Abstract
Early life stress interacts with adult stress to differentially modulate neural systems and vulnerability to various psychiatric illnesses. However, the effects of early life stress and adult stress on addictive behaviors have not been sufficiently investigated. We examined the effects of early life stress in the form of prolonged maternal separation followed in early adulthood by either 10 days of chronic variable stress or no stress on methamphetamine self-administration, extinction, and cue-induced reinstatement. We observed that chronic variable stress in adulthood reduced methamphetamine self-administration in rats with a history of early life stress. These findings add to an emerging body of literature suggesting interactions between and early life and early adulthood stressors on adult behavioral phenotypes.
Keywords: addiction, chronic variable stress, early life stress, maternal separation, methamphetamine, self-administration, rat
INTRODUCTION
Chronic methamphetamine (METH) abuse has dire effects on both society and users’ health. METH is a highly addictive psychostimulant with worldwide abuse rates comparable to both heroin and cocaine combined (United Nations Office on Drugs and Crime, 2007). Therefore, identifying risk factors that contribute to the development of METH addiction is critical. Early life stress is implicated in long term alterations in numerous behavioral and neurobiological systems that contribute to addiction vulnerability. The maternal separation paradigm is a rodent model of early life stress that has been shown to increase the rewarding and reinforcing properties of various drugs of abuse in adulthood (Moffett et al., 2007). While interactions between early life and adult stress differently modulate various neural systems and vulnerability to psychiatric illnesses such as schizophrenia and post-traumatic stress disorder (Choy et al., 2009; Yehuda et al., 2010), investigations into the effects of early life stress in conjunction with adult stress on vulnerability to addiction-related behaviors has largely been unexplored.
The majority of research investigating the effects of early life stress on addiction-like behaviors typically includes a single developmental insult. While this design allows for the detailed examination of effects of adverse early life events alone, it does not take into account the fact that many individuals with a history of early life stress are also exposed to repeated or chronic stress later in adulthood. The purpose of the present study was to evaluate the effects of early life stress, adult chronic variable stress, and their potential interactions on vulnerability towards METH addiction-related behaviors. Therefore, we utilized an established paradigm of early life stress, maternal separation, in conjunction with adult stress in the form of chronic variable stress. We hypothesized the combination of two developmental stressors would increase METH self-administration, delay extinction, and increase the magnitude of cue induced reinstatement compared to rats that either experienced only early life or adult chronic variable stress, or rats with no history of prior stress exposure.
METHODS
Subjects and stressors
Pregnant Long-Evans dams (Charles River Laboratory, Kingston, NY, USA) arrived on gestational day 12. On the day of birth (postnatal day 0, PND0), litters were assigned to 1 of 2 rearing conditions: maternal separation for 180 min/day (Early Life Stress) or 15 min/day (No Stress). The 180 min/day separation was chosen as a commonly utilized early life stressor, and the 15 min/day separation was chosen as control for handling that does not produce neurophysiological stress responses in the pups (Kuhn and Schanberg, 1998), and has previously been utilized for comparison to effects of prolonged separation (Ploj et al., 2003). Separation procedures were conducted on PND2-14. During PND15-20, litters were left undisturbed with the exception of once weekly cage cleaning. Litters were weaned on PND21, and female offspring were then removed from the study. Male offspring were sibling group housed until PND45 after which they were pair housed. On PND55, half the rats were exposed to a 10-day chronic variable stress paradigm (Early Life Stress + Adult Stress or Adult Stress). For 10 consecutive days, rats in the chronic variable stress condition received either 2 daily stressors or one day time stressor and an overnight stressor (see Table 1 for details).
Table 1.
Example Chronic Variable Stress Procedure
| Day | First Stressor | Duration | Second Stressor | Duration | Overnight stressor |
|---|---|---|---|---|---|
| 1 | Loud noise | 2hr | Tail-pinch | 10min | X |
| 2 | Forced swim | 10min | X | Dirty bedding | |
| 3 | Restraint | 2hr | Strobe | 2hr | X |
| 4 | Tail-pinch | 10min | X | Crowding | |
| 5 | Shaker | 2hr | SC Injection | ~2min | X |
| 6 | Loud noise | 2hr | Tail-pinch | 10min | X |
| 7 | Forced swim | 10min | X | Crowding | |
| 8 | Restraint | 2hr | Strobe | 2hr | X |
| 9 | Tail-pinch | 10min | X | Dirty bedding | |
| 10 | SC Injection | ~2min | Shaker | 2hr | X |
Methamphetamine self-administration
After chronic variable stress procedures concluded, all rats were implanted with intravenous catheters into the jugular vein using standard procedures as described elsewhere (Lewis et al., 2013). Rats were allowed five days of recovery prior to commencement of self-administration procedures. Rats were allowed to spontaneously acquire METH self-administration (0.05 mg/kg/infusion, delivered in a volume of 0.06 ml over a 2-s period) by pressing a designated active lever in standard operant conditioning chambers (ENV-008; Med Associates, St. Albans, VT). A fixed-ratio 1 (FR1) schedule of reinforcement was used in 2-h daily sessions for 15 consecutive days. Each infusion was paired with a 2-s auditory stimulus (~65 dB, 2900 Hz) and illumination of a stimulus light located above each response lever. Each drug infusion was followed by a 20-s timeout period during which additional active lever presses were recorded but produced no programmed consequences. Next, all animals were subjected to extinction training, whereby presses on the active lever no longer produced any programmed consequences. Extinction training sessions were 2 h in length and were conducted for 8 consecutive days. On the day immediately following the last extinction session, all rats underwent cue-induced reinstatement testing (2 h duration), whereby presses on the active lever resulted in the presentation of the tone and light previously presented during METH infusions, but did not result in drug infusion.
RESULTS
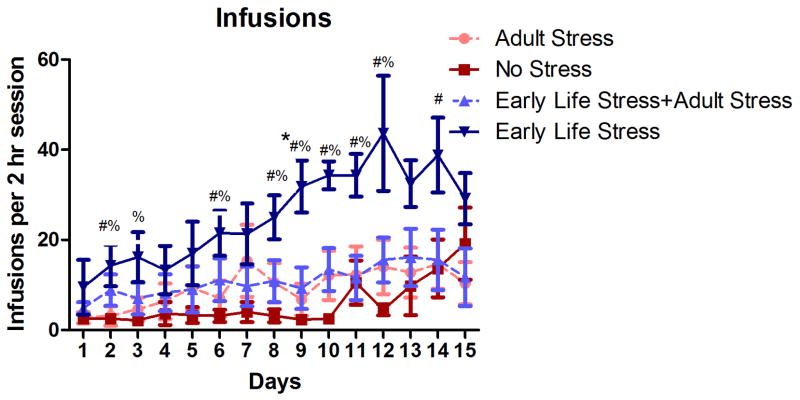
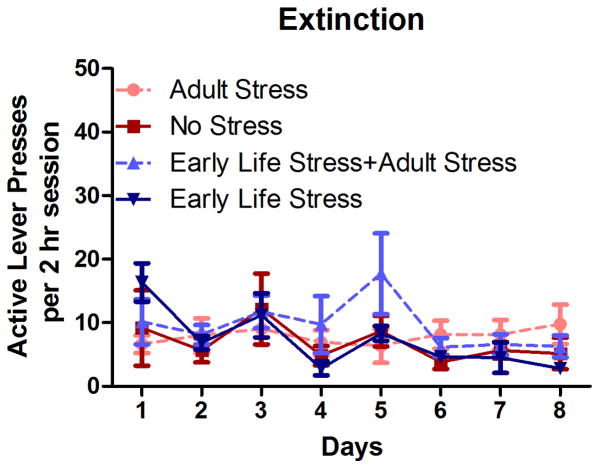
Repeated measures ANOVA revealed a significant effect of time (p < .005) and significant main effects of Early Life Stress on the number of METH infusions obtained per session, F(1,23) = 13.00, p < 0.001, as well as the number of active lever presses per session, F(1,23) = 14.73, p < 0.001. There was no significant main effect of Adult Stress on either the number of METH infusions or active lever presses, but there was a significant interaction between Adult Stress and Early Life Stress with regards to the number of METH infusions obtained, F(1,23) = 4.38, p < 0.05 (Figure 1), and active lever presses, F(1,23) = 3.99, p < 0.05. The effect of Adult Stress was dependent on Early Life Stress history, such that Adult Stress reduced responding for METH in the Early Life Stress group. No group differences in the number of inactive lever presses during self-administration were observed (p > 0.05, data not shown), nor were there any group differences in extinction rates or the number of active (Figure 2) or inactive lever presses during reinstatement testing (all p’s >0.05, data not shown).
Figure 1.
Average number of infusions per 2-h METH self-administration session for 15 consecutive days in No Stress (n=6), Adult Stress (n=10), Early Life Stress (n=6), and Early Life Stress + Adult Stress (n=5) rats. Data points represent group mean ± SEM. p≤0.05: * vs. Early Life Stress + Adult Stress, # vs. Adult Stress, and % vs No Stress.
Figure 2.
Average number of active lever presses per 2-hr extinction training session for eight consecutive days in No Stress (n=6), Adult Stress (n=10), Early Life Stress (n=6), and Early Life Stress + Adult Stress (n=5) rats. Data points represent group mean ± SEM. No significant differences were found between groups.
DISCUSSION
The current study replicates our previous findings that early life stress, induced by prolonged maternal separation, increases adult METH intake compared to handled control rats (Lewis et al., 2013). Additionally, the current results expand on this finding by revealing that chronic variable stress in adulthood attenuates METH self-administration in rats with a history of early life stress. However, adult chronic variable stress had no effect on METH self-administration in animals without a history of early life stress. These findings were unexpected, since instead of demonstrating that the two developmental stressors produced additive effects of increasing METH self-administration, it appears that chronic variable stress in adulthood possibly confers some sort of resiliency towards METH addiction vulnerability. It should be noted, however, that while inactive lever presses did not differ across treatment groups, additional studies are needed to confirm that reductions in general appetitive responding or motor function did not influence these findings. Additional studies are also needed to assess potential stressor interactions in females, as well as in animals with no history of maternal separation to assess potential effects of handling.
There are several possible mechanisms underlying the apparent protective effect of chronic variable stress against METH self-administration in male rats with a history of early life stress. For example, the acute neuroendocrine stress response is considered a predictor for acquisition of psychostimulant self-administration, and adult chronic variable stress decreases the neuroendocrine stress response in rats with a history of early life stress (Ladd et al., 2005; Renard et al., 2007). Adult corticosterone treatment following early life stress desensitizes dopamine receptors without changing the receptor density in nucleus accumbens, a brain region associated with drug reinforcement (Choy et al., 2009). Additionally, chronic social defeat stress in adulthood increases thresholds for brain stimulation reward in rats with a history of early life stress (Der-Avakian and Markou, 2010). Thus, our findings are in agreement with the extant literature suggesting that mild chronic adult stress can potentially reverse detrimental effects of early life stress and modulate the reinforcing properties of psychostimulants. Overall, our results suggest that chronic variable stress alters the reinforcing effects of METH in animals with a history of early life stress but does not alter extinction or cue-induced reinstatement of METH-seeking.
References
- Choy KHC, de Visser YP, van den Buuse M. The effect of “two hit” neonatal and young-adult stress on dopaminergic modulation of prepulse inhibition and dopamine receptor density. Br J Pharmacol. 2009;156:388–96. doi: 10.1111/j.1476-5381.2008.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A. Neonatal maternal separation exacerbates the reward-enhancing effect of acute amphetamine administration and the anhedonic effect of repeated social defeat in adult rats. Neuroscience. 2010;170:1189–98. doi: 10.1016/j.neuroscience.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn CM, Schanberg SM. Responses to maternal separation: mechanisms and mediators. Int J Dev Neurosci. 1998;16:261–70. doi: 10.1016/s0736-5748(98)00034-3. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Thrivikraman KV, Huot RL, Plotsky PM. Differential neuroendocrine responses to chronic variable stress in adult Long Evans rats exposed to handling-maternal separation as neonates. Psychoneuroendocrinology. 2005;30:520–33. doi: 10.1016/j.psyneuen.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Lewis CR, Staudinger K, Scheck L, Olive MF. The effects of maternal separation on adult methamphetamine self-administration, extinction, reinstatement, and MeCP2 immunoreactivity in the nucleus accumbens. Front Psychiatry. 2013;4(55):1–9. doi: 10.3389/fpsyt.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett MC, Vicentic A, Kozel M, Plotsky P, Francis DD, Kuhar MJ. Maternal separation alters drug intake patterns in adulthood in rats. Biochem Pharmacol. 2007;73:321–30. doi: 10.1016/j.bcp.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploj K, Roman E, Nylander I. Long-term effects of maternal separation on ethanol intake and brain opioid and dopamine receptors in male Wistar rats. Neuroscience. 2003;121:787–9. doi: 10.1016/s0306-4522(03)00499-8. [DOI] [PubMed] [Google Scholar]
- Renard GM, Rivarola MA, Suárez MM. Sexual dimorphism in rats: effects of early maternal separation and variable chronic stress on pituitary-adrenal axis and behavior. Intl J Devel Neurosci. 2007;25:373–9. doi: 10.1016/j.ijdevneu.2007.07.001. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime. World drug report. 2007 < http://www.unodc.org/pdf/research/wdr07/WDR_2007.pdf> Retrieved on July 11 2014 n.d.
- Yehuda R, Flory JD, Pratchett LC, Buxbaum J, Ising M, Holsboer F. Putative biological mechanisms for the association between early life adversity and the subsequent development of PTSD. Psychopharmacology (Berl) 2010;212:405–17. doi: 10.1007/s00213-010-1969-6. [DOI] [PubMed] [Google Scholar]