Abstract
Increasing evidences suggest that PI3K/AKT pathway plays an important role in the pathogenesis of inflammatory diseases such as acute pancreatitis. However, the exact effect of PI3K/AKT on thyroid injury associated with acute pancreatitis has not been investigated. This study aimed to investigate the protective effects of wortmannin, PI3K/AKT inhibitor, on thyroid injury in a rat model of severe acute pancreatitis (SAP). Sixty male SD rats were randomly divided into four groups: sham operating group (SO), SAP group, wortmannin treatment (WOR) group and drug control (WOR-CON) group. Serum amylase (AMY), lipase (LIP) and thyroid hormone levels were evaluated. The morphological change of thyroid tissue was analyzed under the light and transmission electron microscopy. AKT, P38MAPK and NF-κB expression in the thyroid tissue was evaluated by immunohistochemical staining. Oxidative stress and inflammatory cytokines were detected. Results showed that wortmannin attenuated the following: (1) serum AMY, LIP and thyroid hormone (2) pancreatic and thyroid pathological injuries (3) thyroid MDA, (4) thyroid ultrastructural change, (5) serum TNF-α, IL-6 and IL-1β (6) AKT, MAPKP38 and NF-κB expression in thyroid tissues. These results suggested that wortmannin attenuates thyroid injury in SAP rats, presumably because of its role on prevent ROS generation and inhibits the activation of P38MAPK, NF-κB pathway. Our findings provide new therapeutic targets for thyroid injury associated with SAP.
Keywords: Severe acute pancreatitis, thyroid injury, PI3K/AKT pathway, wortmannin, P38MAPK, NF-κB
Introduction
Thyroid dysfunction is not uncommon in critically ill patients with no previous intrinsic thyroid disease. Severe non-thyroidal illness such as severe trauma, septic shock, severe acute pancreatitis (SAP) is often associated with hypothyroidism [1]. The presence of non-thyroidal illness syndrome is associated with increased mortality among critically ill patients [2,3]. Hypothyroidism during pancreatitis was first reported in the 1980s [4]. Recent study demonstrated that low serum T4 or low T3 levels seem to be a poor prognostic indicator in hospitalized SAP patients [5].
Many methods of preventing and ameliorating thyroid damage in critically ill have been attempted, including thyroid hormone replacement therapy, metabolic support and placebo management [6]. Only a few studies have examined the use of supplemental thyroid hormone therapy in critically ill general medical patients. Brent and Hershman [7] examined the effect of thyroid hormone therapy in medical intensive care unit patients. Administration of T4 during illness did not prevent hypothyroidism and the T4 replacement was detrimental to the restoration of normal pituitary-thyroid regulation. Another study demonstrated that in organ donors, exogenous thyroid hormones can stabilizes the function of the cardiovascular system. There have been other trials in patients who suffered acute renal failure or underwent renal transplantation that also failed to show any benefit [8]. However, there is no study been reported about the treatment of thyroid injury associated with SAP. No known drug has been shown to prevent SAP associated thyroid damage. Thus, further studies should be required for finding a new drug.
The pathogenesis of SAP is still poorly understood and has not been completely elucidated. The initial enzyme activation in combination with oxygen-free radicals injures the acinar cell and causes a release of cytokines and vasoactive mediators, attracts inflammatory cells and vascular endothelium as well as the expression of adhesion molecules [9]. Increasing evidence has demonstrated that the production and release of inflammatory mediators and other bioactive substances are may play significant role in systemic manifestations of SAP and its associated remote organ dysfunction [10]. Therefore, reducing the pro inflammatory mediators to block the cascade effect of inflammation may be an important part in the treatment strategy of pancreatitis associated thyroid injury.
Genetic or pharmacological blockade of the PI3K/Akt pathway in experimental animals can protect normal tissues against inflammation damage [11]. PI3K is a family of enzymes involved in cellular functions such as cell growth, proliferation, differentiation, and motility [12]. All PI3Ks are inhibited by the drugs wortmannin and LY294002. Wortmannin is a commonly used cell biology reagent and has been previously used to inhibit DNA repair, receptor-mediated endocytosis and cell proliferation [13]. Wortmannin has been demonstrated to be effective in decreased serum cytokine levels and increased the survival of mice subjected to sepsis [14]. Wortmannin, also been shown to markedly reduced the subcellular redistribution of cathepsin B in the acute pancreatitis (AP) rat model [15]. The previous paper also found that doses of wortmannin markedly reduced trypsinogen activation in vitro and in vivo during the early stages of secretagogue-induced pancreatitis [13]. Furthermore, wortmannin treatment decreases inflammatory cytokines in SAP [16].
However, whether PI3K/Akt inhibition is effective in preventing thyroid injury in SAP has not yet been elucidated. We hypothesized that PI3K/Akt is involved in the pathogenesis of thyroid injury associated with SAP. The present study aimed to investigate the protective effect of PI3K/Akt inhibitor on thyroid injury associated with SAP in rats.
Materials and methods
Animals and reagents
Male Sprague-Dawley rats, weighing 200 to 250 g, were obtained from the Center of Experimental Animals of Hubei Academy of Medical Sciences, Wuhan, China. The animals were kept at room temperature and 12 h light-dark cycles, and with free access to water. Rats in this study were maintained in accordance with the principles of the 1983 Declaration of Helsinki by the Ethics Committee of Wuhan University. Wortmannin was obtained from Selleck company (Houston, USA). Sodium taurocholate and dimethyl sulfoxide (DMSO) were purchased from Sigma Aldrich Company (St. Louis, MO). Antibodies purchased from Abcam company (Cambridge, UK).
Induction of severe acute pancreatitis
Rats were fasted overnight and given fresh tap water ad libitum. Anesthesia was administered by intraperitoneal injection of 10% chloraldurat (3 mL/kg). The pancreatic bile duct was cannulated through the duodenum. SAP was induced by a standardized retrograde infusion of a freshly prepared 5% sodium taurocholate solution (1 mg/kg) into the biliary-pancreatic duct. Isotonic saline solution (20 mL/kg) was injected into the back to compensate for fluid loss.
Experimental design
Sixty male rats randomly assigned to four groups (n=15 in each group): sham operating group (SO), severe acute pancreatitis (SAP) group, wortmannin treatment (WOR) group and drug control (WOR-CON) group. In the SAP group, rats received vehicle (10% DMSO, 2 mL/kg) via femoral vein half an hour prior to 5% sodium taurocholate infusion. In the SO group, rats only received the vehicle. In the WOR group, rats received 2 mg/kg wortmannin dissolved in the vehicle (10% DMSO v/v). In the WOR-CON group, rats received wortmannin and saline.
All rats were sacrificed 12 h after SAP induction. Blood was collected via heart puncture. Blood samples were centrifuging, and serum was stored at -20°C. The head of the pancreatic tissue and thyroid tissue were harvested and fixed in 4% PBS-buffered formaldehyde for histopathology and immunohistochemistry. The remaining part of the pancreatic and thyroid tissues were immediately snap frozen in liquid nitrogen and stored at -80°C for assay.
Biochemical assay
Serum amylase (AMY), lipase (LIP) was measured by an automatic biochemistry analyzer with standard techniques (Olympus Optical Ltd., Japan). Serum concentration of the thyroid hormones (T3, T4, FT3 and FT4) was determined by radioimmunoassays (Beijing North Institute of Biological Technology, China). Serum levels of TNF-α, IL-1β and IL-6 were quantified using specific ELISA kits according to the manufacturer’s instructions (Biosource International, Nivelles, Belgium). Serum MDA and superoxide dismutase (SOD) level, a marker of lipid peroxidation, was measured using a commercial MDA, SOD assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Histopathological evaluation
Tissue samples were fixed in 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS), embedded in paraffin, cut into 5-mm sections and stained with hematoxylin and eosin (H&E). All tissue sections were examined microscopically for histopathological changes by an experienced histologist blinded to the study protocol. Histopathological changes of pancreas were evaluated according to the criteria proposed by Schmidt et al [17]. Thyroid gland damage was graded from 1 to 5 according to the following criteria: follicular size, colloid density, height of the follicular epithelium, mesenchymal fibrosis and interstitial vascular proliferation [18].
Immunohistochemistry
After being deparaffinized and pretreated in citrate buffer, sections were incubated with normal goat serum for 15 min at room temperature. Sections were applied with rabbit anti-(AKT/P-AKT, P38/p-P38 and NF-κB) polyclonal antibody (Santa Cruz) and negative control sections with normal rabbit serum and blank control sections with PBS overnight at 4°C. The secondary antibody, biotinylated anti-rabbit immunoglobulin, was applied for 15 min at room temperature and sections were rinsed in PBS. Peroxidase conjugated streptavidin was applied for 15 min, followed by diaminobenzidine (DAB) substrate for 10 min and hematoxylin for 5 min. Finally, sections were rinsed with water, dehydrated, cleared and mounted with permanent mounting medium. Immunohistochemical micrographs were taken with the FSX-100 microscope camera system.
IHC staining was analyzed using Image Pro-Plus (version 6.0; Media Cybernetics, Silver Spring). Briefly, the positive staining area was selected as the area of interest (AOI). The area sum and integrated optical density (IOD) of the AOI were selected as the measurement parameters. The target protein expression was analyzed by comparing the IOD (AKT and P38) and ratio of positive nuclear expression (NF-κB) in different groups. Finally, statistical analysis of the mean expression index for each duplicate was performed.
Transmission electron microscopy (TEM)
Fresh Thyroid specimens were fixed in a mixture of 2% formaldehyde and 2% glutaraldehyde in 0.1 mol/L cacodylate buffer (pH 7.4) overnight, postfixed in 2% osmium tetroxide in the same buffer. Ultrathin sections were cut on Leica EMUC7 ultramicrotome, stained with lead citrate, and changes of the thyroid follicle epithelial cells were examined in a HT7700 transmission electron microscope.
Statistical analysis
Statistical analyses were carried out using SPSS statistical software (PASW Statistics for Windows, version 18.0). Data are expressed as mean ± SD. One-way analysis of variance (ANOVA) was used to investigate Differences among the experimental groups. P<0.05 is considered statistically significant.
Results
Effects of PI3K/AKT inhibition on pancreatic injury and thyroid dysfunction in SAP
Analysis of serum AMY and LIP
As shown in Figure 1, significant hyperamylasemia and hyperlipasemia developed after induction of SAP, it proved that the SAP model was successfully induced. Wortmannin reduced serum AMY and LIP levels in WOR group compared with the SAP group (P<0.05). In WOR-CON group, all serum enzyme levels were not significant compared with the SO group.
Figure 1.
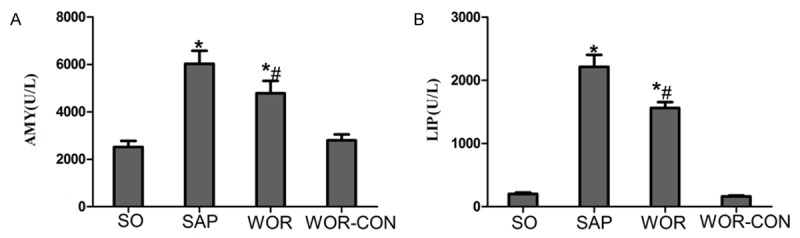
Serum levels of AMY, LIP in all groups of rats. A. Serum AMY level; B. Serum LIP level; *P<0.05 versus SO group. #P<0.05 versus SAP group.
Analysis of serum thyroid hormone
Rats subjected to SAP were associated with a significant decrease in T3, T4, FT3 and FT4 levels (P<0.05), indicating that rats were experiencing aggravated thyroid dysfunction. Wortmannin induced significant increase on the level of serum thyroid hormone 12 h after SAP (P<0.05) (Figure 2).
Figure 2.
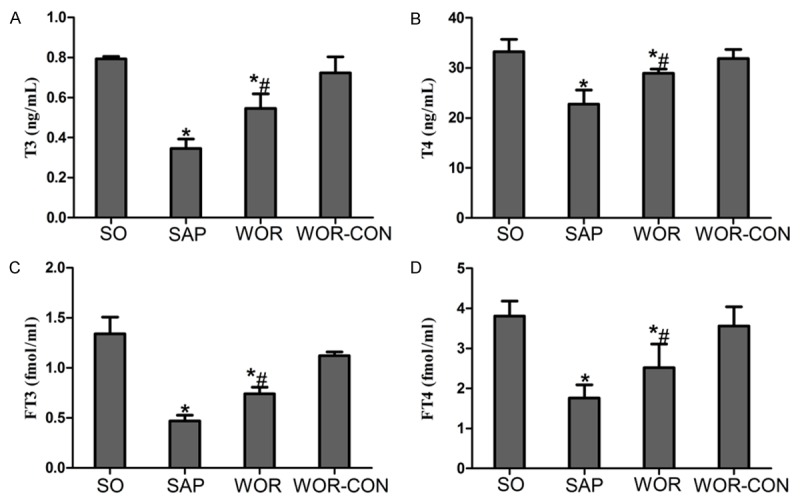
A. Serum level of T3; B. T4; C. FT3; D. FT4. *P<0.05 versus SO group. #P<0.05 versus SAP group.
Inhibition of PI3K/AKT attenuated pancreatic and thyroid histopathology
Representative pathological changes in pancreatic tissue were shown in Figure 3A-D. No morphological changes were found in rats from SO and WOR-CON groups. Interstitial edema, necrosis of acinar cells and infiltration of inflammatory cells were observed in SAP group (Figure 3B). However, treatment with wortmannin markedly decreased pancreatic histological injuries (P<0.05) (Figure 3C and 3I).
Figure 3.
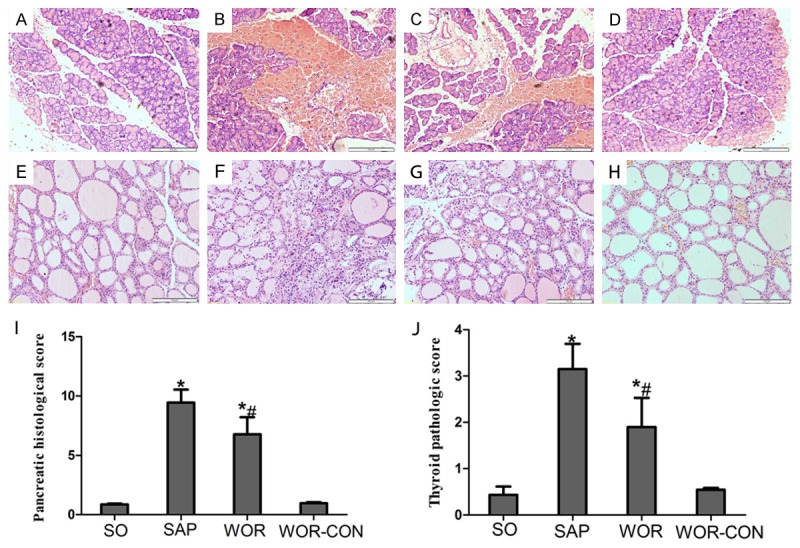
Pancreatic (A-D) and thyroid (E-H) morphologic changes. A. A representative pancreatic figure from SO group; B. A representative pancreatic figure from SAP group; C. A representative pancreatic figure from WOR group; D. A representative pancreatic figure from WOR-CON group; E. A representative thyroid figure from SO group; F. A representative thyroid figure from SAP group; G. A representative thyroid figure from WOR group; H. A representative thyroid figure from WOR-CON group; I. Histological scores of pancreatic tissue; J. Histological scores of thyroid tissue. *P<0.05 versus SO group; #P<0.05 versus SAP group (original magnification ×200).
Pathological changes in thyroid tissue were shown in Figure 3E-H. SO and WOR-CON group represents normal histological structure of thyroid tissue. In contrast, characteristic signs of thyroid injuries including hyperplasia and expansion of the follicles, shedding of epithelial cells, deficient luminal colloid, collapsed follicles, mesenchymal fibrosis, interstitial vascular proliferation, fibrinoid necrosis or even disappearance of the follicular structure were observed in thyroid sections in SAP group (Figure 3F). However, the thyroid pathological grade reduced to a much lower level by pretreatment with wortmannin in the WOR group (P<0.05) (Figure 3J).
Wortmannin treatment reduced oxidative stress and production of proinflammatory cytokines
Thyroid tissue MDA level in SAP group was elevated significantly than SO and WOR-CON group (P<0.05) (Figure 4). The elevation appeared to be significantly inhibited by wortmannin treatment (P<0.05) (Figure 4). Moreover, similar changes were observed for SOD activity in thyroid tissues. This was found to be significantly depleted in SAP rats, probably as a result of oxidative stress processes. In contrast, treatment with wortmannin effectively improved the activity of SOD in thyroid tissue (P<0.05) (Figure 4).
Figure 4.
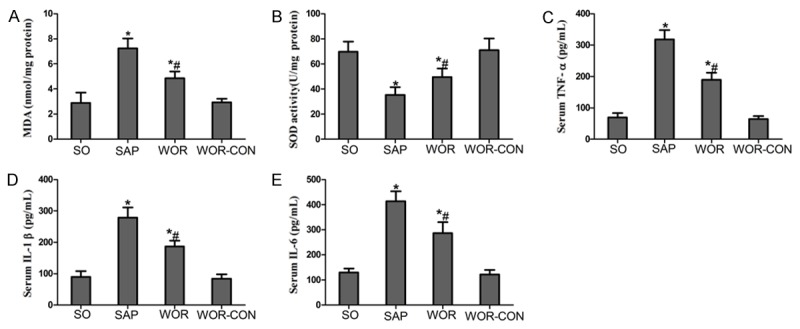
Effects of wortmannin on oxidative stress and pro inflammatory cytokines production; A. Content of MDA; B. SOD activity; C. TNF-α; D. IL-1β; E. IL-6. *P<0.05 versus SO group; #P<0.05 versus SAP group.
Serum concentrations of pro inflammatory cytokines were analyzed to evaluate the effect of PI3K/AKT inhibition on the inflammatory process after SAP. As illustrated in Figure 4, concomitant with the pathological damage incited by the SAP, serum levels of TNF-α, IL-6, and IL-1β were increased significantly than SO and APO-CON group (P<0.05). However, the levels of these cytokines after SAP were markedly decreased by the pharmacological blockade of PI3K/AKT inhibitor wortmannin (P<0.05) (Figure 4).
Inhibition of PI3K/AKT reduced expression of P38MAPK and NF-κB In thyroid tissue after SAP
Effect of wortmannin on AKT/p-AKT expression in thyroid tissue
Immunohistochemical staining of AKT (Figure 5A-D) and phosphorylated AKT (p-AKT) (Figure 5E-H) was used to evaluate whether wortmannin successfully attenuates SAP induced AKT expression in the thyroid tissue. Thyroid sections from the SO and WOR-CON rats showed little expression of AKT and p-AKT (Figure 5A, 5D, 5E and 5H). AKT and p-AKT immunoreactivity was enhanced in SAP rats (Figure 5B and 5F) and Expression of AKT and p-AKT was reduced (Figure 5C and 5G) in the thyroid tissue that had undergone wortmannin pretreatment. Quantitative analysis revealed that, IOD value of both AKT and p-AKT increased significantly in SAP group (P<0.05). IOD value of AKT and p-AKT decreased after wortmannin treatment (P<0.05) (Figure 8A and 8B).
Figure 5.
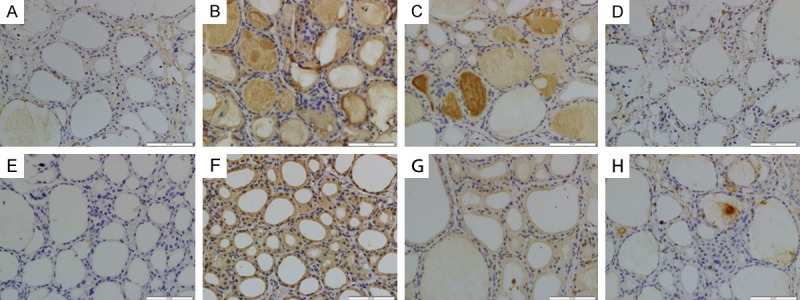
AKT (A-D) and p-AKT (E-H) immunohistochemical staining of cross sections of rat thyroid tissue. The immunohistochemical localization of AKT/ p-AKT appears as brown staining. A. AKT expression in SO group; B. AKT expression in SAP group; C. AKT expression in WOR group; D. AKT expression in WOR-CON group; E. p-AKT expression in SO group; F. p-AKT expression in SAP group; G. p-AKT expression in WOR group; H. p-AKT expression in WOR-CON group. (original magnification ×400).
Figure 8.
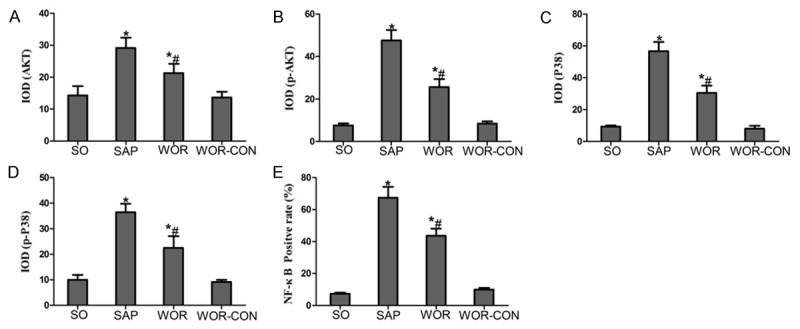
Immunohistochemical analysis. A. AKT; B. p-AKT; C. P38; D. p-P38; E. NF-κB *P<0.05 versus SO group; #P<0.05 versus SAP group.
Effect of wortmannin on P38/p-P38 expression in thyroid tissue
We determined the efficacy of wortmannin on P38MAPK (Figure 6A-D) and phosphorylated P38MAPK (p-P38) (Figure 6E-H) expression in the thyroid tissue by using Immunohistochemical assay. In SO group, the expressions P38 and p-P38 were exceedingly weak in cytoplasm (Figure 6A and 6E). Thyroid expression of P38 in SAP group was significantly increased in the cytoplasm than in the SO group (Figure 6B). In SAP group, p-P38 expression increased in cytoplasm and partly in nucleus (Figure 6F). In addition, inhibition of PI3K/AKT with wortmannin significantly decreased the cytoplasmic expression of P38 and p-P38 in the thyroid tissue (Figure 6C and 6G). Quantitative analysis revealed that, IOD value of both P38 and p-P38 increased significantly in SAP group (P<0.05). IOD value of both P38 and p-P38 decreased after wortmannin treatment (P<0.05) (Figure 8C and 8D).
Figure 6.
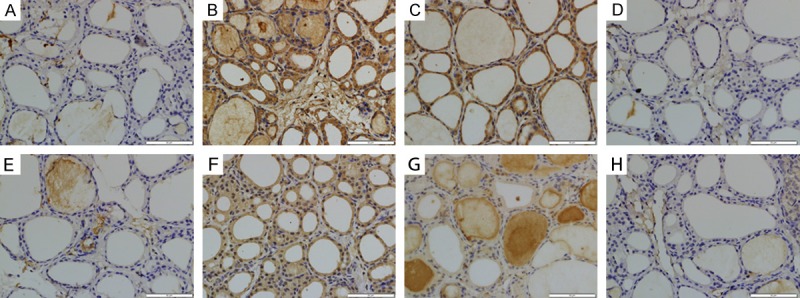
P38 (A-D) and p-P38 (E-H) immunohistochemical staining of cross sections of rat thyroid tissue. The immunohistochemical localization of P38/p-P38 appears as brown staining. (A) P38 expression in SO group; (B) P38 expression in SAP group; (C) P38 expression in WOR group; (D) P38 expression in WOR-CON group; (E) p-P38 expression in SO group; (F) p-P38 expression in SAP group; (G) p-P38 expression in WOR group; (H) p-P38 expression in WOR-CON group. (original magnification ×400).
Effect of wortmannin on NF-κB expression in thyroid tissue
In performing the localization of NF-κB expression, immunohistochemical assay was used. The expressions of NF-κB was concentrated mainly in the cytoplasms in the SO group (Figure 7A). Following SAP, NF-κB immunoreactivity highly expresssed in nucleus (Figure 7B). However, a marked decrease in NF-κB staining was found in the nucleus with the wortmannin pretreatment (Figure 7C), but increase in the cytoplasm. Moreover, WOR-CON group showed weak immunoreactivity (Figure 7D). Quantitative analysis revealed that, ratio of NF-κB positive nucleus in thyroid section from SAP group was increased significantly than in SO group (P<0.05). However, ratio of NF-κB positive nucleus decreased after wortmannin treatment (P<0.05) (Figure 8E).
Figure 7.
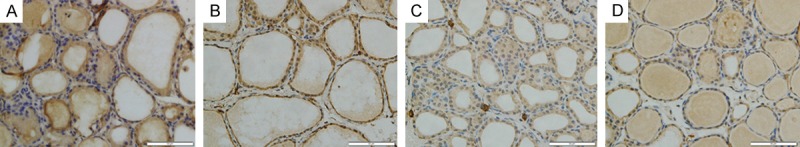
Immunohistochemical staining for NF-κB in thyroid tissue. A. In SO group, immunoreactivity of NF-κB was mainly shown in cytoplasts; B. In SAP group, intense immunoreactivity of NF-κB was shown in nucleus; C. Lower immunoreactivity of NF-κB was shown in nucleus; D. WOR-CON group. (original magnification ×400).
Ultra-structural changes of thyroid follicular cells under transmission electron microscopy (TEM)
TEM analysis of thyroid tissue demonstrated that follicular epithelial cells in SO group was showed an abundance of neatly arranged microvilli on the apical membrane. The morphology of mitochondria, rough endoplasmic reticulum (RER), ribosomes and other cellular organelles were normal and the apical cell areas exhibited numerous secretory vesicles and colloid droplets (Figure 9A). In SAP rats, the follicular cells had many vacuoles and few microvilli, the perinuclear gap was wider than that in follicular cells from SO rats, and secretory vesicles were sparse at the apical pole. Swollen mitochondria and dilated RER cisternae were present in the basal pole (Figure 9B). There was a general loss of subcellular organization and of cellular contents (Figure 9C). Most of the follicular cells had corrugated heterochromatic nuclei and some of them were surrounded by fragmented dilated rough endoplasmic reticulum (Figure 9C). Electron microscope examination of the section from WOR group was revealed that many follicular cells had euchromatic nuclei with peripheral rim of heterochromatin. Their cytoplasm contained moderately dilated cisternae of RER, Golgi saccules and apical electron dense granules. These suggested that ultrastructural damage of follicular cells was ameliorated by wortmannin treatment (Figure 9D).
Figure 9.
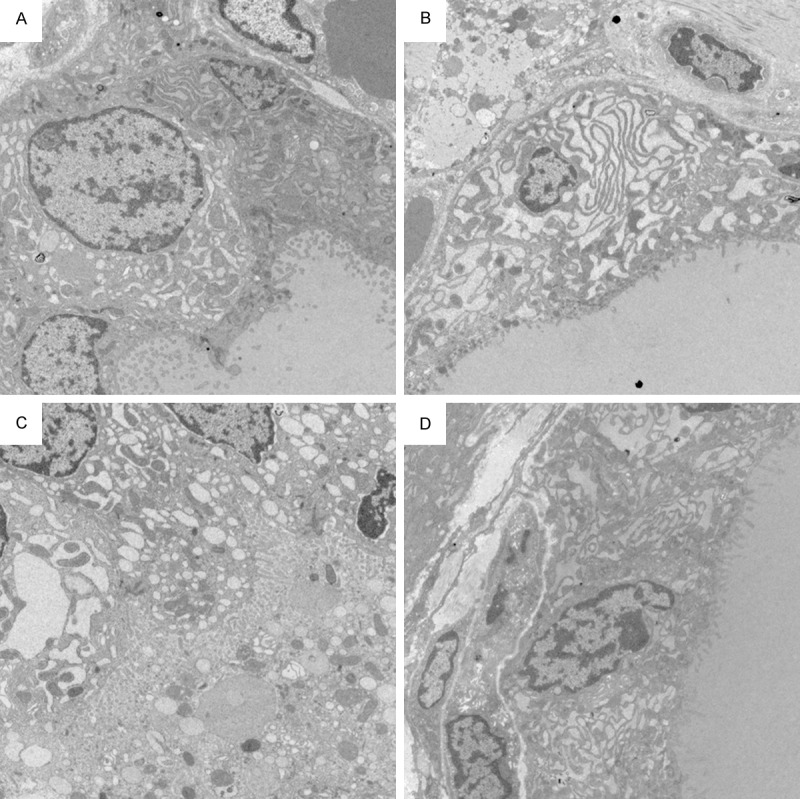
TEM comparison of thyroid follicular cells architecture. A. An electron micrograph from the SO group showing that the follicular cells have basal euchromatic nuclei with thin peripheral rim of heterochromatin. Follicular cells contain abundant microvilli, well developed parallel cisternae of rough endoplasmic reticulum, mitochondria and large moderate electron dense cytoplasmic colloid droplets. Small electron dense secretory granules are observed in their apical cytoplasm. Apical lateral surfaces of follicular cells show tight junctions. (magnification, ×5,000); B. An electron micrograph from the SAP group showed that the follicular cells contained many vacuoles and few microvilli. Some follicular cells have euchromatic nuclei with peripheral rim of heterochromatin and markedly dilated cisternae of rough endoplasmic reticulum (magnification, ×5,000); C. In SAP group, corrugated heterochromatic follicular cells nuclei and desquamated follicular cells within follicular lumen are noticed. The general loss of subcellular organization and cellular contents are also founded (magnification, ×5,000); D. An electron micrograph from the WOR group showing that the follicular cells have euchromatic nuclei with peripheral rim of heterochromatin. Their cytoplasm contains moderately dilated cisternae of rough endoplasmic reticulum, Golgi saccules and apical electron dense granules. (magnification, ×5,000).
Discussion
The PI3K/Akt pathway plays a critical role in the balance between cell survival and apoptosis and the inflammatory response by activating chemokine receptors and promoting leukocyte migration [19,20]. This pathway also has been extensively investigated as potential therapeutic targets in autoimmune, allergic, and many inflammatory diseases [21-23]. The involvement of PI3K/Akt in the pathogenesis of acute pancreatitis was first demonstrated in a study by Singh et al [24] using two unrelated inhibitors of all PI3K isoforms, wortmannin and LY294002, in two different rodent models of acute pancreatitis, one induced by supra-maximal secretagogue stimulation and the other by duct injection. Wortmannin reduced the extent of pancreatic edema, neutrophil sequestration within the pancreas, acinar cell necrosis, and hyperamylasemia in the first model. Wortmannin also reduced pancreatic trypsin activity, acinar cell necrosis and myeloperoxidase activity in the second acute pancreatitis model, which had been induced by retrograde infusion of sodium taurocholate. Other studies by different research groups have further analyzed the specific role of the PI3Kγ isoform in the pathogenesis of acute pancreatitis. Gukovsky et al [25] used PI3Kγ-deficient mice as well as pharmacologic PI3K inhibitors to investigate the role of PI3K in CCK-induced responses in isolated pancreatic acinar cells. Further studies by the same group demonstrated that PI3Kγ regulates calcium signaling in pancreatic acinar cells by inhibiting sarco (endo) plasmic reticulum calcium-ATPase [26,27]. Another study suggested that PI3K/Akt inhibitor wortmannin decreases inflammatory cytokines in SAP rats and suggests its regulatory mechanisms may occur through the suppression on NF-κB and p38MAPK activity [28]. However, to our knowledge, the role of the PI3K/Akt pathway in thyroid dysfunction or SAP-associated thyroid damage has not been examined previously.
The exact mechanism of thyroid dysfunction in critical ill patient such as septic shock and SAP was not been elucidated. The majority of critically ill patients have low serum T3 concentrations, as do some outpatients during illness. Liver and skeletal muscle biopsies obtained within minutes after death from intensive care unit patients demonstrate reduced 5’-monodeiodinase activity and increased 5’-monodeiodinase activity (which converts T4 to rT3) [29]. Moreover, patients with fatal illness have low tissue T4 and T3 concentrations [30]. Several mechanisms can contribute to the inhibition of 5’-monodeiodination and therefore to the low serum T3 concentrations in critically ill patients with non-thyroidal illness [6]: (1) Exogenous glucocorticoid therapy; (2) Circulating inhibitors of deiodinase activity, such as free (non-esterified) fatty acids; (3) Treatment with drugs that inhibit 5’-monodeio-dinase activity, such as amiodarone and high doses of propranolol; (4) Cytokines (such as tumor necrosis factor, interferon-alpha, NF-κB and interleukin-6).
In our study, we found that SAP successfully developed in the rats after 12 h of modeling, characterized by increased serum level of AMY and LIP, pancreatic tissue injury and pathologic changes. The levels of serum T3 and T4 combined with aggravating morphological changes of the thyroid showed obvious thyroid dysfunction and tissue injuries during the progression of SAP. Our data demonstrates that pretreatment of wortmannin attenuated the following: (1) serum AMY and LIP level; (2) reduction of serum thyroid hormone level; (3) morphological change of pancreas and thyroid; (4) pro-inflammatory cytokine production; (5) thyroid MDA (6) expression of AKT, NF-κB and MAPK P38 (7) ultrastructural change of thyroid follicular cells. All of these observations suggesting that PI3K/Akt pathway may participate the process of thyroid damage in SAP and treatment with PI3K inhibition exerts potent anti-inflammatory and anti-oxidative stress effects and ameliorates the degree of SAP and associated thyroid injury in rats.
Previous studies have demonstrated that oxidative stress and its resultant production of ROS play prominent roles in the mechanism of inflammatory responses during AP [31]. In addition, Increased ROS activity has been identified in disturbance of thyroid hormone (TH) homeostasis induced by PCBs and DDT in rats [32]. Oxidative stress results from a disturbance of the normal cell balance between production of reactive oxygen species (ROS) and the capacity to neutralize their actions. When ROS generation exceeds the antioxidant capacity of cells, oxidative stress develops. GSH-Px and SOD as the ROS scavengers are depleted, while MDA as a product of lipid peroxidation is accumulated. In the process of SAP, ROS were overproduced and then induced an imbalance between the ROS and endogenous antioxidants or antioxidant enzymes. The overproduced ROS was able to directly or indirectly damage the thyroid tissues and result in thyroid dysfunction and histological changes. In our current study, we found that oxidative stress occurred after induction of SAP, followed by significant reduction of SOD and accumulation of MDA. The activity of SOD was significantly increased when using the wortmannin treatment in SAP rat. Oppositely, the level of MDA was decreased. These results combined with the levels of TH and morphologic changes of thyroid indicated that wortmannin reduces the level of ROS by inhibiting the PI3K/Akt further ameliorate thyroidal oxidative damage and thyroid dysfunction.
Evidence suggests that inflammatory cell infiltration such as TNF-α, IL-1β and IL-6 is an early and vital event in AP, which will lead to local and systemic complications [33]. Moreover, PI3K/Akt inhibitor wortmannin decreases inflammatory cytokines in SAP rats [28]. Another study shows that, cytokines may be possible mechanism which can cause thyroid dysfunction in critically ill patient [6]. Data from the present study demonstrated increase of these cytokines after SAP. However, serum levels of these cytokines were reduced in rats treated with wortmannin. In the SAP animals, thyroid dysfunction correlated with the increased thyroid injury confirmed by using histological analysis. In the treatment group, we observed milder swelling, necrosis and blurry boundary of epithelial cells, and less inflammatory cell infiltration than those of the SAP rats. Pathological grading of thyroid injury was significantly decreased after pancreatitis in the presence of wortmannin therapy.
There is much evidence confirming that the development and progression of SAP depend on NF-kB transcription, which plays an important role in the regulation of inflammatory mediators. Recent studies have shown that PI3K/Akt is an important mediator for regulating NF-κB-dependent gene expression in various cell types. Furthermore, evidence suggests that PI3K/Akt inhibitor wortmannin decreases inflammatory cytokines in SAP rats and suggests its regulatory mechanisms may occur through the suppression on NF-κB and p38MAPK activity [28]. In addition, PI3K/Akt pathway could regulate NF-κB nuclear activity by affecting its interaction with the cAMP response element-binding protein and CREB-binding protein [34,35]. In this study, we analyzed the expression of NF-κB in SAP rats after administering wortmannin. The result of immunohistochemisty assay indicated that the NF-κB has been shown to translocate from the cytoplasm into the nucleus upon activation in thyroid tissues during SAP. Nuclear expression of NF-κB significantly reduced after wortmannin treatment.
It has been well established that during the progress of AP, the P38MAPK signaling pathway is involved in the regulation of NF-κB activation that plays a crucial role in the inflammatory cascade [36,37]. A recent study showed that the activation of P38MAPK results in phosphorylation of MK2, a downstream protein kinase of P38MAPK, triggers the NF-κB and exaggerates inflammation [38]. Other studies support our observation that P38MAPK, NF-κB and JNK pathway play a crucial role on disturbance of thyroid hormone homeostasis, and activation of these pathways was possible mechanism of thyroid hormone reduction induced by PCBs and DDT [32,39]. In this study, the expression of p-Akt/Akt, p-p38MAPK/p38MAPK increased in SAP rats compared with the SO group. Furthermore, after treatment with wortmannin in SAP rats, p-Akt/Akt, p-p38MAPK/p38MAPK decreased and paralleled the level of pathological injury to the thyroid.
Based on the above data, we found that inhibition of the PI3K/Akt pathway dramatically downregulated the intracellular signaling pathway of the transcription factor NF-κB, p38MAPK and cytokines in SAP rats. The serum assay and the changes in histopathological examination indicated that wortmannin attenuated the severity of SAP induced thyroid injury.
In summary, this work suggests that wortmannin attenuates the severity of SAP induced thyroid injury, through diminish in systemic concentration of cytokines, reduce oxidative stress and deregulate NF-κB and P38MAPK expression. However, its complete molecular mechanism still remains unclear. These works can serve as a basis for further studies on the therapeutic potential of wortmannin in thyroid injury in SAP patients.
Acknowledgements
This study was supported by Natural Science Foundation of Hubei Province (No. 2013CFB458) NSFC (NO. 81500488) and NSFC (NO. 81370562). This research performed in Key Laboratory of Hubei Province for Digestive System Disease.
Disclosure of conflict of interest
None.
References
- 1.Utiger RD. Altered thyroid function in nonthyroidal illness and surgery. To treat or not to treat? N Engl J Med. 1995;333:1562–1563. doi: 10.1056/NEJM199512073332310. [DOI] [PubMed] [Google Scholar]
- 2.Slag MF, Morley JE, Elson MK, Crowson TW, Nuttall FQ, Shafer RB. Hypothyroxinemia in critically ill patients as a predictor of high mortality. JAMA. 1981;245:43–45. [PubMed] [Google Scholar]
- 3.Maldonado LS, Murata GH, Hershman JM, Braunstein GD. Do thyroid function tests independently predict survival in the critically ill? Thyroid. 1992;2:119–123. doi: 10.1089/thy.1992.2.119. [DOI] [PubMed] [Google Scholar]
- 4.Gidaiatov AA, Abasov IT. Concentration of thyroid hormones and thyrotropin in the blood of patients with chronic pancreatitis. Ter Arkh. 1988;60:50–52. [PubMed] [Google Scholar]
- 5.De Sola C, Redondo M, Pallares F, Redondo E, Hortas ML, Morell M. Thyroid function in acute pancreatitis. Rev Esp Enferm Dig. 1998;90:15–22. [PubMed] [Google Scholar]
- 6.Economidou F, Douka E, Tzanela M, Nanas S, Kotanidou A. Thyroid function during critical illness. Hormones (Athens) 2011;10:117–124. doi: 10.14310/horm.2002.1301. [DOI] [PubMed] [Google Scholar]
- 7.Brent GA, Hershman JM. Thyroxine therapy in patients with severe nonthyroidal illnesses and low serum thyroxine concentration. J Clin Endocrinol Metab. 1986;63:1–8. doi: 10.1210/jcem-63-1-1. [DOI] [PubMed] [Google Scholar]
- 8.Acker CG, Flick R, Shapiro R, Scantlebury VP, Jordan ML, Vivas C, Greenberg A, Johnson JP. Thyroid hormone in the treatment of posttransplant acute tubular necrosis (ATN) Am J Transplant. 2002;2:57–61. doi: 10.1034/j.1600-6143.2002.020110.x. [DOI] [PubMed] [Google Scholar]
- 9.Klar E, Werner J. [New pathophysiologic knowledge about acute pancreatitis] Chirurg. 2000;71:253–264. doi: 10.1007/s001040050043. [DOI] [PubMed] [Google Scholar]
- 10.Bhatia M, Neoptolemos JP, Slavin J. Inflammatory mediators as therapeutic targets in acute pancreatitis. Curr Opin Investig Drugs. 2001;2:496–501. [PubMed] [Google Scholar]
- 11.Cantley LC. The Phosphoinositide 3-Kinase Pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 12.Yousif MH. Phosphoinositide 3-kinase contributes to diabetes-induced abnormal vascular reactivity in rat perfused mesenteric bed. Cell Biochem Funct. 2008;26:451–458. doi: 10.1002/cbf.1463. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Shreder KR, Gai W, Corral S, Ferris DK, Rosenblum JS. Wortmannin, a widely used phosphoinositide 3-kinase inhibitor, also potently inhibits mammalian polo-like kinase. Chem Biol. 2005;12:99–107. doi: 10.1016/j.chembiol.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Ryffel B, Yadav UCS, Naura AS, Aguilera-Aguirre L, Boldogh I, Boulares HA, Calhoun WJ, Ramana KV, Srivastava SK. Aldose Reductase Inhibition Prevents Allergic Airway Remodeling through PI3K/AKT/GSK3β Pathway in Mice. PLoS One. 2013;8:e57442. doi: 10.1371/journal.pone.0057442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SH, Jang YW, Hwang P, Kim HJ, Han GY, Kim CW. The reno-protective effect of a phosphoinositide 3-kinase inhibitor wortmannin on streptozotocin-induced proteinuric renal disease rats. Exp Mol Med. 2012;44:45. doi: 10.3858/emm.2012.44.1.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogers Lynette K, Xu P, Wang J, Yang ZW, Lou Xl, Chen C. Regulatory Roles of the PI3K/Akt Signaling Pathway in Rats with Severe Acute Pancreatitis. PLoS One. 2013;8:e81767. doi: 10.1371/journal.pone.0081767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt J, Rattner DW, Lewandrowski K, Compton CC, Mandavilli U, Knoefel WT, Warshaw AL. A better model of acute pancreatitis for evaluating therapy. Ann Surg. 1992;215:44–56. doi: 10.1097/00000658-199201000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Santen HM, Van Dijk JE, Rodermond H, Vansenne F, Meertens N, Haveman J, Endert E, De Vijlder JJ, Vulsma T. The effect of cervical X-irradiation on activity index of thyrocytes and plasma TSH: a pre-clinical model for radiation-induced thyroid damage. J Endocrinol Invest. 2005;28:261–269. doi: 10.1007/BF03345383. [DOI] [PubMed] [Google Scholar]
- 19.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 20.Procko E, McColl SR. Leukocytes on the move with phosphoinositide 3-kinase and its downstream effectors. Bioessays. 2005;27:153–163. doi: 10.1002/bies.20157. [DOI] [PubMed] [Google Scholar]
- 21.Foster JG, Blunt MD, Carter E, Ward SG. Inhibition of PI3K signaling spurs new therapeutic opportunities in inflammatory/autoimmune diseases and hematological malignancies. Pharmacol Rev. 2012;64:1027–1054. doi: 10.1124/pr.110.004051. [DOI] [PubMed] [Google Scholar]
- 22.Fougerat A, Gayral S, Malet N, Briand-Mesange F, Breton-Douillon M, Laffargue M. Phosphoinositide 3-kinases and their role in inflammation: potential clinical targets in atherosclerosis? Clin Sci (Lond) 2009;116:791–804. doi: 10.1042/CS20080549. [DOI] [PubMed] [Google Scholar]
- 23.Ghigo A, Damilano F, Braccini L, Hirsch E. PI3K inhibition in inflammation: Toward tailored therapies for specific diseases. Bioessays. 2010;32:185–196. doi: 10.1002/bies.200900150. [DOI] [PubMed] [Google Scholar]
- 24.Singh VP, Saluja AK, Bhagat L, van Acker GJD, Song AM, Soltoff SP, Cantley LC, Steer ML. Phosphatidylinositol 3-kinase-dependent activation of trypsinogen modulates the severity of acute pancreatitis. J Clin Invest. 2001;108:1387–1395. doi: 10.1172/JCI12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gukovsky I, Cheng JH, Nam KJ, Lee OT, Lugea A, Fischer L, Penninger JM, Pandol SJ, Gukovskaya AS. Phosphatidylinositide 3-kinase γ regulates key pathologic responses to cholecystokinin in pancreatic acinar cells. Gastroenterology. 2004;126:554–566. doi: 10.1053/j.gastro.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 26.Fischer L, Gukovskaya AS, Young SH, Gukovsky I, Lugea A, Buechler A, Penninger JM, Friess H, Pandol SJ. Phosphatidylinositol 3-kinase regulates Ca2 signaling in pancreatic acinar cells through inhibition of sarco(endo)plasmic reticulum Ca2-ATPase. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1200–G1212. doi: 10.1152/ajpgi.00212.2004. [DOI] [PubMed] [Google Scholar]
- 27.Fischer L, Gukovskaya AS, Penninger JM, Mareninova OA, Friess H, Gukovsky I, Pandol SJ. Phosphatidylinositol 3-kinase facilitates bile acid-induced Ca(2+) responses in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G875–886. doi: 10.1152/ajpgi.00558.2005. [DOI] [PubMed] [Google Scholar]
- 28.Xu P, Wang J, Yang ZW, Lou XL, Chen C. Regulatory roles of the PI3K/Akt signaling pathway in rats with severe acute pancreatitis. PLoS One. 2013;8:e81767. doi: 10.1371/journal.pone.0081767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peeters RP, Wouters PJ, van Toor H, Kaptein E, Visser TJ, Van den Berghe G. Serum 3,3’,5’-triiodothyronine (rT3) and 3,5,3’-triiodothyronine/rT3 are prognostic markers in critically ill patients and are associated with postmortem tissue deiodinase activities. J Clin Endocrinol Metab. 2005;90:4559–4565. doi: 10.1210/jc.2005-0535. [DOI] [PubMed] [Google Scholar]
- 30.Peeters RP, Kester MH, Wouters PJ, Kaptein E, van Toor H, Visser TJ, Van den Berghe G. Increased thyroxine sulfate levels in critically ill patients as a result of a decreased hepatic type I deiodinase activity. J Clin Endocrinol Metab. 2005;90:6460–6465. doi: 10.1210/jc.2005-0866. [DOI] [PubMed] [Google Scholar]
- 31.Leung PS, Chan YC. Role of oxidative stress in pancreatic inflammation. Antioxid Redox Signal. 2009;11:135–165. doi: 10.1089/ars.2008.2109. [DOI] [PubMed] [Google Scholar]
- 32.Liu C, Li L, Ha M, Qi S, Duan P, Yang K. The PI3K/Akt and ERK pathways elevate thyroid hormone receptor beta1 and TRH receptor to decrease thyroid hormones after exposure to PCB153 and p,p’-DDE. Chemosphere. 2015;118:229–238. doi: 10.1016/j.chemosphere.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 33.Lupia E, Pigozzi L, Goffi A, Hirsch E, Montrucchio G. Role of phosphoinositide 3-kinase in the pathogenesis of acute pancreatitis. World J Gastroenterol. 2014;20:15190–15199. doi: 10.3748/wjg.v20.i41.15190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ronnelid J, Ahlin E, Nilsson B, Nilsson-Ekdahl K, Mathsson L. Immune complex-mediated cytokine production is regulated by classical complement activation both in vivo and in vitro. Adv Exp Med Biol. 2008;632:187–201. [PubMed] [Google Scholar]
- 35.Kim AH, Khursigara G, Sun X, Franke TF, Chao MV. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol Cell Biol. 2001;21:893–901. doi: 10.1128/MCB.21.3.893-901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papachristou DJ, Papadakou E, Basdra EK, Baltopoulos P, Panagiotopoulos E, Papavassiliou AG. Involvement of the p38 MAPKNF-kappaB signal transduction pathway and COX-2 in the pathobiology of meniscus degeneration in humans. Mol Med. 2008;14:160–166. doi: 10.2119/2007-00138.Papachristou. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu L, Song S, Pi Y, Yu Y, She W, Ye H, Su Y, Hu Q. Cumulated Ca2(+) spike duration underlies Ca2(+) oscillation frequency-regulated NFkappaB transcriptional activity. J Cell Sci. 2011;124:2591–2601. doi: 10.1242/jcs.082727. [DOI] [PubMed] [Google Scholar]
- 38.Tudhope SJ, Finney-Hayward TK, Nicholson AG, Mayer RJ, Barnette MS, Barnes PJ, Donnelly LE. Different mitogen-activated protein kinase-dependent cytokine responses in cells of the monocyte lineage. J Pharmacol Exp Ther. 2008;324:306–312. doi: 10.1124/jpet.107.127670. [DOI] [PubMed] [Google Scholar]
- 39.Liu C, Ha M, Cui Y, Wang C, Yan M, Fu W, Quan C, Zhou J, Yang K. JNK pathway decreases thyroid hormones via TRH receptor: a novel mechanism for disturbance of thyroid hormone homeostasis by PCB153. Toxicology. 2012;302:68–76. doi: 10.1016/j.tox.2012.07.016. [DOI] [PubMed] [Google Scholar]


