Abstract
Cognitive control constrains mental operations to prioritize information that reaches conscious awareness and is essential to flexible, adaptive behavior under conditions of uncertainty. However, these processes can be compromised by neurodevelopmental disorders, such as autism spectrum disorder (ASD), which is characterized by the presence of social and communicative deficits, and restricted interests/repetitive behaviors. Although prior investigations have attempted to elucidate the nature of cognitive control deficits in ASD, whether there is an underlying information processing deficit associated with cognitive control remains unclear. The present study challenged cognitive control in 15 high-functioning adults with ASD and 15 typically developing (TD) controls using three novel tasks designed to systematically manipulate uncertainty. We aimed to investigate the efficiency of cognitive control in sequential information processing, cognitive control of non-sequential information processing across a range of cognitive load, and cognitive control capacity under time constraints. Results demonstrated that the ASD group performed less efficiently under sequential and non-sequential information processing, and had reduced cognitive control capacity under time constraints relative to the TD group. These findings complement existing theories suggesting that inefficient cognitive control of information processing may be a fundamental deficit in ASD.
Keywords: autism, cognitive control, information processing, information theory, executive functions
Introduction
Cognitive control refers to the flexible allocation of mental resources in the service of goal-directed behavior, within the context of input that far exceeds the brain's information-processing capacity (Badre, 2008; Fan, 2014; Kouneiher, Charron, & Koechlin, 2009; Mackie, Van Dam, & Fan, 2013; Miller & Cohen, 2001; Posner & Snyder, 1975). The ability to efficiently process incoming information and rapidly generate responses therefore depends on the integrity of cognitive control. Typically developing (TD) individuals are generally efficient in employing cognitive control, but in cases of neurodevelopmental disorders, cognitive control can be compromised, resulting in functional impairment (Burden et al., 2009; Durston et al., 2003; Minshew & Goldstein, 1998; Minshew, Johnson, & Luna, 2001; Poljac & Bekkering, 2012; Rowe, Lavender, & Turk, 2006; Shapiro, Wong, & Simon, 2013; Solomon, Ozonoff, Cummings, & Carter, 2008; Solomon et al., 2013; Vaidya et al., 2005; van Meel, Heslenfeld, Oosterlaan, & Sergeant, 2007).
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by the presence of symptoms in the domains of social and communicative deficits, and restricted interests/repetitive behaviors (American Psychiatric Association, 2013). Given that individuals with ASD can be extraordinarily inflexible in their behavior, previous work has been devoted to understanding the relationship between cognitive (attributed to cognitive control deficits) and behavioral inflexibility (Bishop, 1993; Bogte, Flamma, van der Meere, & van England, 2008; Damasio & Maurer, 1978; Garcia-Villamisar & Sala, 2002; Geurts, Corbett, & Solomon, 2009; Hughes, Russell, & Robbins, 1994; Lopez, Lincoln, Ozonoff, & Lai, 2005; Ozonoff, Pennington, & Rogers, 1991; Solomon et al., 2008; Turner, 1997, 1999; Yerys et al., 2009). A common approach has been to attempt to isolate specific executive functions (e.g., response inhibition, task-switching, working memory) and test for deficits in child and adult ASD samples. For the most part, results have been inconsistent (Barnard-Brak, 2011; Geurts et al., 2009; Poljac & Bekkering, 2012; Russo et al., 2007; Solomon et al., 2008) and many questions remain regarding the cognitive profile of this disorder. Existing studies have not employed quantitative definitions of information or parametric within-task manipulations of cognitive control, often instead using qualitative differences in difficulty between tasks as an indicator of cognitive load. Parametric manipulations of information, quantified in computational units such as ‘bits’ would result in clearer comparisons between conditions in terms of how much information the cognitive control system is able to efficiently manage. Generally, while the terms “executive functions” and “cognitive control” are used interchangeably in the literature, we are specifically interested in the latter, which is conceptualized as the mental control underlying the ability to perform high-level executive functions (Mackie et al., 2013).
An information theory approach to cognitive control in ASD
Information theory provides a new perspective to the study of cognitive control in ASD (Barbalat, Leboyer, & Zalla, 2014; Fan, 2014; Just, Keller, Malave, Kana, & Varma, 2012), which is concerned with the communication of information under uncertainty (Shannon & Weaver, 1949). Within this framework, cognitive control is a limited-capacity integrative interface between input and response that dynamically facilitates the processing of information (Fan, 2014). Information contained in the occurrence of a certain type of event in a sequence is referred to as surprise; a low frequency event is associated with a high surprise value. For a sequence set that predominantly requires ‘left’ responses (e.g., 87.5% left, 12.5% right), a stimulus requiring a ‘right’ response, would carry a higher surprise value (3 bits) than the ‘left’ response (0.19 bits) (see Methods for the computation). Entropy, on the other hand, is quantified as the information contained in a sequence of events, such that entropy is the weighted average of surprise over events. For the sequence mentioned above, the entropy is 0.54 bits. For a predictable sequence, entropy is low. Entropy would be highest (1 bit) when ‘left’ and ‘right’ responses are equally probable (i.e., 50% left, 50% right; see Fan, 2014 for a review).
From a similar perspective, it has previously been proposed that individuals with ASD have a reduced capacity for information transfer and/or processing due to abnormalities in neural dynamics (Belmonte et al., 2004; Fan et al., 2012; Just, Cherkassky, Keller, & Minshew, 2004; Just et al., 2012). For example, the complex information processing theory of ASD asserts that while basic information processing ability is intact, deficits become apparent as complexity increases (Minshew & Goldstein, 1998; Minshew, Goldstein, & Siegel, 1997; Minshew et al., 2001; Williams, Goldstein, & Minshew, 2006). However, systematic and explicit quantification and manipulation of uncertainty within-tasks to examine cognitive control in ASD are not present in the existing literature.
Uncertainty and cognitive control in ASD
Uncertainty indicates the need for cognitive control to facilitate information processing with dynamic reduction of uncertainty and prioritization of information for further computation (Fan et al., 2014; Mackie et al., 2013; Mushtaq, Bland, & Schaefer, 2011). If cognitive control is less efficient in ASD, dynamically dealing with uncertainty should be problematic, contributing to the clinical presentation of the disorder. For example, the diagnostic domain of effective social communication requires cognitive control for the efficient allocation of brain resources in constraining information to be processed, and to avoid information processing overload (Gomot & Wicker, 2012). Dynamic social situations present incredibly uncertain (high entropy and surprise) conditions. Reciprocal communication requires rapid information processing, idea generation and response, as well as ongoing processing of non-verbal information. Therefore, successful social behavior requires flexible adaptation to variable social contexts (Cashin, Gallagher, Newman, & Hughes, 2012; Cañadas, Rodríguez-Bailón, Milliken, & Lupiáñez, 2013; Dichter & Belger, 2008; Happé & Frith, 2006; Kenworthy, Case, Harms, Martin, &Wallace, 2010). Lower efficiency in sequential and non-sequential information processing, combined with a reduced upper limit of information processing capacity, may negatively impact the cognitive flexibility required for smooth social interaction in dynamic contexts, and therefore set the foundation for the emergence of social and communicative deficits. The other symptom domain of restricted interests/repetitive behaviors may also be explained in terms of uncertainty restriction. Insistence on following routine and constraining interests to a confined set reduces uncertainty associated with novel and dynamic information-rich situations (Baron-Cohen, Ashwin, Ashwin, Tavassoli, & Chakrabarti, 2009). This may serve as a protective mechanism (conscious or unconscious) to avoid the subjective frustration associated with information overload (Hutt, Hutt, Lee, & Ounsted, 1965; Markram, Rinaldi, & Markram, 2007; O'Connor & Kirk, 2008; Valla & Belmonte, 2013).
The present study represents a first step in testing an information theory account of cognitive control deficits in ASD at the level of basic information processing without the influence of social information. We designed a series of tasks that systematically manipulated entropy, surprise, and processing rate to investigate: 1) efficiency of cognitive control in sequential information processing; 2) cognitive control efficiency across a range of uncertainty values of non-sequential events; and 3) the limits of cognitive control capacity under time constraints in a sample of high-functioning adults with ASD in comparison to typically developing (TD) adults. We predicted less efficient cognitive control performance for sequential and non-sequential information processing across the range of uncertainty manipulated, with lower cognitive control capacity under time constraints for the ASD group compared to the TD group, and that greater ASD symptom report would be associated with lower cognitive control performance.
Method
Participants
Fifteen high-functioning adults with ASD and 15 TD adults participated in this study. Participants were recruited at the Seaver Autism Center for Research and Treatment, Icahn School of Medicine at Mount Sinai (ISMMS). Demographic information is presented in Table 1. TD participants were matched with ASD participants on average IQ, age, and gender (all male). Independent sample t-tests confirmed that the there were no significant between group differences in age, full scale IQ, verbal IQ, or performance IQ (all ps > .30).
Table 1.
Means of demographic data (range) of ASD and TD groups.
| Participant characteristics | ASD (n = 15) | TD (n = 15) | t | p |
|---|---|---|---|---|
| Age (years) | 26.0 (18 - 42) | 27.6 (19 - 43) | -.59 | .56 |
| Full Scale IQ | 110 (87- 143) | 115 (96 - 132) | -.87 | .39 |
| Verbal IQ1 | 109 (79 - 148) | 115 (94 - 128) | -.89 | .38 |
| Performance IQ1 | 105 (75 - 146) | 108 (87 - 125) | -.48 | .63 |
| ASD diagnosis | 15 | |||
| ADI-R | ||||
| Social | 18.8 (1 - 28) | |||
| Verbal communication | 15.7 (8 - 22) | |||
| Repetitive behavior | 5.9 (1 - 12) | |||
| Development | 2.9 (0 - 5) | |||
| ADOS-G (n = 14) | ||||
| Communication + Social | 9.7 (5 - 16) | |||
| Communication + Social CSS2 | 7.0 (4 - 10) | |||
| Restricted and repetitive behaviors | 1.4 (0 - 4) | |||
| Restricted and repetitive behaviors CSS | 4.7 (1 - 8) | |||
| Total | 12.1 (8 - 18) | |||
| Total CSS | 6.6 (4 - 9) | |||
Participants with ASD were diagnosed by trained clinicians, according to the Diagnostic and Statistical Manual-IV-Text Revision (prior to release of DSM-5). Diagnoses were confirmed by the Autism Diagnostic Interview – Revised (Lord, Rutter, & Couteur, 1994) and Autism Diagnostic Observation Schedule – Generic (Lord et al., 2000). IQ scores were obtained using the Wechsler Adult Intelligence Scale-III (Wechsler, 1997).
Exclusion criteria included history of epilepsy, use of psychoactive drugs within the past 5 weeks, a lifetime history of substance/alcohol dependence, or Axis I mental disorders (except attention-deficit hyperactivity disorder, n = 2). Additional exclusion criteria included history of encephalitis, phenylketonuria, tuberous sclerosis, fragile X syndrome, anoxia during birth, neurofibromatosis, hypomelanosis of Ito, hypothyroidism, Duchenne muscular dystrophy, and maternal rubella. TD participants were excluded based on medical illness or history in first-degree relatives of developmental disorders, learning disabilities, affective disorders, and anxiety disorders. All participants provided written informed consent, approved by the Institutional Review Board of ISMMS.
Cognitive control tasks
Entropy Variation Task (EVT)
The EVT examines the baseline cognitive control performance effect of both entropy (H) and surprise (I) in a single task for sequential information processing. In Shannon's information theory (Shannon & Weaver, 1949), entropy is defined as: , where p(xi) is the probability of event xi. The surprise, I(xi) = −log2p(xi) quantifies the information conveyed by the occurrence of event xi. The base 2 log transformation results in information quantified in units of bits.
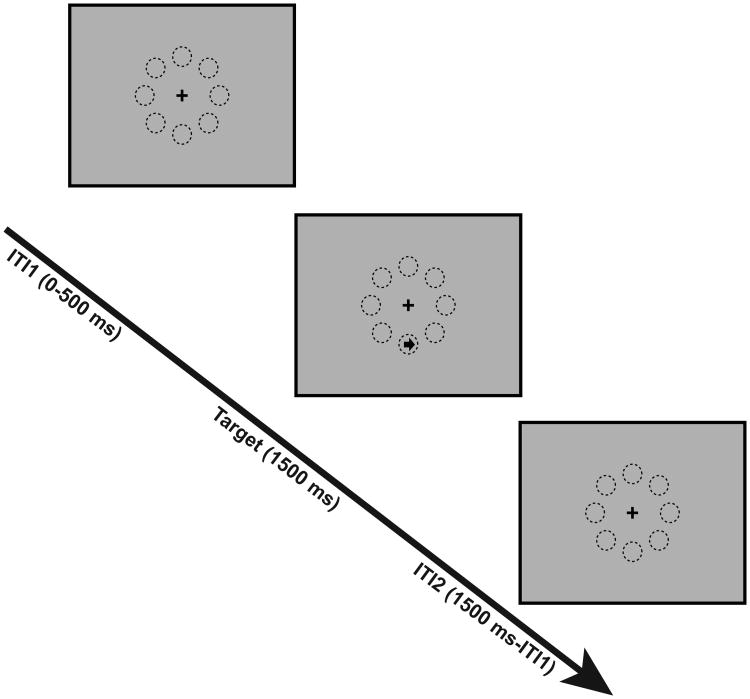
Left- or right-pointing arrows appear randomly at one of eight possible locations arranged around a central fixation cross (Figure 1). Following a 0 to 500 milliseconds (ms) randomly varied fixation interval on each trial, the target arrow appears for 1500 ms, followed by a variable post-target fixation period, with a total trial time of 3000 ms. Participants must indicate the direction of the target arrow.
Figure 1.
Schematic of entropy variation task (EVT). A single arrow appears in one of eight locations arranged around a fixation cross. Participants must respond to the direction of the arrow (left or right).
This is a single-trial and block mixed design. For each block type, entropy has different values, with manipulation of the probability of left-pointing arrows (p), or right-pointing arrows (q), and therefore surprise for each trial type has different values. There are four block types: 1) arrows point in a single direction throughout the entire sequence (H = 0 bits; p/q = 0 or 1; I = 0 bits); 2) alternating sequence of left- and right-pointing arrows, e.g. “LRLRLR…”, and vice versa (H = 0 bits; p = 1; q=1; I = 0 bits); 3) arrows point in one direction more frequently than the other (H = 0.54 bits; p/q = 0.125, I = 3 bits or p/q = 0.825, I = 0.19 bits); and 4) randomly presented left- and right-pointing arrows with equal probability (H = 1 bits; p = q = 0.5; I = 1 bits). Therefore, entropy is manipulated on three sequence levels (0, 0.54, and 1 bit) and surprise on four event type levels (0, 0.19, 1, and 3 bits). There are 8 runs, with 4 blocks each (Latin square counterbalanced), each block has 32 trials, for a total of 1024 trials. Each run lasts approximately 6 minutes, beginning and ending with a 30 s fixation period, with 5 s fixation periods between each block. Total task time is approximately 50 minutes.
Majority Function Task (MFT)
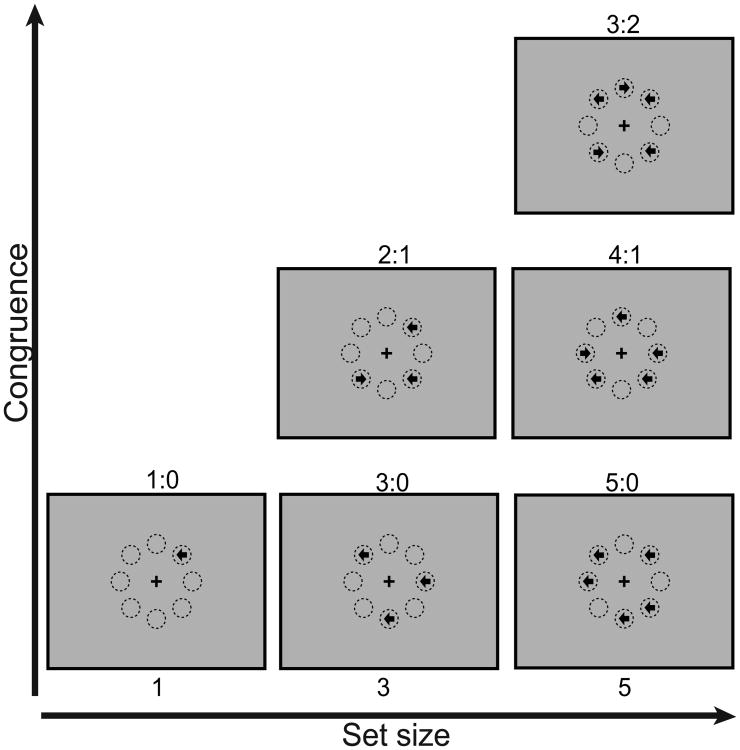
The MFT systematically manipulates uncertainty with computational load (estimated as information entropy) over a wide range to capture the effects of cognitive control for each target event, independent of the sequence (Fan et al., 2014; Fan, Guise, Liu, & Wang, 2008; Wang, Liu, & Fan, 2011). In this task, groups of arrows (set sizes 1, 3 or 5, corresponding to 3 types of blocks) are randomly presented at 8 possible locations arranged around a central fixation cross (Figure 2). The arrows are presented simultaneously, pointing either left or right, and participants must indicate the direction in which the majority of arrows point. There are six conditions, indicating the ratios of arrows pointing in the same direction to arrows pointing in the opposite direction: 1:0 for set size 1; 3:0 and 2:1 for set size 3; and 5:0, 4:1, and 3:2 for set size 5. Trials begin with a variable fixation period of 0 to 1000 ms. Stimuli are then presented for 2500 ms, followed by a variable 1500 to 2500 ms post-stimulus fixation period. Each trial lasts 5 seconds. There are six runs with six blocks each (two for each set size), each block has 12 trials with the same set size, and each run has 72 trials, lasting 395 s. There are 5 s fixation periods at the beginning and end of each run, as well as 10 s between blocks in each run. The order of the blocks is counterbalanced with reversed repetition for each run. Total trial number is 432, with a total time of approximately 40 minutes. Previous algorithmic and computational modeling analyses of MFT performance revealed estimated computational loads for the six conditions are 1.00, 2.00, 3.58, 2.58, 3.91, and 5.91 bits, respectively, including an additive 1 bit for the response (Fan et al., 2008; Wang et al., 2011).
Figure 2.
Schematic of majority function task (MFT). Arrows of set size 1, 3, or 5 are presented randomly in 8 possible locations arranged around a fixation cross. The arrows point either left or right and are presented simultaneously, and participants' task is to indicate the majority direction of the arrows. Computational load is manipulated via set size and the proportion of arrows pointing in either direction. Circles presented in the figure above are to illustrate the possible locations only, and are not presented during the experiment.
Dual Conflict Task (DCT)
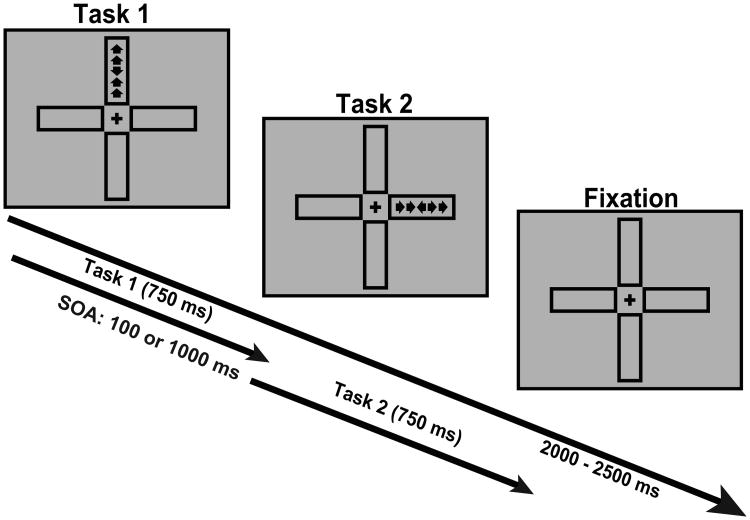
This task examines the impact of the bottleneck of cognitive control capacity by manipulating both conflict processing and time constraints. On each trial, following a 0 to 500 ms randomly varied fixation interval, two tasks (Task 1 and Task 2) are presented sequentially for 750 ms each with a variable Task 1 to Task 2 stimulus onset asynchrony (SOA) of 100 and 1000 ms (Figure 3). The 750 ms task duration is used to avoid the attentional blink effect, which would interfere with detection of the second target if a shorter (e.g., 500 ms) duration were used. For Task 1, the stimulus is presented in one of two locations, aligned vertically, either above or below the central fixation cross, and consists of a central target arrow, flanked by 4 direction-congruent or incongruent arrows (2 on each side), pointing either up or down. For Task 2, the stimulus is presented either to the left or right of the central fixation cross and similarly includes a central target arrow flanked by 4 direction-congruent or incongruent arrows, pointing either left or right. Task 2 is followed by a variable post-target fixation (2000 – 2500 ms), with total trial time of 5000 ms. Participants must make an up/down response to the central arrows for Task 1 using the left hand buttons, and a left/right response for Task 2 using the right hand buttons, sequentially. There are 8 blocks, with 64 trials per block. Each block lasts approximately 6 minutes, and the total task time is approximately 50 minutes.
Figure 3.
Schematic of dual conflict task (DCT). Participants are presented with two flanker tasks in succession, and must indicate the direction of the center arrow and ignore the flanker arrows, which may either be congruent or incongruent with the target. SOA is manipulated such that Task 2 appears either 100 or 1000 ms after Task 1, and under the 100 ms condition, Task 1 and Task 2 often interfere with each other.
Computational loads for Tasks 1 and 2 are approximately 1 bit (which is log22 for 2 possible response directions) respectively under the congruent condition, and greater than 1 but less than 2 under the incongruent condition. The conflict resulting from task-irrelevant flankers can be estimated as less than or equal to a difference of 1 bit between conflict and no-conflict conditions (Fan, 2014; Wang et al., 2011). The two possible locations of the target for each task contribute to a computational load of 1 bit. Therefore for Task 1 and Task 2, the minimum and maximum computational loads are 2 and 3, respectively. In a previous pilot study it was demonstrated that under the 1000 ms SOA the computational load of Task 2 is not significantly affected by Task 1, indicating that the information processing involved in each task does not overlap, resulting in sequential task processing. However, under the 100 ms SOA, the tasks occur in much quicker succession, resulting in task processing overlap, with the computational load during Task 2 processing approaching the sum of the computational load of Tasks 1 and 2, an additive effect based on RT pattern. Therefore, under the 100 ms SOA, the minimum (Task 1 congruent, Task 2 congruent, CC) and maximum (Task 1 incongruent, Task 2 incongruent, II) computational loads for Task 2 are 4 and 6 bits, respectively. Therefore, for the whole task (including both 1000 and 100 ms SOA conditions), the minimum and maximum computation loads are 2 bits for the 1000 SOA CC condition and 6 bits for the 100 SOA II condition, respectively. The estimated loads for Task 2 under different conditions are shown in Table S4.
Procedure
All participants completed: 1) EVT; 2) MFT; and 3) DCT, in the same order. Participants were instructed to respond as quickly and accurately as possible and took self-initiated and self-terminated breaks as needed between runs within each task, as well as between each task, to control for fatigue.
Data Analysis
The independent variable was information (cognitive load) in bits. The primary dependent variable was efficiency (Accuracy/RT, reflecting the probability of a correct response per unit time in seconds), taking both speed and accuracy into account. Average efficiency >1 typically indicates high accuracy and/or RT <1 s. Efficiency <1 typically reflects lower accuracy and/or RT >1s. For group comparisons, a higher efficiency score indicates better performance. Means (±SD) of RT and accuracy were also calculated and analyzed for each task, and are presented in Supplementary Materials (SM). Data were tested for normal distributions and homogeneity of variance (Levene's test). For within-subjects factors where Mauchley's test indicated a violation of the assumption of sphericity, univariate analyses of variance with Greenhouse-Geisser correction are reported.
We estimated the best-fit regression line for all tasks to obtain the slope and intercept of the regression line of performance as a function of cognitive load for each participant, and used independent t-tests to examine group differences in both baseline performance and rates of change in performance. A lower efficiency intercept indicates a lower level of cognitive control efficiency at baseline. With efficiency scores plotted against information entropy in bits, efficiency scores decrease as information increases, resulting in a negative slope. A more negative number is indicative of a faster rate of decline in performance with increasing information. For each task Group by entropy mixed analyses of variance (ANOVA) were conducted. Non-parametric correlation analyses (Kendall's tau) with Bonferroni correction were performed to assess the relationship between efficiency on the three tasks and symptom report on ADI-R and ADOS-G (both non-continuous variables) for the ASD group. Prior to correlation analyses, outliers (+/- 3 SD on efficiency) were excluded, which was limited to 1 case in the EVT analysis. One-tailed statistical tests were utilized to test our directional predictions.
Results
In the interest of readability, only ANOVA results for efficiency are reported below. All RT and accuracy ANOVA results are presented in the SM.
Cognitive control for sequential stimuli: Results of the EVT
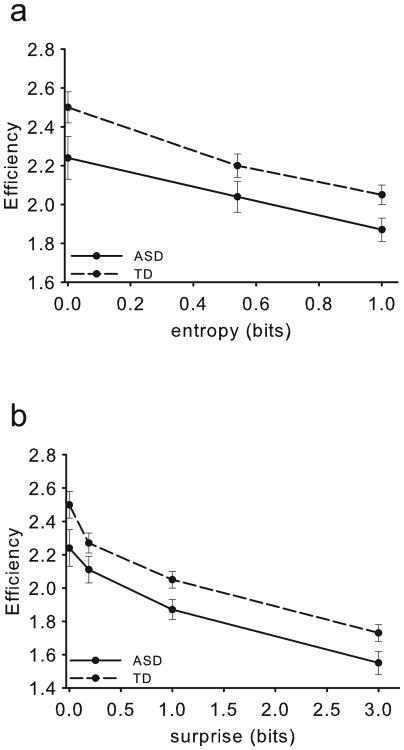
For this analysis, we excluded one TD participant whose overall accuracy on this task was 62%, drastically below the group accuracy mean of 96% (final n = 15, ASD; n = 14 TD). This participant's performance was within normal limits relative to his group on both the MFT and DCT. Efficiency performance on the EVT for both entropy and surprise is presented in Figure 4. RT and accuracy performance are presented in Figure S1 and Table S1 of SM.
Figure 4.
EVT efficiency results, with error bars depicting the standard error of the mean (SEM). The ASD group demonstrated less efficient performance than the TD group for both (a) entropy and (b) surprise. ASD efficiency intercepts were also both significantly lower for entropy and surprise relative to controls.
Cognitive control efficiency for sequential stimuli – entropy
The main effect of Group was significant (F(1,27) = 4.18, p = .05, ηp2= .13), reflecting less efficient performance in the ASD group (M = 1.97±0.28) than the TD group (M = 2.17±0.22). For entropy, sphericity had been violated, χ2(2) = 19.07, p < .001, ε = .66, and with correction the main effect was significant (F(1.32, 35.53) = 81.88, p < .001, ηp2 = .75). Pairwise comparisons (Bonferroni-corrected) revealed significant decreases in efficiency for each entropy value point, all ps < .001). There was no significant Group by entropy interaction (F < 1). Intercept was significantly lower for the ASD group (M = 2.24±0.41, R2= .92) than for the TD group (M = 2.48±0.29, R2= .95), t(27) = -1.85, p < .05. There was no significant difference in slope between groups (t(27) = 0.981, p = .34).
Cognitive control efficiency for sequential stimuli – surprise
The main effect of Group was significant (F(1,27) = 5.32, p < .05, ηp2 = .16), with less efficient performance in the ASD group (M = 1.94±0.41) than the TD group (M = 2.14±0.37). For surprise, sphericity was violated, χ2(5) = 71.80, p < .001, ε = .41, and with correction the main effect was significant (F(1.23, 33.19) = 81.06, p < .001, ηp2 = .75). Pairwise comparisons with Bonferroni corrections revealed significant decreases in efficiency for each surprise value point (all ps < .001). The Group by surprise interaction for efficiency was not significant (F < 1). Intercept was significantly lower for the ASD group (M = 2.17±0.36, R2= .89) than for the TD group (M = 2.38±0.26, R2= .87), t(27) = -1.77, p < .05. There were no significant group differences in slope (t(27) = 0.26, p = .80).
Cognitive control for non-sequential stimuli: Results of the MFT
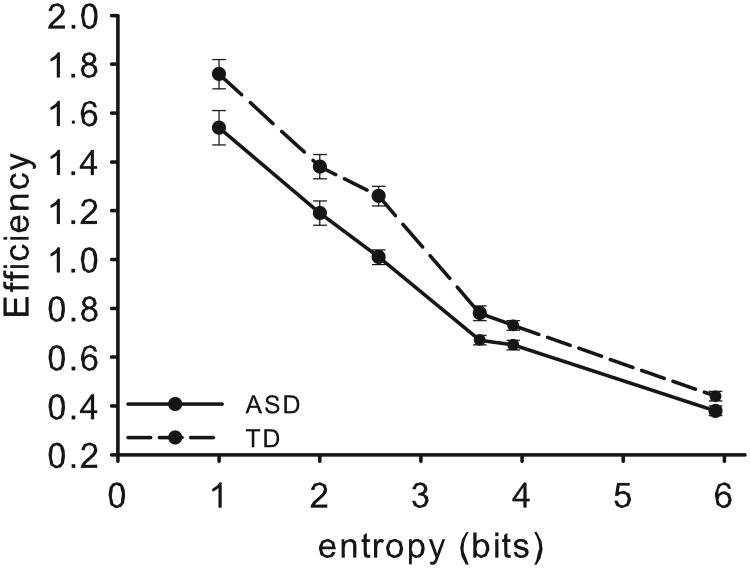
Performance efficiency is presented Figure 5. RT and accuracy performance are presented in Figure S2 and Table S2 of SM. The main effect of Group was significant (F(1,28) = 12.70, p < 0.001, ηp2 = .31), such that the ASD group (M = 0.91±0.12) demonstrated decreased overall performance efficiency compared to the TD group (M = 1.06±0.11). Sphericity was violated for entropy, χ2(14) = 103.46, p < 0.001, ε = .41, and with correction, the main effect was significant, F(2.1, 57.5) = 509.25, p < 0.001, ηp2 = .95. The Group by entropy interaction was significant, F(2.1,57.5) = 3.68, p < 0.05, ηp2 = .12. Pairwise comparisons (Bonferroni-corrected) indicated the TD group had significantly higher performance on each MFT condition compared to the ASD group. For the six conditions of 1:0, 3:0, 2:1, 5:0, 4:1, and 3:2, ts(28) = 2.38, 2.73, 4.61, 3.18, 2.97, and 2.17, respectively, all ps < .05. We also found a significant difference in slope between the two groups t(28) = 2.37, p < .05, such that TD group (M = -0.28±0.04, R2 = .92) had a higher rate of decreasing performance efficiency than ASD group (M = -0.24±0.05, R2 = .91). The ASD group had a significantly lower intercept (M = 1.42±0.20) than the TD group (M = 1.66±0.20), t(28) = -3.18, p < .01.
Figure 5.
MFT efficiency results as a function of computational load (quantified as entropy) in bits. Error bars represent SEM. Overall, the ASD group was significantly less efficient across the range of values of entropy, had a lower efficiency intercept, and showed a slower slope decrease relative to controls.
Cognitive control capacity under time constraints: Results of the DCT
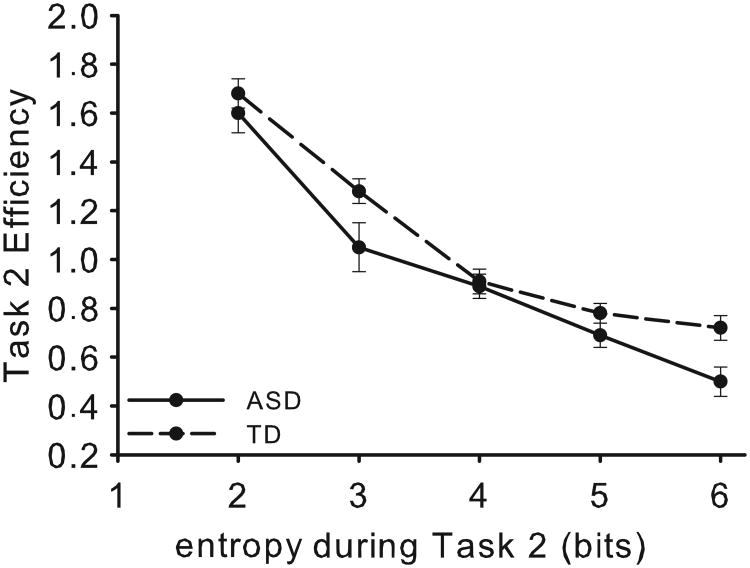
DCT performance efficiency as a function of computational load of Task 2 is shown in Figure 6. Behavioral performance for all conditions of the DCT are presented in Table S3 and Figure S3 of SM. Additional analyses are also presented in SM. The main effect of Group was not significant (F(1,28) = 2.72, p = .11, ηp2= .09). Sphericity was violated for entropy χ2(9) = 44.65, p < .001, ε = .67, and with correction there was a significant main effect (F(2.70,75.52) = 376.16, p < .001, ηp2= .93). Pairwise comparisons (Bonferroni-corrected) indicated significant decreases in efficiency for each increasing value of entropy, all ps > .001. The interaction between entropy and Group was significant (F(2.70,75.52) = 4.78, p < .01, ηp2= .15), with greater efficiency decrease in the ASD group compared to the TD group as a function of cognitive load. There were no significant group differences for intercept (t(28) = -0.64, p = 0.26) or slope (t(28) = -1.08, p = 0.14). The groups did not differ significantly in performance at baseline (Task 1, CC 1000 ms SOA, 2 bits) (F < 1), but the ASD group performed significantly less efficiently than the TD group at high cognitive load(Task 2, II 100 ms SOA, 6 bits), F(1,28) = 7.48, p < .05.
Figure 6.
DCT efficiency results as a function of computational load of Task 2. Error bars represent SEM. Baseline performance was not significantly different between groups, but the ASD group performance decreased to a greater extent than the TD with increasing computational load.
Relationship between task performance and symptom domains
Correlation coefficients are presented in Table 2. Because the value for efficiency slope is negative, a negative correlation suggests that a more negative (steeper) efficiency slope, or faster rate of decrease in efficiency as a function of cognitive load (entropy), was associated with greater ASD symptom report. Due in part to small sample size and restricted variance of symptom scores, none of these correlations survived false discovery rate correction.
Table 2.
Kendall's tau correlation coefficients for relationships between behavioral efficiency and ADI-R and ADOS-G subscales.
| Efficiency | Social | ADI-R | Behavior/Interest | Communication | ADOS-G | Behavior/Interest |
|---|---|---|---|---|---|---|
|
|
|
|||||
| Communication | Social | |||||
| EVT1 | ||||||
| entropy slope | -.27 | -.41* | .15 | -.21 | -.44* | .15 |
| entropy intercept | -.12 | .16 | -.17 | .23 | .00 | -.37* |
| surprise slope | -.10 | -.33* | -.03 | -.14 | -.27 | .12 |
| surprise intercept | -.29 | .06 | -.09 | .14 | -.12 | -.42* |
| Overall | -.36* | -.07 | -.12 | .35 | -.18 | -.24 |
| MFT | ||||||
| Slope | .53** | .20 | .07 | .11 | .12 | .57** |
| Intercept | -.53** | -.06 | -.19 | -.04 | -.17 | -.49* |
| Overall | -.43* | -.06 | -.26 | .04 | -.10 | -.30 |
| DCT2 | ||||||
| Slope | .32* | .32* | .08 | .23 | .01 | .11 |
| Intercept | -.31 | -.31 | -.19 | -.09 | -.07 | -.12 |
| Overall | -.23 | -.22 | -.15 | .11 | -.02 | -.12 |
p < .05,
p < .01 (one-tailed)
Note:
n = 14, except for overall efficiency analysis;
n = 14 for ADOS-G. None of the correlations survived false discovery rate correction.
The correlations between performance in efficiency on the three tasks can be found in Table S5 of SM.
Discussion
In this study we tested our hypothesis that individuals with ASD implement cognitive control less efficiently than TD adults with a lower capacity for cognitive control, and that these deficits possibly contribute to the clinical presentation of the disorder. On tasks designed to systematically manipulate uncertainty and test cognitive control efficiency, the ASD group showed a lower baseline efficiency of information processing for sequential events, a general reduction in efficiency for non-sequential events over a wide range of uncertainty, and a larger decline in performance when capacity limits were pushed by time constraints.
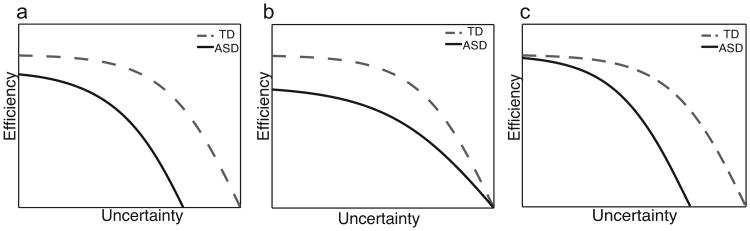
We aimed to test three hypothetical efficiency performance models (Figure 7): (a) The ASD group has a lower baseline than controls, both groups decrease in efficiency as uncertainty increases, and the ASD group has a lower upper limit than TD (Figure 7a, Model A); (b) The ASD group has a lower baseline than controls, the performance difference remains somewhat constant across increasing uncertainty values, with performance finally converging with TD at the highest levels of uncertainty (Figure 7b, Model B); and (c) The ASD group and controls have similar baseline performance and then diverge as uncertainty increases, with ASD efficiency beginning to decrease at a lower uncertainty level compared to TD (Figure 7c, Model C).
Figure 7.
Hypothetical efficiency performance models for the ASD and TD groups. The EVT results fit best with Model A (a), MFT results fit best with Model B (b), and DCT results fit best with Model C (c).
The EVT results fit best with Model A, with relatively lower efficiency in the ASD group across the full range of uncertainty values when the task involved sequential information. The MFT results fit Model B, for non-sequential information processing, performance was less efficient for the ASD group at low uncertainty levels, converging toward a similar level as TD at high uncertainty levels. At first glance, the EVT and MFT results might appear to be at odds with existing information processing theories that predict equivalent group performance at low load levels (e.g., (Minshew & Goldstein, 1998; Minshew et al., 2001). However, we speculate that differences at baseline may be explained by additional uncertainty due to spatial location (1/8 possible locations for ∼3 bits) in these tasks. This possibility is highlighted in the DCT results, which best fit Model C, and show that the ASD group performed less efficiently than controls at the high capacity condition rather than at the low capacity condition. In the EVT and MFT, participants are required to shift attention from fixation to one of eight possible target locations, whereas in the DCT, target stimuli appear in fewer possible locations (4 possible locations for Tasks 1 and 2 combined). It is possible then, that the additional attentional orienting requirement contributed to group differences at baseline for the EVT and MFT, but not for the DCT. These results are consistent with theories that propose that deficits in ASD arise at more demanding levels of information processing, and provide further support for the idea of a greater limitation on information processing capacity in ASD compared to TD (Belmonte et al., 2004; Just et al., 2012). Taken together, it appears that Model C is the most plausible, and can best explain performance in these experiments.
The observed group differences in cognitive control under uncertainty suggest that deficits in control of information processing may contribute to ASD symptoms, as social and communicative processing involves dealing with uncertainty at various levels, such as decoding a linguistic message or inferring the mental states/intentions of others. According to uncertainty reduction theory (based upon information theory (Berger & Calabrese, 1975)), people undertake several steps to reduce uncertainty in social situations. In ASD, lower efficiency in sequential and non-sequential information processing in concert with a reduced upper limit of information processing capacity, may negatively impact the ability to effectively engage in this uncertainty reduction, resulting in social and communication deficits. Furthermore, deficits in the domain of restricted interests and repetitive behaviors may serve to compensate for a diminished uncertainty-reduction capacity. This is supported by strong correlation with a large effect size between MFT performance and the restricted interests repetitive behaviors domain in this study (though this did not survive false discovery rate multiple comparison correction). By restricting interests to predictable sequences and familiar domains, individuals with ASD are able to avoid cognitive control overload.
Cognitive control of information processing is supported by the frontoparietal network, with the anterior cingulate (ACC) and anterior insular (AIC) cortices as core regions, in addition to other regions such as the frontal eye fields and near/along the intraparietal sulcus (Fan, 2014; Fan et al., 2014). Reduced cognitive control efficiency in ASD may be related to a deficiency in this network. We have previously shown that there is a lack of activation of the ACC in ASD during conflict processing (Fan et al., 2012). ACC is involved in baseline state uncertainty monitoring, and abnormal recruitment of this crucial network hub could underlie the inefficient performance demonstrated in our experiments. In addition to region-specific neural differences, there is also evidence for differences in connectivity within the frontoparietal network in ASD, resulting in inefficient information transfer between frontal and parietal areas (Matthew K. Belmonte et al., 2004; Just et al., 2004; Just et al., 2012; Kana, Keller, Cherkassky, Minshew, & Just, 2006), contributing to symptom presentation (Uddin et al., 2014).
Limitations and alternative explanations
A primary limitation in this study is the relatively small size of the sample. Further, given that participants in the ASD group were relatively high-functioning and all male, there are limits to the generalizability of these findings. However, one might expect that lower-functioning individuals would show even greater relative impairment on the tasks described in this study. ASD is often comorbid with other conditions, and two of our participants were previously diagnosed with ADHD. We do not believe that this significantly affected the results due to the small proportion of participants involved. Ideally, these findings should be replicated in a larger sample, free of comorbidity, to address these limitations.
One could argue that the differences we observed in performance might be attributed to motor slowing previously documented in ASD (Kenworthy, Yerys, Weinblatt, Abrams, & Wallace, 2013; Williams, Goldstein, & Minshew, 2013). While we did not directly examine motor speed, it is notable that there were no significant group differences in overall RT for the DCT; EVT overall RT difference trended toward significance; and MFT overall RT was significantly faster for the ASD group. This unclear pattern of RT differences makes it difficult to draw conclusions about generalized slowing in the ASD group. Previous work has suggested that slowness to respond in ASD may result from use of psychoactive medications (Bogte et al., 2008). However, our sample was unmedicated at the time of participation in the study. Additionally, we presented a large number of trials across tasks, which could potentially result in differential fatigue effects between groups. However, failure to find group differences in RT on the third task, the DCT, rules out this possibility.
Conclusion
This study represents a preliminary step in the investigation of cognitive control in ASD and the relationship between cognitive control deficits and the symptom presentation of ASD. We found participants with ASD had a generally lower efficiency of cognitive control, with a reduced capacity under time constraints, compared to controls. Of three possible explanations of group differences in performance, the model in which the ASD group is less efficient than controls as cognitive control load increases best fit the present data. While this study has targeted the processing of stimuli not typically encountered in daily life, it would be beneficial for future studies to investigate the role of cognitive control deficits as they arise in more ecologically valid tasks that require higher-level cognition, such as language and theory of mind, which map more representatively onto the established symptom domains.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Numbers of R01 MH094305 and R21 MH083164. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would like to thank the Seaver Autism Center for participant recruitment and evaluation, including Latha Soorya, Ph.D., Evdokia Anagnostou, M.D., Alexander Kolevzon, M.D., David Grodberg, M.D., and Ting Wang, Ph.D. We would also like to thank Yunsoo Park for help with data collection, and Alfredo Spagna Ph.D. and Laura Egan Ph.D. for edits.
Grant sponsor: National Institutes of Health
Grant number: R01 MH094305 and R21 MH083164
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- American Psychiatric Association, A.P. Diagnostic and statistical manual of mental disorders. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Badre D. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends in Cognitive Sciences. 2008;12(5):193–200. doi: 10.1016/j.tics.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Barbalat G, Leboyer M, Zalla T. A specific impairment in cognitive control in individuals with high-functioning autism. Journal of Psychiatric Research. 2014;58:26–35. doi: 10.1016/j.jpsychires.2014.07.013. [DOI] [PubMed] [Google Scholar]
- Barnard-Brak L. The difference between autism and ADHD is in the eye of the cognitive task? Personality and Individual Differences. 2011;50(8):1305–1308. doi: 10.1016/j.paid.2011.02.007. [DOI] [Google Scholar]
- Baron-Cohen S, Ashwin E, Ashwin C, Tavassoli T, Chakrabarti B. Talent in autism: hyper-systemizing, hyper-attention to detail and sensory hypersensitivity. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364(1522):1377–1383. doi: 10.1098/rstb.2008.0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper Ra, Webb SJ. Autism and abnormal development of brain connectivity. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24(42):9228–9231. doi: 10.1523/jneurosci.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte MK, Cook EH, Anderson GM, Rubenstein JLR, Greenough WT, Beckel-Mitchener a, Tierney E, et al. Autism as a disorder of neural information processing: directions for research and targets for therapy. Molecular psychiatry. 2004;9(7):646–663. doi: 10.1038/sj.mp.4001499. [DOI] [PubMed] [Google Scholar]
- Berger CR, Calabrese RJ. Some explorations in initial interaction and beyond: Toward a developmental theory of interpersonal communication. Human communication research. 1975;1(2):99–112. [Google Scholar]
- Bishop DVM. Annotation: Autism, Executive Functions and Theory of Mind: A Neuropsychological Perspective. Journal of Child Psychology and Psychiatry. 1993;34(3):279–293. doi: 10.1111/j.1469-7610.1993.tb00992.x. [DOI] [PubMed] [Google Scholar]
- Bogte H, Flamma B, van der Meere J, van England H. Cognitive flexibility in adults with high functioning autism. Journal of Clinical and Experimental Neuropsychology. 2008;30(1):33–41. doi: 10.1080/13803390601186668. [DOI] [PubMed] [Google Scholar]
- Burden MJ, Andrew C, Saint-Amour D, Meintjes EM, Molteno CD, Hoyme HE, Jacobson SW, et al. The effects of fetal alcohol syndrome on response execution and inhibition: An event-related potential study. Alcoholism: Clinical and Experimental Research. 2009;33(11):1994–2004. doi: 10.1111/j.1530-0277.2009.01038.x. [DOI] [PubMed] [Google Scholar]
- Cashin A, Gallagher H, Newman C, Hughes M. Autism and the cognitive processing triad: a case for revising the criteria in the diagnostic and statistical manual. Journal of child and adolescent psychiatric nursing: official publication of the Association of Child and Adolescent Psychiatric Nurses, Inc. 2012;25(3):141–148. doi: 10.1111/j.1744-6171.2012.00337.x. [DOI] [PubMed] [Google Scholar]
- Cañadas E, Rodríguez-Bailón R, Milliken B, Lupiáñez J. Social categories as a context for the allocation of attentional control. Journal of experimental psychology General. 2013;142(3):934–943. doi: 10.1037/a0029794. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Maurer RG. A neurological model for childhood autism. Archives of Neurology. 1978;35(12):777–786. doi: 10.1001/archneur.1978.00500360001001. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Belger A. Atypical modulation of cognitive control by arousal in autism. Psychiatry Research: Neuroimaging. 2008;164(3):185–197. doi: 10.1016/j.pscychresns.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Tottenham NT, Thomas KM, Davidson MC, Eigsti IM, Yang Y, Casey BJ, et al. Differential patterns of striatal activation in young children with and without ADHD. Biological Psychiatry. 2003;53(10):871–878. doi: 10.1016/S0006-3223(02)01904-2. [DOI] [PubMed] [Google Scholar]
- Fan J. An information theory account of cognitive control. Frontiers in Human Neuroscience. 2014;8:680. doi: 10.3389/fnhum.2014.00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Bernardi S, Dam NT, Anagnostou E, Gu X, Martin L, Hof PR, et al. Functional deficits of the attentional networks in autism. Brain and behavior. 2012;2(5):647–660. doi: 10.1002/brb3.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Dam NTV, Gu X, Liu X, Wang H, Tang CY, Hof PR. Quantitative characterization of functional anatomical contributions to cognitive control under uncertainty. Journal of cognitive neuroscience. 2014 doi: 10.1162/jocn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Guise KG, Liu X, Wang H. Searching for the Majority: Algorithms of Voluntary Control. PLoS One. 2008;3(10) doi: 10.1371/journal.pone.0003522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Villamisar D, Sala SD. Dual-task performance in adults with autism. Cognitive Neuropsychiatry. 2002;7(1):63–74. doi: 10.1080/13546800143000140. [DOI] [PubMed] [Google Scholar]
- Geurts HM, Corbett B, Solomon M. The paradox of cognitive flexibility in autism. Trends in Cognitive Sciences. 2009;13(2):74–82. doi: 10.1016/j.tics.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomot M, Wicker B. A challenging, unpredictable world for people with Autism Spectrum Disorder. International Journal of Psychophysiology. 2012;83(2):240–247. doi: 10.1016/j.ijpsycho.2011.09.017. [DOI] [PubMed] [Google Scholar]
- Happé F, Frith U. The weak coherence account: detail-focused cognitive style in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2006;36(1):5–25. doi: 10.1007/s10803-005-0039-0. [DOI] [PubMed] [Google Scholar]
- Hughes C, Russell J, Robbins TW. Evidence for executive dysfunction in autism. Neuropsychologia. 1994;32(4):477–492. doi: 10.1016/0028-3932(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Hus V, Lord C. The Autism Diagnostic Observation Schedule, Module 4: Revised Algorithm and Standardized Severity Scores. Journal of Autism and Developmental Disorders. 2014;44(8):1996–2012. doi: 10.1007/s10803-014-2080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutt SJ, Hutt C, Lee D, Ounsted C. A behavioural and electroencephalographic study of autistic children. Journal of Psychiatric Research. 1965;3(3):181–197. doi: 10.1016/0022-3956(65)90028-2. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller Ta, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain: a journal of neurology. 2004;127(Pt 8):1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Just MA, Keller TA, Malave VL, Kana RK, Varma S. Autism as a neural systems disorder: A theory of frontal-posterior underconnectivity. Neuroscience & Biobehavioral Reviews. 2012;36(4):1292–1313. doi: 10.1016/j.neubiorev.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller Ta, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain: a journal of neurology. 2006;129(Pt 9):2484–2493. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenworthy L, Case L, Harms MB, Martin A, Wallace GL. Adaptive behavior ratings correlate with symptomatology and IQ among individuals with high-functioning autism spectrum disorders. Journal of Autism and Developmental Disorders. 2010;40(4):416–423. doi: 10.1007/s10803-009-0911-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenworthy L, Yerys BE, Weinblatt R, Abrams DN, Wallace GL. Motor Demands Impact Speed of Information Processing in Autism Spectrum Disorders. Neuropsychology. 2013 doi: 10.1037/a0033599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouneiher F, Charron S, Koechlin E. Motivation and cognitive control in the human prefrontal cortex. Nature Neuroscience. 2009;12(7):939–947. doi: 10.1038/nn.2321. [DOI] [PubMed] [Google Scholar]
- Lopez BR, Lincoln AJ, Ozonoff S, Lai Z. Examining the Relationship between Executive Functions and Restricted, Repetitive Symptoms of Autistic Disorder. Journal of Autism and Developmental Disorders. 2005;35(4):445–460. doi: 10.1007/s10803-005-5035-x. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook E, Jr, Leventhal B, DiLavore P, Rutter M, et al. The Autism Diagnostic Observation Schedule—Generic: A Standard Measure of Social and Communication Deficits Associated with the Spectrum of Autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. doi: 10.1023/a:1005592401947. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/bf02172145. [DOI] [PubMed] [Google Scholar]
- Mackie MA, Van Dam NT, Fan J. Cognitive control and attentional functions. Brain and Cognition. 2013;82(3):301–312. doi: 10.1016/j.bandc.2013.05.004. doi: http://dx.doi.org/10.1016/j.bandc.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Rinaldi T, Markram K. The intense world syndrome--an alternative hypothesis for autism. Frontiers in neuroscience. 2007;1(1):77–96. doi: 10.3389/neuro.01.1.1.006.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E, Cohen J. An integrative theory of prefrontal cortex function. Annual Review Of Neuroscience. 2001;2001(24):167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Goldstein G. Autism as a disorder of complex information processing. Mental Retardation and Developmental Disabilities Research Reviews. 1998;4:129–136. [Google Scholar]
- Minshew NJ, Goldstein G, Siegel DONJ. Neuropsychologic functioning in autism: profile of a complex information processing disorder. Journal of the International Neuropsychological Society. 1997;3(04):303–316. doi:null. [PubMed] [Google Scholar]
- Minshew NJ, Johnson C, Luna B. The Cognitive and Neural Basis of Autism. A Disorder of Complex Information Processing and Dysfunction of Neocortical Systems. 2001;23 [Google Scholar]
- Mushtaq F, Bland AR, Schaefer A. Uncertainty and Cognitive Control. Frontiers in Psychology. 2011;2 doi: 10.3389/fpsyg.2011.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor K, Kirk I. Brief report: atypical social cognition and social behaviours in autism spectrum disorder: a different way of processing rather than an impairment. Journal of Autism and Developmental Disorders. 2008;38(10):1989–1997. doi: 10.1007/s10803-008-0559-5. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Pennington BF, Rogers SJ. Executive function deficits in high-functioning autistic individuals: relationship to theory of mind. Journal Of Child Psychology And Psychiatry, And Allied Disciplines. 1991;32(7):1081–1105. doi: 10.1111/j.1469-7610.1991.tb00351.x. [DOI] [PubMed] [Google Scholar]
- Poljac E, Bekkering H. A review of intentional and cognitive control in autism. Frontiers in Psychology. 2012;3 doi: 10.3389/fpsyg.2012.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Snyder CRR. Attention and cognitive control. In: Solso RL, editor. Information processing and cognition: The Loyola symposium. Lawrence Erlbaum; 1975. [Google Scholar]
- Rowe J, Lavender A, Turk V. Cognitive executive function in Down's syndrome. British Journal of Clinical Psychology. 2006;45(1):5–17. doi: 10.1348/014466505X29594. [DOI] [PubMed] [Google Scholar]
- Russo N, Flanagan T, Iarocci G, Berringer D, Zelazo PD, Burack JA. Deconstructing executive deficits among persons with autism: Implications for cognitive neuroscience. Brain and Cognition. 2007;65(1):77–86. doi: 10.1016/j.bandc.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Shannon CE, Weaver W. The Mathematical Theory of Communication. Urbana: University of Illinois Press; 1949. [Google Scholar]
- Shapiro HM, Wong LM, Simon TJ. A cross-sectional analysis of the development of response inhibition in children with Chromosome 22q11.2 Deletion Syndrome. Frontiers in Psychiatry. 2013;4 doi: 10.3389/fpsyt.2013.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M, Ozonoff SJ, Cummings N, Carter CS. Cognitive control in autism spectrum disorders. International Journal of Developmental Neuroscience. 2008;26(2):239–247. doi: 10.1016/j.ijdevneu.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M, Yoon JH, Ragland JD, Niendam Ta, Lesh Ta, Fairbrother W, Carter CS. The Development of the Neural Substrates of Cognitive Control in Adolescents with Autism Spectrum Disorders. Biological Psychiatry. 2013:1–10. doi: 10.1016/j.biopsych.2013.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M. Autism as an executive disorder. New York, NY, US: Oxford University Press; 1997. Towards an executive dysfunction account of repetitive behaviour in autism; pp. 57–100. [Google Scholar]
- Turner M. Generating novel ideas: fluency performance in high-functioning and learning disabled individuals with autism. Journal of child psychology and psychiatry, and allied disciplines. 1999;40(2):189–201. [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Lynch CJ, Cheng KM, Odriozola P, Barth ME, Menon V, et al. Brain State Differentiation and Behavioral Inflexibility in Autism. Cerebral Cortex. 2014 doi: 10.1093/cercor/bhu161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya CJ, Bunge SA, Dudukovic NM, Zalecki CA, Elliott GR, Gabrieli JDE. Altered neural substrates of cognitive control in childhood ADHD: Evidence from functional magnetic resonance imaging. American Journal of Psychiatry. 2005;162(9):1605–1613. doi: 10.1176/appi.ajp.162.9.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valla JM, Belmonte MK. Detail-oriented cognitive style and social communicative deficits, within and beyond the autism spectrum: Independent traits that grow into developmental interdependence. Developmental Review. 2013 doi: 10.1016/j.dr.2013.08.004. [DOI] [Google Scholar]
- van Meel CS, Heslenfeld DJ, Oosterlaan J, Sergeant JA. Adaptive control deficits in attention-deficit/hyperactivity disorder (ADHD): The role of error processing. Psychiatry Research. 2007;151(3):211–220. doi: 10.1016/j.psychres.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Wang H, Liu X, Fan J. Cognitive control in majority search: a computational modeling approach. Frontiers in Human Neuroscience. 2011;5(16) doi: 10.3389/fnhum.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale - Third Edition. 3. San Antonio, TX: Harcourt Assessment; 1997. [Google Scholar]
- Williams DL, Goldstein G, Minshew N. The modality shift experiment in adults and children with high functioning autism. Journal of autism and developmental disorders. 2013;43(4):794–806. doi: 10.1007/s10803-012-1618-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, Goldstein G, Minshew NJ. Neuropsychologic functioning in children with autism: further evidence for disordered complex information-processing. Child neuropsychology: a journal on normal and abnormal development in childhood and adolescence. 2006;12(4-5):279–298. doi: 10.1080/09297040600681190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerys BE, Wallace GL, Harrison B, Celano MJ, Giedd JN, E KL. Set-shifting in children with autism spectrum disorders: reversal shifting deficits on the Intradimensional/Extradimensional Shift Test correlate with repetitive behaviors. Autism. 2009;13:523–538. doi: 10.1177/1362361309335716. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.