Abstract
PFTK1 was identified as a member of the cyclin-dependent kinase (CDK) family and it is frequently upregulated in many types of tumors. However, its expression and role in pancreatic cancer has not been yet reported. In this study, we aimed to explore the expression and function in pancreatic cancer. The present study verified that PFTK1 was highly expressed in pancreatic cancer cell lines. The in vitro experiments demonstrated that knockdown of PFTK1 inhibited the proliferation, migration and invasion of pancreatic cancer cells as well as the epithelial-to-mesenchymal transition (EMT) progress. Finally, knockdown of PFTK1 inhibited the expression of p-PI3K and p-Akt in pancreatic cancer cells. In summary, the present study has provided further evidence that knockdown of PFTK1 inhibited the proliferation and invasion of pancreatic cancer cells as well as the EMT progress by suppressing the PI3K/Akt signaling pathway. Therefore, these findings reveal that PFTK1 might potentially become a novel strategy for targeting pancreatic cancer.
Keywords: PFTK1, pancreatic cancer, invasion, epithelial-to-mesenchymal transition (EMT)
Introduction
Pancreatic carcinoma is the fourth leading cause of cancer related death in the world. It is reported that the overall 5 year survival rates to be below 6% [1]. In People’s Republic of China, pancreatic cancer is the sixth leading cause of cancer-related death with a lower 5-year survival rate of 1%-3% [2,3]. Over the past years, although substantial progress has been made in our understanding of the biology of pancreatic cancer, there is no obvious improvement on survival of this malignancy. The dismal prognosis of pancreatic cancer is mainly due to its high metastatic potential, the late manifestation. Thus, identifying additional novel and effective agents to manage this disease is of urgent need.
Cyclin-dependent kinases (CDKs) are crucial regulators of the eukaryotic cell cycle whose activities are controlled by associated cyclins [4]. The PFTK1 gene, also known as PFTAIRE1, is a new member of the CDK family. Recent reports demonstrated that PFTK1 interacts with cyclin D3 (CCND3) and cyclin Y (CCNY) to regulate cell cycle progression and cell proliferation [5,6], and also regulates several pathways and cellular mechanisms as an oncogene [7,8]. For example, Pang et al. reported that PFTK1 promotes invasiveness and cell motility in hepatocellular carcinoma (HCC) [7]. However, its expression and role in pancreatic cancer has not been yet reported. In this study, we aimed to explore the expression and function in pancreatic cancer. Our results showed that the expression of PFTK1 was up-regulated in pancreatic cancer cells. Moreover, in vitro experiments proved that knockdown of PFTK1 inhibited cell proliferation, migration, invasion and epithelial-to-mesenchymal transition (EMT) progress in the pancreatic cancer cells.
Materials and methods
Cell culture
Four human pancreatic cancer cell lines (Patu8988, PANC-1, BxPC-3, and Capan-1) and the nonmalignant hTERT-HPNE were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS; Sijiqing biochemical, Hangzhou, China) at 37°C in a humidified 5% CO2 atmosphere.
Real-time quantitative PCR analysis
Total RNA was extracted from pancreatic cancer cells using Trizol Reagent according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). Then, Single-strand cDNA was prepared from the purified RNA using oligo (dT) priming (Thermoscript RT kit; Invitrogen), followed by SYBR-Green real-time PCR (Qiagen, Hilden, Germany). The following primers were used: PFTK1, 5’-CCAAGGAGTTGCTGCTTTTC-3’ (sense) and 5’-GAATGAACTCCAGGCCATGT-3’ (anti-sense); and β-actin 5’-CCGTGAAAAGATGACCCAGATC-3’ (sense), 5’-CACAGCCTGGATGGCTACGT-3’ (antisense). The PCR procedure was as follows: 94°C for 4 min; 94°C for 20 s, 55°C for 30 s, and 72°C for 20 s; 2 s for plate reading for 35 cycles; and melting curve from 65 to 95°C. Expression levels of the relative genes were calculated using the 2-ΔΔct method [9] and with the β-actin mRNA as an internal control.
Western blot
Pancreatic cancer cells were lysed on ice in RIPA lysis buffer supplemented with protease inhibitor (Beyotime, Shanghai, China), and protein concentrations were measured by using the Bradford method. Equal amounts of protein (30 μg protein each lane) were separated by SDS-PAGE, transferred to PVDF membranes (Millipore, Boston, MA, USA). Immunoblots were blocked with 5% skim milk in TBS/Tween 20 (0.05%, v/v) at room temperature for 1 h. Then, the membrane was immunoblotted with primary antibodies overnight at 4°C. The following primary antibodies were used: anti-E-cadherin, anti-N-cadherin, anti-vimentin, anti-PI3K, anti-p-PI3K, anti-Akt, anti-p-Akt and HRP-conjugated anti-GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Subsequently, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. The blots were developed using an enhanced chemiluminescence western blotting detection system (Amersham Bioscience, UK).
Small interfering RNA and cell transfection
For the siRNA-knockout experiment, a double-stranded RNA duplex that targeted the human PFTK1 gene was used (sense: 5’-GTTCATTCTTTACCACATT-3’, antisense: 5’-AGGTTGCATCTTTGTTGAA-3’); negative control siRNA was also synthesized. At 60% confluency, cells were treated with plasmid or siRNA-PFTK1 using Lipofectamine 2000 Transfection Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions.
Cell proliferation assay
Cell proliferation was measured using MTT assay. The transiently transfected cells were seeded in a 96-well plate at a cell density of 1.0 × 104 cells/well and then cultured at 24 h intervals for 4 days. Then, MTT solution (0.2 mg/ml, Sigma-Aldrich, St Louis, MO, USA) was added to each well and incubated for an additional 4 h. Following that, the solution was carefully aspirated, and 150 μL DMSO was added into each well to disolve the crystal. The absorbance was determined at 490 nm using a microplate reader (Bio-Tek Instruments, Winooski, VT, USA).
Cell migration and invasion assays
For the migration assay, 1.0 × 105 cells were suspended in serum-free medium and plated on chambers (Corning Costar, NY, USA). In brief, medium containing 10% FBS was added to the lower chamber as a chemoattractant. After incubating for 24 h at 37°C with 5% CO2, cells were fixed in methanol for 15 min and stained with 0.05% crystal violet in PBS for 15 min and then counted under a microscope (Olympus, Tokyo, Japan). Noninvasive cells in the upper chamber were removed by wiping with a cotton swab, and invasive cells were fixed with 4% formaldehyde in PBS and were stained with 1% crystal violet in 2% ethanol. Cells in the lower surface of the filter were photographed under a light microscope (100 × magnification).
For the invasion assay, the same procedures described above were used, except that the filters were precoated with 100 ml Matrigel (BD Biosciences, CA, USA) at a 1:4 dilution in DMEM to form a genuine reconstituted basement membrane.
Statistical analysis
All experiments were performed independently at least three times. Differences between groups were analyzed by Student’s t-test and P<0.05 was considered to indicate a statistically significant difference.
Results
PFTK1 is highly expressed in pancreatic cancer cell lines
We investigated the expression of PFTK1 in pancreatic cancers. The results demonstrated that PFTK1 mRNA expression was highly expressed in pancreatic cancer cell lines, as compared with the nonmalignant hTERT-HPNE cell line (Figure 1A). Similarly, we observed that the expression of PFTK1 protein was also highly expressed in pancreatic cancer cell lines (Figure 1B). And PANC-1 and BxPC-3 cell lines displayed a higher expression level of PFTK1. So, we used PANC-1 and BxPC-3 cells to investigate the effect of PFTK1 on pancreatic cancer cell proliferation, invasion and EMT.
Figure 1.
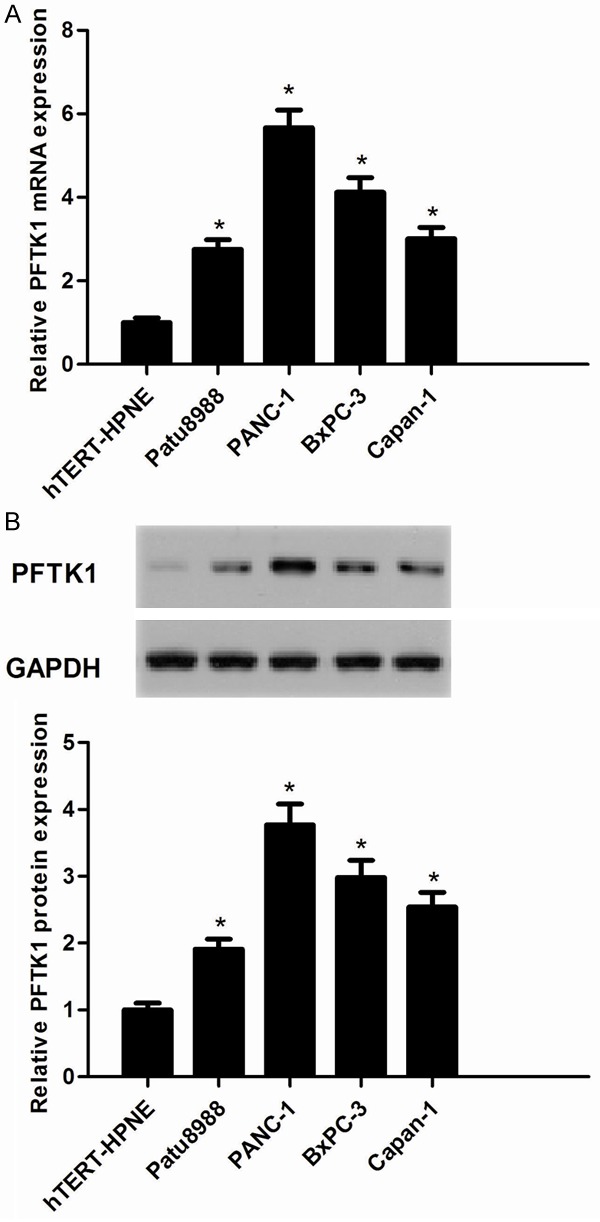
PFTK1 is highly expressed in pancreatic cancer cell lines. A. mRNA expression of PFTK1 was analyzed by RT-PCR. PFTK1 mRNA levels in human pancreatic cancer cell lines (Patu8988, PANC-1, BxPC-3, and Capan-1) were obviously higher than that in normal cell line (hTERT-HPNE); B. Representative Western image of PFTK1 protein in human pancreatic cancer cell lines. All experiments were repeated at least three times. Data are presented as mean ± SD. *P<0.05 compared to the hTERT-HPNE group.
Knockdown of PFTK1 inhibits pancreatic cancer cell proliferation
To test the functional role of PFTK1 in pancreatic cancer, PANC-1 and BxPC-3 cells were transfected with siRNA-PFTK1 or mock for 24 h. As shown in Figure 2A and 2C, RT-qPCR and Western blot results confirmed a remarkable downregulation of PFTK1 expression in PANC-1 cells after transfection with siRNA-PFTK1. Similarly, siRNA-PFTK1 also obviously decreased the expression of PFTK1 in BxPC-3 cells (Figure 2B and 2D). These results confirm that PFTK1 downregulating cells were successfully established. Then, we investigated the effect of PFTK1 on the proliferation of pancreatic cancer cells. As indicated in Figure 3, compared with the mock group, knockdown of PFTK1 significantly inhibited the proliferation in PANC-1 (Figure 3A) and BxPC-3 cells (Figure 3B), respectively, exhibiting a time-dependent manner.
Figure 2.
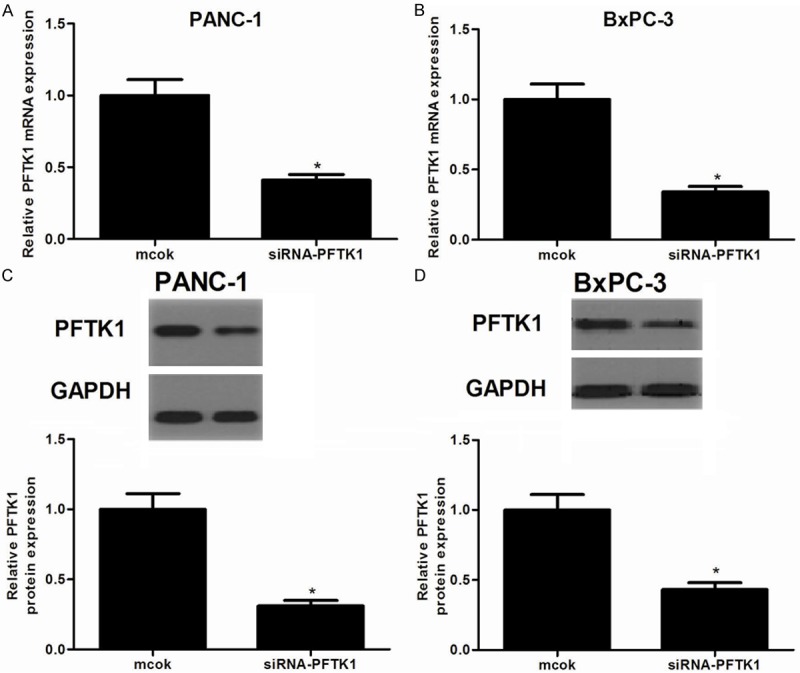
Transfection efficiency was determined by RT-qPCR and Western blot. A, B. The mRNA levels of PFTK1 in siRNA-PFTK1-transfected PANC-1 and BxPC-3 cells, respectively; C, D. The protein levels of PFTK1 in siRNA-PFTK1-transfected PANC-1 and BxPC-3 cells, respectively. All experiments were repeated at least three times. Data are presented as mean ± SD. *P<0.05 compared to the mock group.
Figure 3.

Knockdown of PFTK1 inhibits cell proliferation and colony formation. PANC-1 and BxPC-3 cells were infected with siRNA-PFTK1 or mock for 24, 48, 72 and 96 h. A. siRNA-PFTK1 inhibited the proliferation of PANC-1 cells in a time-dependent manner; B. siRNA-PFTK1 inhibited the proliferation of BxPC-3 cells in a time-dependent manner. All experiments were repeated at least three times. Data are presented as mean ± SD. *P<0.05 compared to the mock group.
Knockdown of PFTK1 suppresses EMT in pancreatic cancer cells
Then, we investigated the effect of PFTK1 on the EMT progression of pancreatic cancer cells. As indicated in Figure 4A and 4B, PANC-1 and BxPC-3 cells transfected with siRNA-PFTK1 showed higher mRNA expression levels of epithelial markers E-cadherin, while lower mRNA expression levels of mesenchymal markers vimentin and N-cadherin, compared with the mock group. Consistent with mRNA expression, western blot analysis demonstrated that knockdown of PFTK1 increased E-cadherin and decreased the protein levels of vimentin and N-cadherin in PANC-1 (Figure 4C) and BxPC-3 cells (Figure 4D), respectively.
Figure 4.
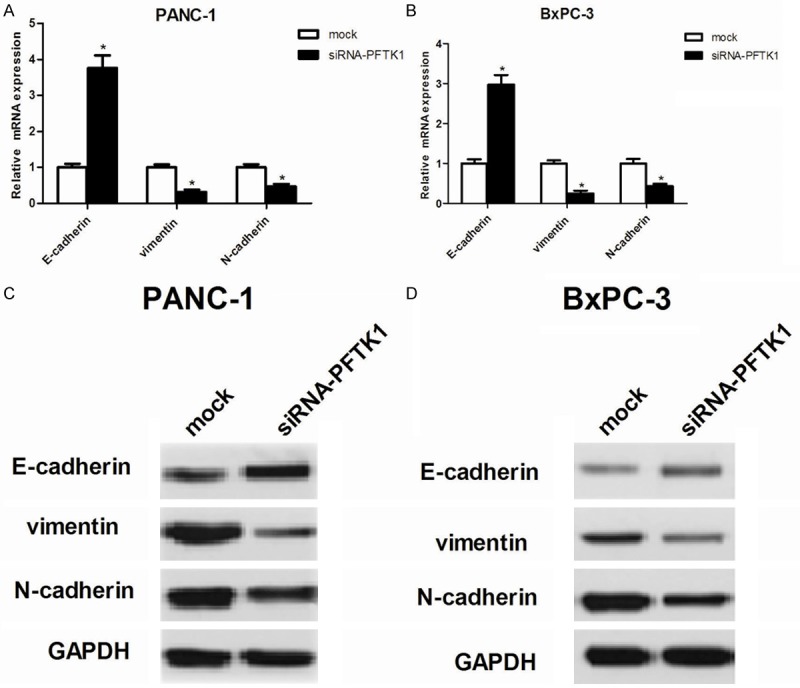
Knockdown of PFTK1 suppresses EMT in pancreatic cancer cells. PANC-1 and BxPC-3 cells were infected with siRNA-PFTK1 or mock for 24 h. A, B. RT-qPCR analysis for N-cadherin, E-cadherin and Vimentin in siRNA-PFTK1-transfected PANC-1 and BxPC-3 cells, respectively. C, D. Western blot analysis for N-cadherin, E-cadherin and Vimentin in siRNA-PFTK1-transfected PANC-1 and BxPC-3 cells, respectively. All experiments were repeated at least three times. Data are presented as mean ± SD. *P<0.05 compared to the mock group.
Knockdown of PFTK1 inhibits pancreatic cancer cell migration and invasion
To determine whether siRNA-PFTK1-prevented EMT had decreased invasiveness, we performed Transwell migration assay and Boyden chamber invasion assay. As indicated in Figure 5A and 5B, PANC-1 and BxPC-3 cells transfected with siRNA-PFTK1 showed a significantly lower rate of migrating capacity, compared with the mock group. In addition, knockdown of PFTK1 also significantly inhibited cell invasion in PANC-1 (Figure 5C) and BxPC-3 cells (Figure 5D).
Figure 5.

Knockdown of PFTK1 inhibits cell migration and invasion. PANC-1 and BxPC-3 cells were infected with siRNA-PFTK1 or mock for 24 h. A, B. Cell migration of PANC-1 and BxPC-3 cells transfected with siRNA-PFTK1 was measured by Transwell migration assay. C, D. Cell invasion of PANC-1 and BxPC-3 cells transfected with siRNA-PFTK1 was assessed by the Matrigel invasion chamber. All experiments were repeated at least three times. Data are presented as mean ± SD. *P<0.05 compared to the mock group.
Knockdown of PFTK1 regulates activation of the PI3K/Akt signaling pathway in pancreatic cancer cells
The PI3K/Akt signaling plays a major role in tumorigenesis by regulating cell proliferation, colony formation, cell cycle, cell survival, cell invasion, and metabolism. To further investigate the underlying mechanism of siRNA-PFTK1-inhibited cell proliferation and invasion, we investigated the effect of PFTK1 on phosphorylation levels of PI3K and Akt in pancreatic cancer cells. As shown in Figure 6, knockdown of PFTK1 significantly inhibited the phosphorylation of PI3K and Akt in pancreatic cancer cells.
Figure 6.
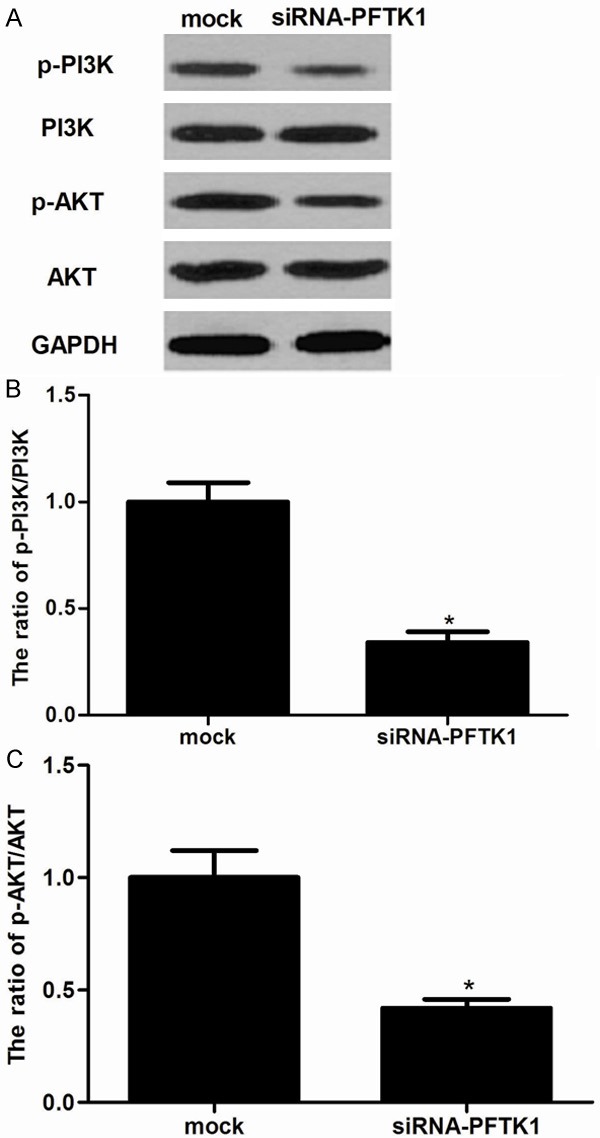
Knockdown of PFTK1 regulates activation of the PI3K/Akt signaling pathway. PANC-1 cells were infected with siRNA-PFTK1 or mock for 24 h. A. Western blot measurement of phosphorylation levels of PI3K and Akt in siRNA-PFTK1-transfected PANC-1 cells; B, C. The relative protein expression levels of p-PI3K and p-AKT were quantified in PANC-1 cells. All experiments were repeated at least three times. Data are presented as mean ± SD. *P<0.05 compared to the mock group.
Discussion
The present study verified that PFTK1 was highly expressed in pancreatic cancer cell lines. The in vitro experiments demonstrated that knockdown of PFTK1 inhibited the proliferation, migration and invasion of pancreatic cancer cells as well as the EMT progress. Finally, knockdown of PFTK1 inhibited the expression of p-PI3K and p-Akt in pancreatic cancer cells.
PFTK1 belongs to the CDK family. It has been reported that the expression of PFTK1 was highly in glioma cells and breast cancer cells [10,11]. In line with the results, the present data also showed that PFTK1 was highly expressed in pancreatic cancer tissues and cell lines. These results indicated that PFTK1 was an oncogene, which was required for the progression of pancreatic cancer.
Increasing evidence indicates that PFTK1 shows abilities to promote cell proliferation in various tissues [5]. Gu et al. showed that knockdown of PFTK1 attenuated breast cancer cell proliferation, whereas exogenous expression of PFTK1 might promote breast cancer cell proliferation [11]. Moreover, development of cell cycle inhibitors has emerged for the treatment of pancreatic cancer [12]. A recent study showed that inhibition of CDK5 reduces pancreatic cancer growth and progression [13].
EMT plays a critical role in driving tumor invasion and metastasis [14]. It has been reported that the forced expression of E-cadherin suppresses cancer metastasis, and an E-cadherin mutation in a tumor decreases cellular adhesion and increases cellular motility, invasion and metastasis [15,16]. In addition, vimentin has been identified as a mechanical transducer between cell surface integrins and the nucleus, thus, potentially controls cell migration through the regulation of cell adhesion stability [17,18]. In the present study, we found that knockdown of PFTK1 increased E-cadherin expression and decreased vimentin expression; siRNA-PFTK1 also inhibited pancreatic cancer cell migration and invasion. These results suggest that siRNA-PFTK1 inhibit pancreatic cancer cell invasion by suppressing EMT.
PI3K/Akt signaling pathway plays an important role in cell proliferation, migration, invasion, and metastasis of cancer cells [19-21]. The serine/threonine kinase Akt, the most studied signaling molecule downstream of PI3K, is involved in the stimulation of cell proliferation, inhibition of apoptosis, alteration of the cell cycle, and promotion of invasiveness as well as induction of EMT [22,23]. In pancreatic cancer, dysregulation of the PI3K/Akt pathway is quite common [24,25]. It was reported that HS-527, a new PI3-kinase inhibitor, inhibited the cell growth and proliferation of the pancreatic cancer in a time- and dose-dependent manner; it also promoted pancreatic cancer cell apoptosis [26]. Most interestingly, one study demonstrated that combined inhibition of cyclin-Dependent Kinases (dinaciclib) and AKT (MK-2206) blocks pancreatic tumor growth and metastases in patient-derived xenograft models [27]. In the present study, we found that knockdown of PFTK1 significantly inhibited the phosphorylation of PI3K and Akt in pancreatic cancer cells. These data suggest that knockdown of PFTK1 inhibited the proliferation and invasion of pancreatic cancer cells as well as the EMT progress by suppressing the PI3K/Akt signaling pathway.
In summary, the present study has provided further evidence that knockdown of PFTK1 inhibited the proliferation and invasion of pancreatic cancer cells as well as the EMT progress by suppressing the PI3K/Akt signaling pathway. Therefore, these findings reveal that PFTK1 might potentially become a novel strategy for targeting pancreatic cancer.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Guo X, Cui Z. Current diagnosis and treatment of pancreatic cancer in China. Pancreas. 2005;31:13–22. doi: 10.1097/01.mpa.0000168220.97967.d1. [DOI] [PubMed] [Google Scholar]
- 3.Li L, Zhao F, Lu J, Li T, Yang H, Wu C, Liu Y. Notch-1 signaling promotes the malignant features of human breast cancer through NF-kappaB activation. PLoS One. 2014;9:e95912. doi: 10.1371/journal.pone.0095912. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18:2699–2711. doi: 10.1101/gad.1256504. [DOI] [PubMed] [Google Scholar]
- 5.Shu F, Lv S, Qin Y, Ma X, Wang X, Peng X, Luo Y, Xu BE, Sun X, Wu J. Functional characterization of human PFTK1 as a cyclin-dependent kinase. Proc Natl Acad Sci U S A. 2007;104:9248–9253. doi: 10.1073/pnas.0703327104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang M, Gao Y, Yang T, Zhu X, Chen J. Cyclin Y, a novel membrane-associated cyclin, interacts with PFTK1. FEBS Lett. 2009;583:2171–2178. doi: 10.1016/j.febslet.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Pang EY, Bai AH, To KF, Sy SM, Wong NL, Lai PB, Squire JA, Wong N. Identification of PFTAIRE protein kinase 1, a novel cell division cycle-2 related gene, in the motile phenotype of hepatocellular carcinoma cells. Hepatology. 2007;46:436–445. doi: 10.1002/hep.21691. [DOI] [PubMed] [Google Scholar]
- 8.Sun T, Co NN, Wong N. PFTK1 interacts with cyclin Y to activate non-canonical Wnt signaling in hepatocellular carcinoma. Biochem Biophys Res Commun. 2014;449:163–168. doi: 10.1016/j.bbrc.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 10.Fan S, Zhao C, Zhang L, Dai S, Ren J, Zhang X, Ban N, He X, Yang L, Bao Z, Chen W, Sun J, Gao Y, Tao T. Knockdown of PFTK1 Inhibits the Migration of Glioma Cells. J Mol Neurosci. 2015;57:257–264. doi: 10.1007/s12031-015-0600-z. [DOI] [PubMed] [Google Scholar]
- 11.Gu X, Wang Y, Wang H, Ni Q, Zhang C, Zhu J, Huang W, Xu P, Mao G, Yang S. Upregulated PFTK1 promotes tumor cell proliferation, migration, and invasion in breast cancer. Med Oncol. 2015;32:195. doi: 10.1007/s12032-015-0641-8. [DOI] [PubMed] [Google Scholar]
- 12.Bayraktar S, Rocha Lima CM. Emerging cellcycle inhibitors for pancreatic cancer therapy. Expert Opin Emerg Drugs. 2012;17:571–582. doi: 10.1517/14728214.2012.739606. [DOI] [PubMed] [Google Scholar]
- 13.Feldmann G, Mishra A, Hong SM, Bisht S, Strock CJ, Ball DW, Goggins M, Maitra A, Nelkin BD. Inhibiting the cyclin-dependent kinase CDK5 blocks pancreatic cancer formation and progression through the suppression of Ras-Ral signaling. Cancer Res. 2010;70:4460–4469. doi: 10.1158/0008-5472.CAN-09-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwatsuki M, Mimori K, Yokobori T, Ishi H, Beppu T, Nakamori S, Baba H, Mori M. Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer Sci. 2010;101:293–299. doi: 10.1111/j.1349-7006.2009.01419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 16.Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–193. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Diab IH, Zehner ZE. ZBP-89 represses vimentin gene transcription by interacting with the transcriptional activator, Sp1. Nucleic Acids Res. 2003;31:2900–2914. doi: 10.1093/nar/gkg380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eckes B, Dogic D, Colucci-Guyon E, Wang N, Maniotis A, Ingber D, Merckling A, Langa F, Aumailley M, Delouvée A. Impaired mechanical stability, migration and contractile capacity in vimentin-deficient fibroblasts. J Cell Sci. 1998;111:1897–1907. doi: 10.1242/jcs.111.13.1897. [DOI] [PubMed] [Google Scholar]
- 19.Shukla S, MacLennan GT, Hartman DJ, Fu P, Resnick MI, Gupta S. Activation of PI3K-Akt signaling pathway promotes prostate cancer cell invasion. Int J Cancer. 2007;121:1424–1432. doi: 10.1002/ijc.22862. [DOI] [PubMed] [Google Scholar]
- 20.Chang F, Lee J, Navolanic P, Steelman L, Shelton J, Blalock W, Franklin R, McCubrey J. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia. 2003;17:590–603. doi: 10.1038/sj.leu.2402824. [DOI] [PubMed] [Google Scholar]
- 21.Wong KK, Engelman JA, Cantley LC. Targeting the PI3K signaling pathway in cancer. Curr Opin Genet Dev. 2010;20:87–90. doi: 10.1016/j.gde.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grille SJ, Bellacosa A, Upson J, Klein-Szanto AJ, Van Roy F, Lee-Kwon W, Donowitz M, Tsichlis PN, Larue L. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 2003;63:2172–2178. [PubMed] [Google Scholar]
- 23.Cheung M, Testa JR. Diverse mechanisms of AKT pathway activation in human malignancy. Curr Cancer Drug Targets. 2013;13:234–244. doi: 10.2174/1568009611313030002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanno S, Tanno S, Mitsuuchi Y, Altomare DA, Xiao GH, Testa JR. AKT activation up-regulates insulin-like growth factor I receptor expression and promotes invasiveness of human pancreatic cancer cells. Cancer Res. 2001;61:589–593. [PubMed] [Google Scholar]
- 25.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase-AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 26.Ryu YL, Jung KH, Son MK, Yan HH, Kim SJ, Shin S, Hong S, Hong SS. Anticancer activity of HS-527, a novel inhibitor targeting PI3-kinase in human pancreatic cancer cells. Cancer Lett. 2014;353:68–77. doi: 10.1016/j.canlet.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Hu C, Dadon T, Chenna V, Yabuuchi S, Bannerji R, Booher R, Strack P, Azad NA, Nelkin BD, Maitra A. Combined Inhibition of Cyclin-Dependent Kinases (Dinaciclib) and AKT (MK-2206) Blocks Pancreatic Tumor Growth and Metastases in Patient-Derived Xenograft Models. Mol Cancer Ther. 2015;14:1532–539. doi: 10.1158/1535-7163.MCT-15-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]


