Abstract
The DNA methyltransferase inhibitor 5-azacytidine is being evaluated clinically as an oral formulation to treat various solid tumors. A sensitive, reliable method was developed to quantitate 5-azacytidine using LC-MS/MS to perform detailed pharmacokinetic studies. The drug of interest was extracted from plasma using Oasis MCX ion exchange solid-phase extraction 96-well plates. Chromatographic separation was achieved with an YMC J'sphere M80 C18 column and isocratic elution with a methanol-water-formic acid (15:85:0.1, v/v/v) mobile phase over a 7 minute total analytical run time. An AB Sciex 5500 triple quadrupole mass spectrometer operated in positive electrospray ionization mode was used for the detection of 5-azacytidine. The assay range was 5 to 500 ng/mL and proved to be accurate (97.8-109.1%) and precise (%CV ≤9.8%). Tetrahydrouridine was used to stabilize 5-azacytidine in blood/plasma samples. With the addition of tetrahydrouridine, long-term frozen plasma stability for 5-azacytidine at -70°C has been determined for at least 323 days. The method was applied for the measurement of total plasma concentrations of 5-azacytidine in a cancer patient receiving a 300 mg oral daily dose.
Keywords: 5-azacytidine, DNA methyltransferase inhibitor, LC/MS/MS, Pharmacokinetics
Introduction
5-azacytidine is a DNA methyltransferase inhibitor and is approved for the treatment of myelodysplastic syndromes (MDS) at a subcutaneous dose of 75 mg/m2 (Kaminskas et al. 2005). Recently an oral formulation with 12.7% relative bioavailability was developed (Laille et al. 2014).
5-azacytidine undergoes spontaneous hydrolysis and is metabolized by cytidine deaminase (Chabner et al. 1973, Notari et al. 1975, Kissinger et al. 1986). The only published LC-MS/MS method achieved a lower limit of quantitation (5 ng/mL) and has been used extensively to characterize the pharmacokinetics (Zhao et al. 2004, Rudek et al. 2005).
The aim of this study was to improve upon current method for the determination of 5-azacytidine utilizing an isocratic LC-MS/MS and to apply it to pharmacokinetic analyses in patients receiving oral 5-azacytidine.
Experimental
Chemical and Reagents
5-azacytidine was obtained from MP Biomedicals (Santa Ana, CA) and 5-methyl-2′-deoxycytidine (5-Me-2′DC), the internal standard, from Chem-Impex International (Wood Dale, IL) (Supplemental Fig 1).
Sample Preparation
Calibration standards and quality control samples were prepared by spiking blank human sodium heparin plasma containing 25 μg/mL tetrahydrourine (THU), a cytidine deaminase inhibitor. Plasma (200 μL) was vortex-mixed with 20 μL of IS. The mixture was transferred to an activated Waters Oasis MCX LP 60 μm (30 mg) ion exchange solid phase extraction (SPE) 96-well plate. After a wash with 1 mL 0.1 N HCl followed by 1 mL methanol, analytes were eluted using 0.5 mL acetonitrile-water-ammonium hydroxide (85:10:5, v/v/v) twice. Eluents were evaporated to dryness and reconstituted with 100 μL water.
Chromatographic Conditions
Chromatographic analysis was performed using a Waters Acquity UPLC system (Milford, MA). Separation was performed on a J'sphere ODS-M80 (250×2.1 mm i.d., YMC Co. Ltd., Kyoto, Japan) column. The mobile phase consisted of methanol-water-formic acid (15:85:0.1, v/v/v) delivered isocratically at a flow-rate of 0.15 mL/min.
Mass spectroscopic Conditions
The column effluent was monitored using an AB Sciex 5500 triple quadrapole™ (Foster City, CA) operating in positive electrospray ionization mode. Mass transitions (m/z) were monitored using a dwell time of 300 ms for 5-azacytidine (245.0/112.9) and IS (242.0/126.0). Collision energy for 5-azacytidine was 14 V and 8 V for the IS.
Method validation
The validation of this method includes precision and accuracy, sensitivity and selectivity, stock and plasma stability and matrix effects according to the FDA guidelines (US Food and Drug Administration May 2001).
Application of method
The method was applied to a patient who received 5-azacytidine administered orally, once daily, in combination with romidepsin on an ongoing Phase I clinical trial (NCT01537744). The protocol was approved by the Institutional Review Board of The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins (Baltimore, MD).
Results and Discussion
Separation and detection
A LC-MS/MS method was developed and validated to determine 5-azacytidine concentrations in human plasma containing 25 μg/mL THU. THU was added to minimize degradation by cytidine deaminase (Wentworth et al. 1975). 5-azacytidine and its internal standard eluted at 4.8 and 3.7 minutes respectively, with a total run time of 7 minutes (Figure 1).
Figure 1.
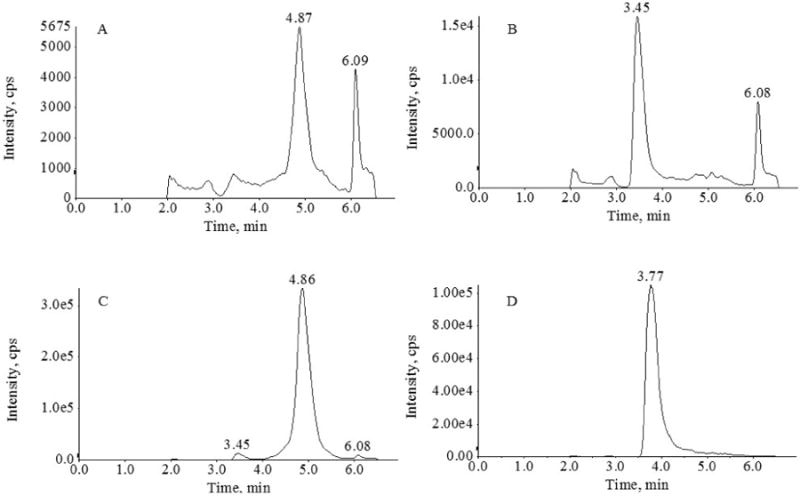
Chromatograms of plasma for 5-azacytidine (A) LLOQ (5 ng/mL), (B) predose patient sample, (C) patient sample with 446 ng/mL of 5-azacytidine, and (D) internal standard (5-methyl-2′-deoxycytine).
Calibration curves
The calibration curve for 5-azacytidine was constructed from the peak ratio of 5-azacytidine to its internal standard using the least-squares quadratic regression analysis with 1/x2 weight. The r2 ≥ 0.99 and the calibration range was 5 ng/mL to 500 ng/mL.
Precision and Accuracy
The accuracy, within run and between run precision at the LLOQ (5 ng/mL) was 109.1%, 9.8%, and no difference when performing the assay in different runs, respectively (Supplemental Table 2). The accuracy, within run and between run precision for the QCs ranged 97.8-104.4%, 5.1-6.4%, and 5.0-6.4%, respectively (Supplemental Table 2).
Sensitivity and Selectivity
The average signal to noise ratio of the LLOQ (5 ng/mL) was 89 was determined by the Analyst software. No major interferences were observed from 6 different blank plasma lots spiked with 25 μg/mL THU (Figure 1).
Matrix Effects
Matrix effects, absolute recovery and combined recovery were evaluated in 3 QC concentration levels (low, medium, and high). Sample concentrations were analyzed in neat solutions, post extraction spiked samples, and pre-spiked samples. Matrix effects were also assessed using the previously published sample preparation method to compare the differences between methods (Zhao et al. 2004). Matrix effects were found with 51-55% suppression and a combined recovery was 15-17% when compared to the previously assay the combined recovery ranged from 1-4% (Supplemental Table 3). For the internal standard, the overall ion suppression was 57% and the combined recovery was 35%.
Stability
The master stock solution of 5-azacytidine was stable for 46 days in 50% methanol at -20°C. Plasma containing 25 μg/mL THU was stable for 323 days at -70°C (Supplemental Table 2).
Application to pharmacokinetic study
The LC/MS/MS was applied to a single patient receiving 300 mg dose with a resultant Cmax of 446 ng/mL which occurred at 1.5 h with an AUC∞ of 822 ng*h/mL.
Conclusion
An analytical assay method was developed and validated to quantify 5-azacytidine levels in human plasma containing THU. The validated method improves previous extraction methods using current instrumentation, shortens the runtime, and is automated. The method described in this paper meets all requirements in the FDA guidelines and has been used to analyze clinical trial samples.
Supplementary Material
Acknowledgments
We would like to thank Dr. Ming Zhao for his original analytical method which laid the foundation for our work, and Linping Xu for her quality assurance review of the data. The project described was supported by Celgene Corporation, the Flight Attendants Medical Research Institution (FAMRI) Center for Excellence at the Johns Hopkins University School of Medicine, and by the Analytical Pharmacology Core of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins (NIH grants P30 CA006973 and UL1TR001079, and the Shared Instrument Grant (1S10RR026824-01)). The project described was supported by Grant Number UL1TR001079 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research, and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCATS or NIH.
Disclosure: This research was funded in part by Celgene Corporation.
References
- Chabner BA, Drake JC, Johns DG. Deamination of 5-azacytidine by a human leukemia cell cytidine deaminase. Biochem Pharmacol. 1973;22:2763–2765. doi: 10.1016/0006-2952(73)90137-8. [DOI] [PubMed] [Google Scholar]
- Kaminskas E, Farrell A, Abraham S, Baird A, Hsieh LS, Lee SL, Leighton JK, Patel H, Rahman A, Sridhara R, Wang YC, Pazdur R Fda. Approval summary: azacitidine for treatment of myelodysplastic syndrome subtypes. Clin Cancer Res. 2005;11:3604–3608. doi: 10.1158/1078-0432.CCR-04-2135. [DOI] [PubMed] [Google Scholar]
- Kissinger LD, Stemm NL. Determination of the antileukemia agents cytarabine and azacitidine and their respective degradation products by high-performance liquid chromatography. J Chromatogr. 1986;353:309–318. doi: 10.1016/s0021-9673(01)87101-6. [DOI] [PubMed] [Google Scholar]
- Laille E, Savona MR, Scott BL, Boyd TE, Dong Q, Skikne B. Pharmacokinetics of different formulations of oral azacitidine (CC-486) and the effect of food and modified gastric pH on pharmacokinetics in subjects with hematologic malignancies. J Clin Pharmacol. 2014;54:630–639. doi: 10.1002/jcph.251. [DOI] [PubMed] [Google Scholar]
- Notari RE, DeYoung JL. Kinetics and mechanisms of degradation of the antileukemic agent 5-azacytidine in aqueous solutions. J Pharm Sci. 1975;64:1148–1157. doi: 10.1002/jps.2600640704. [DOI] [PubMed] [Google Scholar]
- Rudek MA, Zhao M, He P, Hartke C, Gilbert J, Gore SD, Carducci MA, Baker SD. Pharmacokinetics of 5-azacitidine administered with phenylbutyrate in patients with refractory solid tumors or hematologic malignancies. J Clin Oncol. 2005;23:3906–3911. doi: 10.1200/JCO.2005.07.450. [DOI] [PubMed] [Google Scholar]
- Wentworth DF, Wolfenden R. On the interaction of 3,4,5,6-tetrahydrouridine with human liver cytidine deaminase. Biochemistry. 1975;14:5099–5105. doi: 10.1021/bi00694a012. [DOI] [PubMed] [Google Scholar]
- Zhao M, Rudek MA, He P, Hartke C, Gore S, Carducci MA, Baker SD. Quantification of 5-azacytidine in plasma by electrospray tandem mass spectrometry coupled with high-performance liquid chromatography. J Chromatogr B. 2004;813:81–88. doi: 10.1016/j.jchromb.2004.09.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


