Abstract
Acute myeloid leukemia is known as one of the most malignant diseases. We aimed at exploring the effect of portulacerebroside A (PCA) on the apoptosis in human leukemia HL60 cells and clarify the possible mechanisms involved in. By MTT analysis, we found that PCA (1-100 μM) inhibited the cell viability in a time- and dose-dependent manner, and cell cycle was arrested at G0/G1 period. PCA treatment from 5 to 50 μM dose-dependently induced apoptosis from 12.7 ± 1.56% to 52.7 ± 6.214% of HL60 cells. Mitochondrial membrane potential (MMP) was decreased and reactive oxygen species (ROS) accumulated obviously. mRNA expressions and protein levels of Bax/Bcl-2, caspase-3 and caspase-9 were elevated significantly. ERK1/2, JNK1/2 and p38 MAPK pathway were blocked detected by western blot analysis. In conclusion, PCA can act as a new agent for leucocythemia treatment.
Keywords: Portulacerebroside A, p38/JNK, leucocythemia, apoptosis
Introduction
Acute myeloid leukemia (AML) characterized by the rapid growth and accumulation of white blood cells in the bone marrow, is a cancer of the myeloid line of blood cells, which interferes with the production of normal blood cells [1,2]. It is identified as one of the most malignant diseases diagnosed in young people. Chemotherapy is one of the main treatments for leukemia, but most of the patients cannot be treated thoroughly. Most people suffered from leukemia tend to die from relapse or drug resistance eventually. As a result, natural products play an important role in the treatment of these hematological malignancies.
Portulaca oleracea L., widely distributed in the temperate and tropical zones of the world, has been used as both foods and medicines. Its aerial part (Chinese name Ma-Chi-Xian) has been applied for the treatment of diarrhea, urinary tract infection and diabetes for a rather long history in China. It has wide pharmacological properties, such as antibacterial, regulating lipidemia, anti-aging, anti-oxidative, anti-inflammatory, wound-healing, analgesic and antitumor activities [3,4]. A cerebroside compound named as portulacerebroside A from Portulaca oleracea L. shows property of antitumor [5].
In this study, we attempted to explore the effects of PCA on the proliferation, cell cycle distribution, apoptosis, mitochondrial membrane potential (MMP) and reactive oxygen species (ROS) of human human leukemia HL60 cells and clarify the possible mechanisms involved in.
Methods and materials
Cell culture
Human HL60 cell line was obtained from Shanghai Institute of Cell Biology (Shanghai, China). Cells were cultured in RPMI-1640 medium with 10% FBS (Gibco BRL, Rockville, MD, USA), 100 U/ml penicillin G and 100 μg/mL streptomycin in an incubator (37°C, 100% humidity and 5% CO2).
PCA
Portulacerebroside A (PCA) was isolated and purified from the aerial parts of Portulaca oleracea L. according to the previous report and appeared as white powder [6]. It was dissolved in an appropriate amount of dimethylsulfoxide (DMSO) and diluted to the desired concentrations before utilization, with the final concentration of DMSO kept below 0.5%.
MTT assay
The MTT assay was used to assess the effect of PCA on cell viability. In brief, the cells were seeded in 96-well culture plates and treated without or with PCA (1, 2, 5, 10, 25, 50 and 100 μM) for 6, 12, 24 and 48 h. Subsequently the cell viability was evaluated by MTT assay. The absorbance was measured at 490 nm test wavelength and 570 nm reference wavelength with an automated Bio-Rad 550 microtiter plate reader (Rome, Italy).
DNA fragmentation assay
Following treatment with PCA (5, 10 and 50 μM) for 24 h, the cells were harvested and fixed for 5 minutes in 3% Para formaldehyde in phosphate buffered saline. After air dying, cells were stained for 10 minutes with Hoechst 33258 (10 mL), mounted in 50% glycerol containing 20 mmol/L citric acid and 50 mmol/L orthophosphate, and stored at -20°C before analysis. Nuclear morphology was evaluated using a fluorescence microscope (DMI3000B, Leica, Gemany).
Measurement of apoptotic cells by flow cytometry
HL60 cells were collected after treatment with PCA (5, 10 and 50 μM) for 24 h and fixed with 75% ethanolover night at 4°C, and stained with Annexin V and propidium iodide (PI). Cell apoptosis was evaluated using FAC flow cytometry (San Jose, CA).
Mitochondria membrane potential (MMP)
Rhodamine-123 (Rho-123) dye (Sigma) was used to detect the changes in MMP. Cells (5 × 104 cells/well) were cultured in 24-well plate. After a period of exposure (24 h) with various concentrations of PCA (5, 10 and 50 μM), cells were washed with PBS, incubated with Rho-123 (10 mg/mL) and subsequently subjected to flowcytometry.
Detection of reactive oxygen species (ROS)
Detection of ROS was performed by flow cytometric analysis as described previously. In brief, (5 × 104 cells/well) were cultured in 24-well plate, after a period of exposure (12 h) with various concentrations of PCA (5, 10 and 50 μM), cells were washed with PBS and resuspended in complete medium followed by incubation with 0.5 μM dihydrorhodamine 123 (Sigma) for 30 min at 37°C. ROS fluorescence intensity was determined by cytometry with excitation at 490 nm and emission at 520 nm.
Western blot assay
Cells were seeded at a density of 5 × 105 cells per well in 6-well plates, cultured overnight and then treated with PCA (5, 10 and 50 μM) for 1 and 24 h. Cell protein lysates were separated in 10% sodium dodecyl sulfate-polyacrylamide gels, electroblotted onto to a polyvinylidene fluoride membrane (Roche Diagnostics, Mannheim, Germany), then detected with JNK, phosphorylated (P-) JNK, p38 MAPK, P-p38 MAPK, Bax, Bcl-2, casepase-3 and caspase-8 proteins. Protein loading was estimated using mouse anti-GAPDH monoclonal antibody. Lab Works Image Acquisition and Analysis Software (UVP, Upland, CA, USA) were used to quantify band intensities. Antibodies were purchased from Univ-bio Inc (Shanghai, China).
Fluorescent quantitative reverse transcription-PCR (FQRT-PCR)
Total mRNA was isolated from HL60 cells using TRIzol Reagent (Gibco-BRL, Gaithersburg, MD) according to the previous report (11). Briefly, a 4 μg aliquot of RNA was reversely transcribed to cDNA by Thermoscript RT-PCR System reagent (Gibco-BRL). The primers and probes were designed using the Primer Express design software (Applied Biosystems) based on the sequence of cDNA encoding the human Bcl-2, Bax, caspase-3 and caspase-9 proteins. The primers for each gene were listed as following: 5’-AGACCGAAGTCCGCAGAACC-3’ and 5’-GAGACCACACTGCCCTGTTG-3’ for Bcl-2 (product: 113 bps); 5’ -GCGACTGATGTCCCTGTCTC-3’ and 5’-GGCCTCAGCCCATCTTCTTC-3’ for Bax (product: 132 bps); 5’-AACTGGACTGTGGCATTGAG-3’ and 5’-ACAAAGCGACTGGATGAACC-3’ for Caspase 3 (product: 161 bps); 5’-ATCACTGCCACCCAGAAG-3’ and 5’-TCCACGACGGACACATTG-3’ for GAPDH (product: 191 bps). PCR amplification consisted of 35 cycles: 15 s at 94°C for denaturing, 30 s at 58°C for annealing and 45 s (7 min in the final cycle) at 72°C for elongation. Relative expression of mRNA (%) = 2-ΔCT(1, 2, 3, 4) × 100%, where CT represents threshold cycle, ΔCT1 = CT(Bax) - CT(GAPDH), ΔCT2 = CT(Bcl-2) - CT(GAPDH), ΔCT3 = CT(caspase-9) - CT(GAPDH), ΔCT3 = CT(caspase-3) - CT(GAPDH).
Statistics
Values were expressed as means ± SD. One-way analysis of variance (ANOVA) followed by Dunnett’s test was used for statistical analysis. Probability (p) values less than 0.05 were considered significant.
Results
PCA inhibited proliferation and induced cell cycle arrest of HL60 cells
MTT assay was performed to determine the cell proliferation of HL60 cells under the influence of PCA. PCA (1, 2, 5, 10, 20, 50 and 100 μM) were added to the culture medium for 12, 24 and 48 h. The result showed that PCA inhibited HL60 cells proliferation in a time- and dose-dependent manner (Figure 1). The doses of 5, 10 and 50 μM were determined to carry out further investigations. In addition, cell cycle distribution was determined by flow cytometry. The Figure 2 showed that cell cycle of HL60 was arrested at G0/G1 period by PCA (10, 50 and 100 μM).
Figure 1.
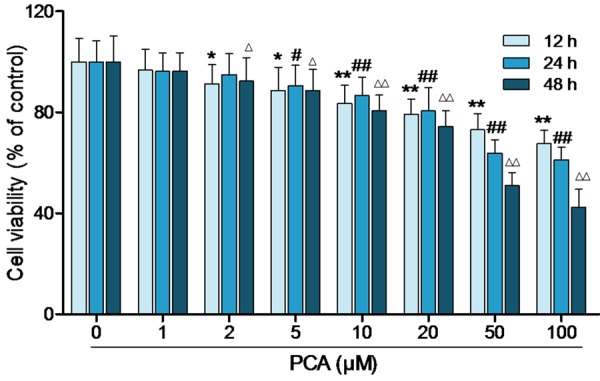
PCA inhibited the proliferation of HL60 cells. After treated with PCA (1, 2, 5, 10, 20, 50 and 100 μM) for 12, 24 and 48 h, cell viability was detected by MTT. Data was presented as mean ± SD (n = 6). *,#,ΔP<0.05, **,##,ΔΔP<0.01, compared with the control group.
Figure 2.
PCA caused cell cycle arrest of HL60 cells HL60 cells were treated with PCA (5, 10 and 50 μM) for 24 h, cell cycle distribution was identified by flow cytometry. Data was presented as mean ± SD (n = 6). **P<0.01, compared with the control group.
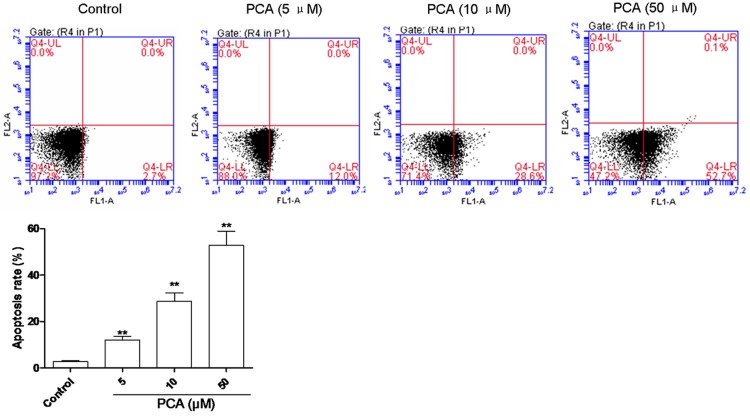
PCA induced apoptosis of HL60 cells
Flow cytometry assay was carried out to substantiate cell apoptosis induced by PCA treatment under various concentrations. The number of apoptotic cells was counted as late apoptotic cells shown in the upper right (Q4-UR) quadrant and early apoptotic cells as shown in lower right (Q4-LR) quadrant of the histograms. As shown in Figure 3, treatment of PCA at the dose of 5, 10 and 50 μM for 24 h significantly increased the number of early apoptotic cells (Q4-LR), respectively, from 12 ± 1.56% to 52.7 ± 6.214% (n = 3) in a dose-dependent manner compared with control cells with that of 2.7 ± 0.3%. The significant induction of apoptosis indicated the anticancer effect of PCA against HL60 cells.
Figure 3.
PCA induced apoptosis in HL60 cells. HL60 cells were treated with PCA PCA (5, 10 and 50 μM) for 24 h, and cell apoptosis was assessed by flow cytometry. Data was presented as mean ± SD (n = 6). **P<0.01, compared with the control group.
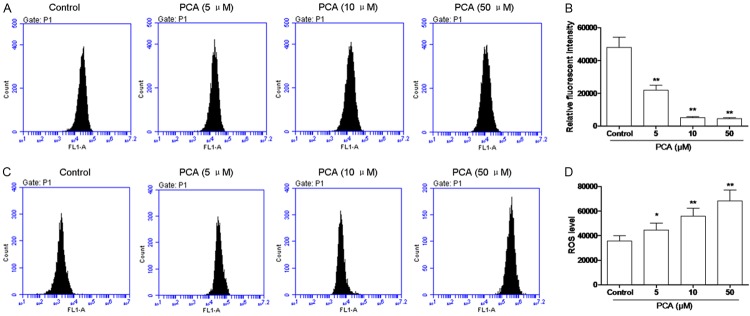
PCA induced apoptosis in the mitochondrial pathway
Loss of MMP is related to the mitochondrial apoptotic pathway. To assess the effect of PCA on the changes of MMP in HL60 cells, FCM analysis was carried out to detect the fluorescence intensity of Rho-123. As shown in Figure 4A and 4B, treatment of HL60 cells with PCA at the concentrations of 5, 10 and 50 μM for 24 h caused a moderate depolarization of MMP in a dose-dependent manner.
Figure 4.
Effects of PCA on MMP and ROS in HL60 cells. A, B. Cells were treated with PCA for 24 h at 5, 10, and 50 μM respectively, then incubated with Rhodamine 123 and analyzed by flow cytometry. C, D. Cells were treated with PCA for 24 h at 5, 10, and 50 μM respectively, and fluorescence probe DCFH-DA was used to determine the levels of ROS production. Data was presented as mean ± SD (n = 3). *P<0.05, **P<0.01, compared with the control group.
On the other hand, ROS generation is also linked to mitochondria. Fluorescence probe DCFH-DA was used to determine the levels of ROS production in HL60 cells. As shown in Figure 4C and 4D, HL60 cells exposed to PCA at 5, 10 and 50 μM for 12 h caused a significant increase in the intracellular accumulation of ROS in a dose-dependent manner.
Expression of Bax/Bcl-2, casepase-3 and casepase-9
To clarify the mechanism of HL60 cell apoptosis induced by PCA, apoptosis-related molecules were determined by real-time PCR. As shown in Figure 5A and 5B, mRNA expressions of Bax/Bcl-2, casepase-3 and casepase-9 were significantly increased in a dose-dependent manner, after PCA treatment for 12 h. Western blot analysis showed that protein expressions of Bax/Bcl-2, casepase-3 and casepase-9 were also increased by PCA treatment for 24 h (Figure 5B, 5C, 5E and 5F).
Figure 5.
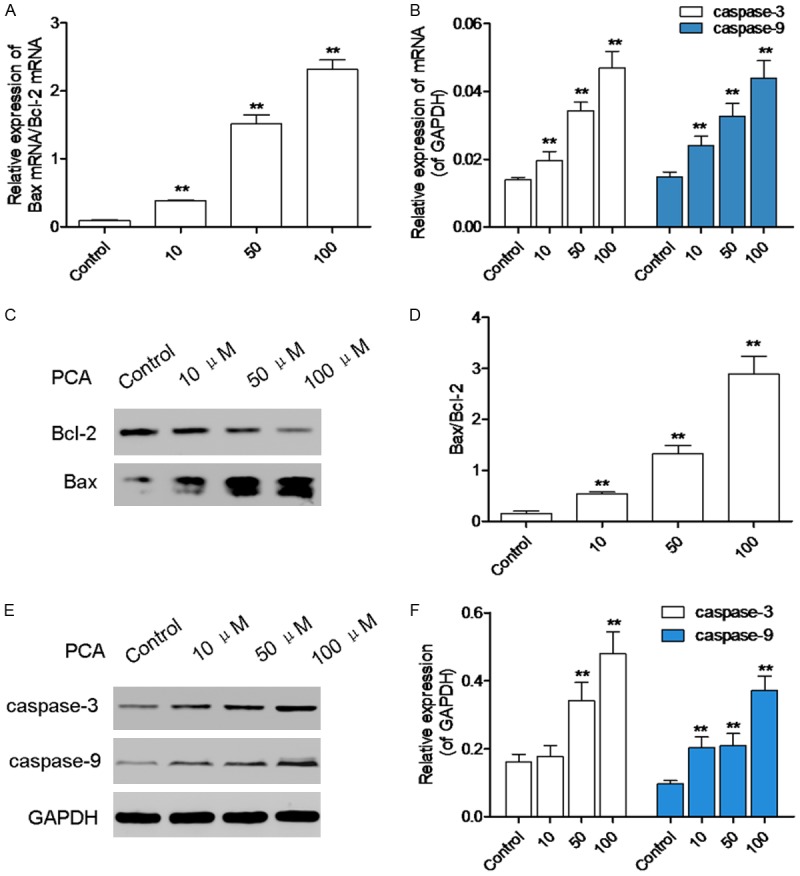
PCA surpressed the Bax/Bcl-2, caspase-3 and caspase-9 expression. A, B. HL60 cells were treated with PCA (5, 10, and 50 μM) for 12 h, mRNA expressions of Bax/Bcl-2, caspase-3 and caspase-9 were detected by real time PCR. C-F. HL60 cells were treated with PCA (5, 10, and 50 μM) for 24 h, protein levels of Bax/Bcl-2, caspase-3 and caspase-9 were detected by western blot analysis. Data was presented as mean ± SD (n = 6). *P<0.05, **P<0.01, compared with the control group.
PCA surpressed the phosphorylation of ERK1/2 and p38 MAPK
MAPK signaling draws plenty of attention in recent years, which is stimulated by cytokines and involved in cell proliferation, differentiation, apoptosis, immunoregulation and other important biological processes [7,8]. After PCA treatment for 3 h, western blot was performed to identify the protein expressions of JNK, P-JNK, p38 MAPK, P-p38 MAPK. As shown in Figure 6A and 6B, P-JNK and P-p38 MAPK was decreased by PCA treatment compared with the control group.
Figure 6.
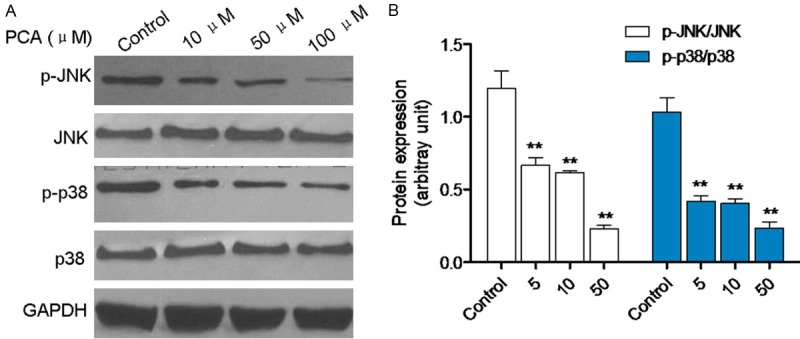
PCA blocked the JNK and p38 signaling. A, B. HL60 cells were treated with PCA (5, 10, and 50 μM) for 3 h, protein levels of P-JNK, JNK, P-p38 and p38 were identified by western blot analysis. Data was presented as mean ± SD (n = 6). *P<0.05, **P<0.01, compared with the control group.
Discussion
Many reports have suggested that PCA exhibited antitumor activity against liver cancer cells, tongue carcinoma cells [9]. In the present study, we investigated the inhibitory effect of PCA on human leukemia cells HL60 and elucidated the possible molecular mechanism involved. The results showed that PCA inhibited HL60 cell proliferation in a time- and dose-dependent manner. The result of flow cytometry analysis by Annexin V/PI staining showed that PCA treatment from 10 to 100 μM dose-dependently induced apoptosis. Our data regarding cell viability, cell cycle distribution and cell apoptosis suggested that PCA could penetrate HL60 cells, destroy mitochondria membrane integrity and increase the ROS, which consequently caused cell apoptosis.
Mitochondria play a crucial role in the complex process of cell apoptosis [10,11]. During this process, mitochondrial membrane pores are opened, resulting in the loss of mitochondrial membrane potential (MMP). The loss of MMP causes an increase in the permeability of the mitochondrial membrane, followed by the release of pro-apoptotic molecules such as cytochrome c. Cytochrome c releasing from mitochondrial interacts with ATP, Apaf-1 and caspase-9, and subsequently activates caspase-3, which consequently elicits caspase-dependent apoptotic cell death [12-14]. The western blot and FQRT-PCR analysis results suggest that the protein and mRNA expression levels of caspase-3 and caspase-9 were increased after treatment with OA.
Bcl-2 family members are crucial to regulating the mitochondrial death pathway [15] and include anti-apoptotic proteins Bcl-2 and Bcl-xL and pro-apoptotic proteins Bax, Bak and Bid [16,17]. Apoptosis inhibitory protein Bcl-2 residing on the outer mitochondrial membrane suppresses cytochrome c release through inhibiting mitochondrial permeability transition and/or stabilizing the outer mitochondrial membrane barrier function. When an apoptotic stimulus is sensed via upstream apoptotic signaling pathways such as MAPK, pro-apoptotic protein Bax residing in the cytosol can translocate to mitochondria, which promotes cytochrome c release via antagonization of apoptosis inhibitory protein effects. In the current study, PCA evidently decreased Bcl-2 protein expression, increased Bax protein expression indicating that PCA increases mitochondrial membrane permeability by modulating the translocation of Bax and expression of Bcl-2 and Bax, which results in the loss of MMP and subsequent release of cytochrome c and AIF.
Accumulating evidence indicates that activation of JNK and p38 signaling is associated with cell cycle arrest and apoptosis induction [18,19]. The activation of endogenous p38/JNK will promote tumor cell proliferation, survival and invasion [20,21]. In the present study, the phosphorylation levels of JNK and p38 were detected by western blot. We found that JNK and p38 were markedly decreased in HL60 cells exposed to PCA, indicating that p38/JNK signal pathway was inactivated by PCA.
Collectively, we found that PCA, a compound from Portulaca oleracea L. induces apoptosis in human leucocythemia cells HL60 via inactivating p38/JNK signal pathway. It also indicated that PCA is a promising agent for the prevention of liver cancer.
References
- 1.Kobayashi Y, Yamauchi T, Kiyoi H, Sakura T, Hata T, Ando K, Watabe A, Harada A, Taube T, Miyazaki Y, Naoe T. Phase I trial of volasertib, a Polo-like kinase inhibitor, in Japanese patients with acute myeloid leukemia. Cancer Sci. 2015 doi: 10.1111/cas.12814. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harada M, Benito J, Yamamoto S, Kaur S, Arslan D, Ramirez S, Jacamo R, Platanias L, Matsushita H, Fujimura T, Kazuno S, Kojima K, Tabe Y, Konopleva M. The novel combination of dual mTOR inhibitor AZD2014 and pan-PIM inhibitor AZD1208 inhibits growth in acute myeloid leukemia via HSF pathway suppression. Oncotarget. 2015;6:37930–47. doi: 10.18632/oncotarget.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharafati CR, Rafieian KM, Salehi E. Bioactivity of Apium petroselinum and Portulaca oleracea Essential Oils as Natural Preservatives. Jundishapur J Microbiol. 2015;8:e20128. doi: 10.5812/jjm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu XF, Zheng CG, Shi HG, Tang GS, Wang WY, Zhou J, Dong LW. Ethanol extract from portulaca oleracea L. attenuated acetaminopheninduced mice liver injury. Am J Transl Res. 2015;7:309–318. [PMC free article] [PubMed] [Google Scholar]
- 5.Ji Q, Zheng GY, Xia W, Chen JY, Meng XY, Zhang H, Rahman K, Xin HL. Inhibition of invasion and metastasis of human liver cancer HCCLM3 cells by portulacerebroside A. Pharm Biol. 2015;53:773–780. doi: 10.3109/13880209.2014.941505. [DOI] [PubMed] [Google Scholar]
- 6.Hai-Liang X, Yin-Huan H, Yan-Feng XU, Xiao-Qiang Y, Min LI, Jin-Cai LU, Chang-Quan L. Portulacerebroside A: new cerebroside from Portulaca oleracea L. Chinese Journal of Natural Medicines. 2008;6:401–403. [Google Scholar]
- 7.Lan CW, Chen MJ, Tai KY, Yu DC, Yang YC, Jan PS, Yang YS, Chen HF, Ho HN. Functional microarray analysis of differentially expressed genes in granulosa cells from women with polycystic ovary syndrome related to MAPK/ERK signaling. Sci Rep. 2015;5:14994. doi: 10.1038/srep14994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jalmi SK, Sinha AK. ROS mediated MAPK signaling in abiotic and biotic stress- striking similarities and differences. Front Plant Sci. 2015;6:769. doi: 10.3389/fpls.2015.00769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ji Q, Zheng GY, Xia W, Chen JY, Meng XY, Zhang H, Rahman K, Xin HL. Inhibition of invasion and metastasis of human liver cancer HCCLM3 cells by portulacerebroside A. Pharm Biol. 2015;53:773–780. doi: 10.3109/13880209.2014.941505. [DOI] [PubMed] [Google Scholar]
- 10.Sze CI, Kuo YM, Hsu LJ, Fu TF, Chiang MF, Chang JY, Chang NS. A cascade of protein aggregation bombards mitochondria for neurodegeneration and apoptosis under WWOX deficiency. Cell Death Dis. 2015;6:e1881. doi: 10.1038/cddis.2015.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salimi A, Roudkenar MH, Sadeghi L, Mohseni A, Seydi E, Pirahmadi N, Pourahmad J. Ellagic acid, a polyphenolic compound, selectively induces ROS-mediated apoptosis in cancerous B-lymphocytes of CLL patients by directly targeting mitochondria. Redox Biol. 2015;6:461–471. doi: 10.1016/j.redox.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia Y, Lee KW, Swerdloff R, Hwang D, Cobb LJ, Sinha HA, Lue YH, Cohen P, Wang C. Interaction of insulin-like growth factor-binding protein-3 and BAX in mitochondria promotes male germ cell apoptosis. J Biol Chem. 2010;285:1726–1732. doi: 10.1074/jbc.M109.046847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radogna F, Albertini MC, De Nicola M, Diederich M, Bejarano I, Ghibelli L. Melatonin promotes Bax sequestration to mitochondria reducing cell susceptibility to apoptosis via the lipoxygenase metabolite 5-hydroxyeicosatetraenoic acid. Mitochondrion. 2015;21:113–121. doi: 10.1016/j.mito.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Lu YY, Chen TS, Wang XP, Qu JL, Chen M. The JNK inhibitor SP600125 enhances dihydroartemisinin-induced apoptosis by accelerating Bax translocation into mitochondria in human lung adenocarcinoma cells. FEBS Lett. 2010;584:4019–4026. doi: 10.1016/j.febslet.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Pirouzpanah MB, Sabzichi M, Pirouzpanah S, Chavoshi H, Samadi N. Silibilin-induces apoptosis in breast cancer cells by modulating p53, p21, Bak and Bcl-XL pathways. Asian Pac J Cancer Prev. 2015;16:2087–2092. doi: 10.7314/apjcp.2015.16.5.2087. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Y, Liu QH, Liu CL, Lin L. Calycosin induces apoptosis in human ovarian cancer SKOV3 cells by activating caspases and Bcl-2 family proteins. Tumour Biol. 2015;36:5333–5339. doi: 10.1007/s13277-015-3194-8. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Yin RF, Teng JS. Wogonoside induces cell cycle arrest and mitochondrial mediated apoptosis by modulation of Bcl-2 and Bax in osteosarcoma cancer cells. Int J Clin Exp Pathol. 2015;8:63–72. [PMC free article] [PubMed] [Google Scholar]
- 18.Wu X, Li L, Zhang L, Wu J, Zhou Y, Zhou Y, Zhao Y, Zhao J. Inhibition of thioredoxin-1 with siRNA exacerbates apoptosis by activating the ASK1-JNK/p38 pathway in brain of a stroke model rats. Brain Res. 2015;1599:20–31. doi: 10.1016/j.brainres.2014.12.033. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Wang X, Wu T, Li B, Liu T, Wang R, Liu Q, Liu Z, Gong Y, Shao C. Isoliensinine induces apoptosis in triple-negative human breast cancer cells through ROS generation and p38 MAPK/JNK activation. Sci Rep. 2015;5:12579. doi: 10.1038/srep12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanaji N, Nelson A, Wang X, Sato T, Nakanishi M, Gunji Y, Basma H, Michalski J, Farid M, Rennard SI, Liu X. Differential roles of JNK, ERK1/2, and p38 mitogen-activated protein kinases on endothelial cell tissue repair functions in response to tumor necrosis factor-alpha. J Vasc Res. 2013;50:145–156. doi: 10.1159/000345525. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q, Wang J, Duan MT, Han SP, Zeng XY, Wang JY. NF-kappaB, ERK, p38 MAPK and JNK contribute to the initiation and/or maintenance of mechanical allodynia induced by tumor necrosis factor-alpha in the red nucleus. Brain Res Bull. 2013;99:132–139. doi: 10.1016/j.brainresbull.2013.10.008. [DOI] [PubMed] [Google Scholar]