tissue factor in venous thrombosis. Annu. Rev. Physiol. 2011;73:515–525. doi: 10.1146/annurev-physiol-042210-121137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manly DA, Wang J, Glover SL, Kasthuri R, Liebman HA, Key NS, Mackman N. Increased microparticle tissue factor activity in cancer patients with venous thromboembolism. Thromb. Res. 2010;125:511–512. doi: 10.1016/j.thromres.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mann KG, Nesheim ME, Church WR, Haley P, Krishnaswamy S. Surface-dependent reactions of the vitamin K-dependent enzyme complexes. Blood. 1990;76:1–16. [PubMed] [Google Scholar]
- 32.Monkovic DD, Tracy PB. Functional characterization of human platelet-released factor V and its activation by factor Xa and thrombin. J. Biol. Chem. 1990;265:17132–17140. [PubMed] [Google Scholar]
- 33.Morrissey JH. Tissue factor modulation of factor VIIa activity: use in measuring trace levels of factor VIIa in plasma. Thromb. Haemost. 1995;74:185–188. [PubMed] [Google Scholar]
- 34.Okorie UM, Denney WS, Chatterjee MS, Neeves KB, Diamond SL. Determination of surface tissue factor thresholds that trigger coagulation at venous and arterial shear rates: amplification of 100 fM circulating tissue factor requires flow. Blood. 2008;111:3507–3513. doi: 10.1182/blood-2007-08-106229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qu Z, Krishnamurthy V, Haller CA, Dorr BM, Marzec UM, Hurst S, Hinds MT, Hanson SR, Liu DR, Chaikof EL. Immobilization of actively thromboresistant assemblies on sterile blood-contacting surfaces. Adv. Healthc. Mater. 2014;3:30–35. doi: 10.1002/adhm.201300110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qu Z, Muthukrishnan S, Urlam MK, Haller CA, Jordan SW, Kumar VA, Marzec UM, Elkasabi Y, Lahann J, Hanson SR, Chaikof EL. A biologically active surface enzyme assembly that attenuates thrombus formation. Adv. Funct. Mater. 2011;21(24):4736–4743. doi: 10.1002/adfm.201101687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rawala-Sheikh R, Ahmad SS, Ashby B, Walsh PN. Kinetics of coagulation factor X activation by platelet-bound factor IXa. Biochemistry. 1990;29:2606–2611. doi: 10.1021/bi00462a025. [DOI] [PubMed] [Google Scholar]
- 38.Rezaie AR, Olson ST. Calcium enhances heparin catalysis of the antithrombin-factor Xa reaction by promoting the assembly of an intermediate heparin-antithrombin-factor Xa bridging complex. Demonstration by rapid kinetics studies. Biochemistry. 2000;39:12083–12090. doi: 10.1021/bi0011126. [DOI] [PubMed] [Google Scholar]
- 39.Rosing J, Tans G, Govers-Riemslag JW, Zwaal RF, Hemker HC. The role of phospholipids and factor Va in the prothrombinase complex. J. Biol. Chem. 1980;255:274–283. [PubMed] [Google Scholar]
- 40.Serruys PW, van Hout B, Bonnier H, Legrand V, Garcia E, Macaya C, Sousa E, van der Giessen W, Colombo A, Seabra-Gomes R, Kiemeneij F, Ruygrok P, Ormiston J, Emanuelsson H, Fajadet J, Haude M, Klugmann S, Morel MA. Randomised comparison of implantation of heparin-coated stents with balloon angioplasty in selected patients with coronary artery disease (Benestent II). Lancet. 1998;352:673–681. doi: 10.1016/s0140-6736(97)11128-x. [DOI] [PubMed] [Google Scholar]
- 41.Shen F, Kastrup CJ, Ismagilov RF. Using microfluidics to understand the effect of spatial distribution of tissue factor on blood coagulation. Thromb. Res. 2008;122(Suppl 1):S27–S30. doi: 10.1016/S0049-3848(08)70015-X. [DOI] [PubMed] [Google Scholar]
- 42.Solymoss S, Tucker MM, Tracy PB. Kinetics of inactivation of membrane-bound factor Va by activated protein C. Protein S modulates factor Xa protection. J. Biol. Chem. 1988;263:14884–14890. [PubMed] [Google Scholar]
- 43.Tseng PY, Jordan SW, Sun XL, Chaikof EL. Catalytic efficiency of a thrombomodulin-functionalized membrane-mimetic film in a flow model. Biomaterials. 2006;27:2768–2775. doi: 10.1016/j.biomaterials.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 44.Tseng PY, Rele SM, Sun XL, Chaikof EL. Fabrication and characterization of heparin functionalized membrane-mimetic assemblies. Biomaterials. 2006;27:2627–2636. doi: 10.1016/j.biomaterials.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 45.Tseng PY, Rele SS, Sun XL, Chaikof EL. Membrane–mimetic films containing thrombomodulin and heparin inhibit tissue factor-induced thrombin generation in a flow model. Biomaterials. 2006;27:2637–2650. doi: 10.1016/j.biomaterials.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 46.von Bruhl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, Khandoga A, Tirniceriu A, Coletti R, Kollnberger M, Byrne RA, Laitinen I, Walch A, Brill A, Pfeiler S, Manukyan D, Braun S, Lange P, Riegger J, Ware J, Eckart A, Haidari S, Rudelius M, Schulz C, Echtler K, Brinkmann V, Schwaiger M, Preissner KT, Wagner DD, Mackman N, Engelmann B, Massberg S. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 2012;209:819–835. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walpoth BH, Rogulenko R, Tikhvinskaia E, Gogolewski S, Schaffner T, Hess OM, Althaus U. Improvement of patency rate in heparin-coated small synthetic vascular grafts. Circulation. 1998;98:319–323. [PubMed] [Google Scholar]
- 48.Wang N-HL, Keller K. Augmented transport of extracellular solutes in concentrated erythrocyte suspensions in Couette flow. J. Colloid Interface Sci. 1985;103:210–225. [Google Scholar]
- 49.Zarnitsina V, Pokhilko A, Ataullakhanov F. A mathematical model for the spatio-temporal dynamics of intrinsic pathway of blood coagulation. I. The model description. Thromb. Res. 1996;84:225–236. doi: 10.1016/s0049-3848(96)00182-x. [DOI] [PubMed] [Google Scholar]
- 50.Zarnitsina V, Pokhilko A, Ataullakhanov F. A mathematical model for the spatio-temporal dynamics of intrinsic pathway of blood coagulation. II. Results. Thromb. Res. 1996;84:333–344. doi: 10.1016/s0049-3848(96)00197-1. [DOI] [PubMed] [Google Scholar]
- 51.Zwicker JI, Liebman HA, Neuberg D, Lacroix R, Bauer KA, Furie BC, Furie B. Tumor-derived tissue factor-bearing microparticles are associated with venous thromboembolic events in malignancy. Clin. Cancer Res. 2009;15:6830–6840. doi: 10.1158/1078-0432.CCR-09-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zwicker JI, Trenor CC, 3rd, Furie BC, Furie B. Tissue factor-bearing microparticles and thrombus formation. Arterioscler. Thromb. Vasc. Biol. 2011;31:728–733. doi: 10.1161/ATVBAHA.109.200964. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.