Abstract
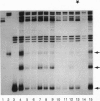
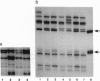
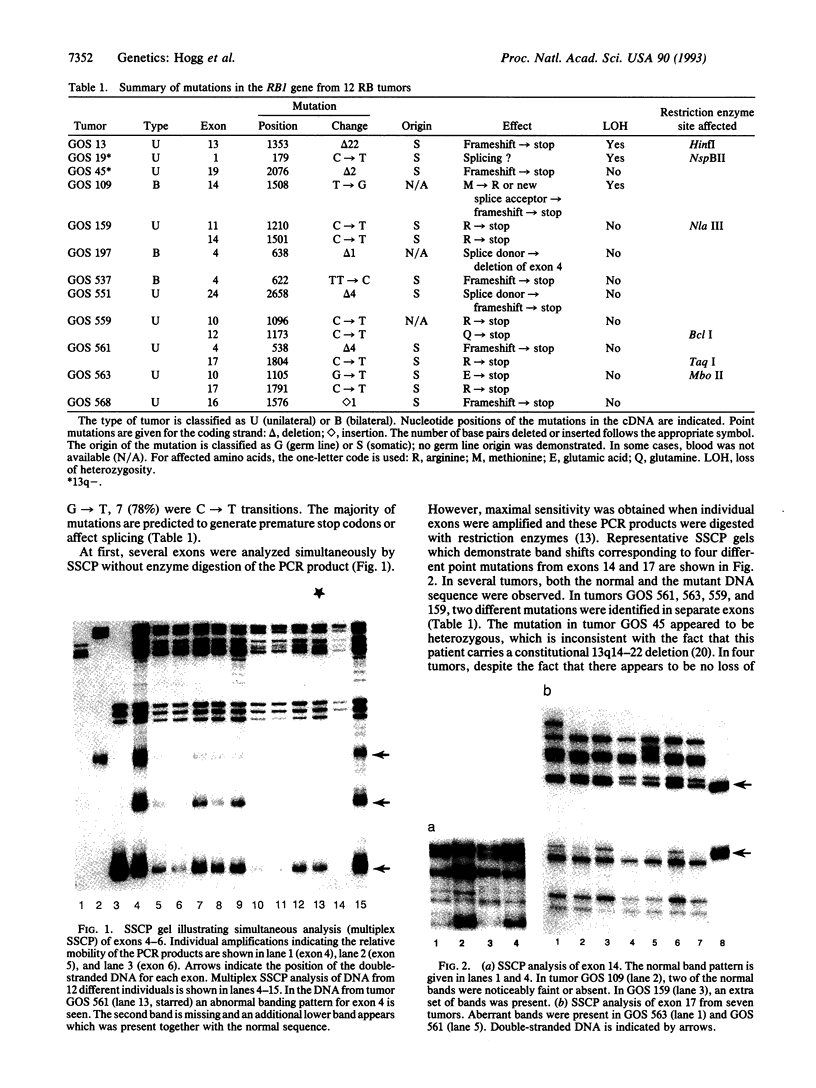
The RB1 gene from 12 human retinoblastoma tumors has been analyzed exon-by-exon with the single-strand conformation polymorphism technique. Mutations were found in all tumors, and one-third of the tumors had independent mutations in both alleles neither of which were found in the germ line, confirming their true sporadic nature. In the remaining two-thirds of the tumors only one mutation was found, consistent with the loss-of-heterozygosity theory of tumorigenesis. Point mutations, the majority of which were C-->T transitions, were the most common abnormality and usually resulted in the conversion of an arginine codon to a stop codon. Small deletions were the second most common abnormality and most often created a downstream stop codon as the result of a reading frameshift. Deletions and point mutations also affected splice junctions. Direct repeats were present at the breakpoint junctions in the majority of deletions, supporting a slipped-mispairing mechanism. Point mutations generally produced DNA sequences which resulted in perfect homology with endogenous sequences which lay within 14 bp.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird P. N., Groves N., Haber D. A., Housman D. E., Cowell J. K. Identification of mutations in the WT1 gene in tumours from patients with the WAGR syndrome. Oncogene. 1992 Nov;7(11):2141–2149. [PubMed] [Google Scholar]
- Caron de Fromentel C., Soussi T. TP53 tumor suppressor gene: a model for investigating human mutagenesis. Genes Chromosomes Cancer. 1992 Jan;4(1):1–15. doi: 10.1002/gcc.2870040102. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Cawthon R. M., Weiss R., Xu G. F., Viskochil D., Culver M., Stevens J., Robertson M., Dunn D., Gesteland R., O'Connell P. A major segment of the neurofibromatosis type 1 gene: cDNA sequence, genomic structure, and point mutations. Cell. 1990 Jul 13;62(1):193–201. doi: 10.1016/0092-8674(90)90253-b. [DOI] [PubMed] [Google Scholar]
- Cole W. G., Chiodo A. A., Lamande S. R., Janeczko R., Ramirez F., Dahl H. H., Chan D., Bateman J. F. A base substitution at a splice site in the COL3A1 gene causes exon skipping and generates abnormal type III procollagen in a patient with Ehlers-Danlos syndrome type IV. J Biol Chem. 1990 Oct 5;265(28):17070–17077. [PubMed] [Google Scholar]
- Comings D. E. A general theory of carcinogenesis. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3324–3328. doi: 10.1073/pnas.70.12.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H., Farabaugh P. J., Gilbert W. Molecular basis of base substitution hotspots in Escherichia coli. Nature. 1978 Aug 24;274(5673):775–780. doi: 10.1038/274775a0. [DOI] [PubMed] [Google Scholar]
- Cowell J. K., Hogg A. Genetics and cytogenetics of retinoblastoma. Cancer Genet Cytogenet. 1992 Nov;64(1):1–11. doi: 10.1016/0165-4608(92)90314-x. [DOI] [PubMed] [Google Scholar]
- Cowell J. K., Hungerford J., Rutland P., Jay M. Genetic and cytogenetic analysis of patients showing reduced esterase-D levels and mental retardation from a survey of 500 individuals with retinoblastoma. Ophthalmic Paediatr Genet. 1989 Jun;10(2):117–127. doi: 10.3109/13816818909088352. [DOI] [PubMed] [Google Scholar]
- Dryja T. P., Cavenee W., White R., Rapaport J. M., Petersen R., Albert D. M., Bruns G. A. Homozygosity of chromosome 13 in retinoblastoma. N Engl J Med. 1984 Mar 1;310(9):550–553. doi: 10.1056/NEJM198403013100902. [DOI] [PubMed] [Google Scholar]
- Dunn J. M., Phillips R. A., Zhu X., Becker A., Gallie B. L. Mutations in the RB1 gene and their effects on transcription. Mol Cell Biol. 1989 Nov;9(11):4596–4604. doi: 10.1128/mcb.9.11.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Bernards R., Rogelj S., Weinberg R. A., Rapaport J. M., Albert D. M., Dryja T. P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986 Oct 16;323(6089):643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- Godbout R., Dryja T. P., Squire J., Gallie B. L., Phillips R. A. Somatic inactivation of genes on chromosome 13 is a common event in retinoblastoma. Nature. 1983 Aug 4;304(5925):451–453. doi: 10.1038/304451a0. [DOI] [PubMed] [Google Scholar]
- Green M. R. Biochemical mechanisms of constitutive and regulated pre-mRNA splicing. Annu Rev Cell Biol. 1991;7:559–599. doi: 10.1146/annurev.cb.07.110191.003015. [DOI] [PubMed] [Google Scholar]
- Green P. M., Montandon A. J., Bentley D. R., Ljung R., Nilsson I. M., Giannelli F. The incidence and distribution of CpG----TpG transitions in the coagulation factor IX gene. A fresh look at CpG mutational hotspots. Nucleic Acids Res. 1990 Jun 11;18(11):3227–3231. doi: 10.1093/nar/18.11.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T., Takahashi R., Yandell D. W., Xu H. J., Hu S. X., Gunnell S., Benedict W. F. Characterization of intragenic deletions in two sporadic germinal mutation cases of retinoblastoma resulting in abnormal gene expression. Oncogene. 1991 Mar;6(3):463–469. [PubMed] [Google Scholar]
- Hogg A., Onadim Z., Baird P. N., Cowell J. K. Detection of heterozygous mutations in the RB1 gene in retinoblastoma patients using single-strand conformation polymorphism analysis and polymerase chain reaction sequencing. Oncogene. 1992 Jul;7(7):1445–1451. [PubMed] [Google Scholar]
- Horowitz J. M., Park S. H., Bogenmann E., Cheng J. C., Yandell D. W., Kaye F. J., Minna J. D., Dryja T. P., Weinberg R. A. Frequent inactivation of the retinoblastoma anti-oncogene is restricted to a subset of human tumor cells. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2775–2779. doi: 10.1073/pnas.87.7.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson A. G., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971 Apr;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczak M., Cooper D. N. Gene deletions causing human genetic disease: mechanisms of mutagenesis and the role of the local DNA sequence environment. Hum Genet. 1991 Mar;86(5):425–441. doi: 10.1007/BF00194629. [DOI] [PubMed] [Google Scholar]
- Lohmann D., Horsthemke B., Gillessen-Kaesbach G., Stefani F. H., Höfler H. Detection of small RB1 gene deletions in retinoblastoma by multiplex PCR and high-resolution gel electrophoresis. Hum Genet. 1992 Apr;89(1):49–53. doi: 10.1007/BF00207041. [DOI] [PubMed] [Google Scholar]
- McGee T. L., Yandell D. W., Dryja T. P. Structure and partial genomic sequence of the human retinoblastoma susceptibility gene. Gene. 1989 Aug 1;80(1):119–128. doi: 10.1016/0378-1119(89)90256-4. [DOI] [PubMed] [Google Scholar]
- Michaud J., Brody L. C., Steel G., Fontaine G., Martin L. S., Valle D., Mitchell G. Strand-separating conformational polymorphism analysis: efficacy of detection of point mutations in the human ornithine delta-aminotransferase gene. Genomics. 1992 Jun;13(2):389–394. doi: 10.1016/0888-7543(92)90258-t. [DOI] [PubMed] [Google Scholar]
- Onadim Z. O., Mitchell C. D., Rutland P. C., Buckle B. G., Jay M., Hungerford J. L., Harper K., Cowell J. K. Application of intragenic DNA probes in prenatal screening for retinoblastoma gene carriers in the United Kingdom. Arch Dis Child. 1990 Jul;65(7 Spec No):651–656. doi: 10.1136/adc.65.7_spec_no.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onadim Z., Hogg A., Baird P. N., Cowell J. K. Oncogenic point mutations in exon 20 of the RB1 gene in families showing incomplete penetrance and mild expression of the retinoblastoma phenotype. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6177–6181. doi: 10.1073/pnas.89.13.6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Powell S. M., Zilz N., Beazer-Barclay Y., Bryan T. M., Hamilton S. R., Thibodeau S. N., Vogelstein B., Kinzler K. W. APC mutations occur early during colorectal tumorigenesis. Nature. 1992 Sep 17;359(6392):235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- Rideout W. M., 3rd, Coetzee G. A., Olumi A. F., Jones P. A. 5-Methylcytosine as an endogenous mutagen in the human LDL receptor and p53 genes. Science. 1990 Sep 14;249(4974):1288–1290. doi: 10.1126/science.1697983. [DOI] [PubMed] [Google Scholar]
- Ripley L. S. Frameshift mutation: determinants of specificity. Annu Rev Genet. 1990;24:189–213. doi: 10.1146/annurev.ge.24.120190.001201. [DOI] [PubMed] [Google Scholar]
- Satokata I., Tanaka K., Miura N., Miyamoto I., Satoh Y., Kondo S., Okada Y. Characterization of a splicing mutation in group A xeroderma pigmentosum. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9908–9912. doi: 10.1073/pnas.87.24.9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffner M., Münger K., Byrne J. C., Howley P. M. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5523–5527. doi: 10.1073/pnas.88.13.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shew J. Y., Chen P. L., Bookstein R., Lee E. Y., Lee W. H. Deletion of a splice donor site ablates expression of the following exon and produces an unphosphorylated RB protein unable to bind SV40 T antigen. Cell Growth Differ. 1990 Jan;1(1):17–25. [PubMed] [Google Scholar]
- Sparkes R. S., Murphree A. L., Lingua R. W., Sparkes M. C., Field L. L., Funderburk S. J., Benedict W. F. Gene for hereditary retinoblastoma assigned to human chromosome 13 by linkage to esterase D. Science. 1983 Feb 25;219(4587):971–973. doi: 10.1126/science.6823558. [DOI] [PubMed] [Google Scholar]
- Streisinger G., Okada Y., Emrich J., Newton J., Tsugita A., Terzaghi E., Inouye M. Frameshift mutations and the genetic code. This paper is dedicated to Professor Theodosius Dobzhansky on the occasion of his 66th birthday. Cold Spring Harb Symp Quant Biol. 1966;31:77–84. doi: 10.1101/sqb.1966.031.01.014. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Vogel F. Genetics of retinoblastoma. Hum Genet. 1979 Nov 1;52(1):1–54. doi: 10.1007/BF00284597. [DOI] [PubMed] [Google Scholar]
- Vulliamy T., Mason P., Luzzatto L. The molecular basis of glucose-6-phosphate dehydrogenase deficiency. Trends Genet. 1992 Apr;8(4):138–143. doi: 10.1016/0168-9525(92)90372-B. [DOI] [PubMed] [Google Scholar]
- Weir-Thompson E., Condie A., Leonard R. C., Prosser J. A familial RB1 mutation detected by the HOT technique is homozygous in a second primary neoplasm. Oncogene. 1991 Dec;6(12):2353–2356. [PubMed] [Google Scholar]
- Wiggs J., Nordenskjöld M., Yandell D., Rapaport J., Grondin V., Janson M., Werelius B., Petersen R., Craft A., Riedel K. Prediction of the risk of hereditary retinoblastoma, using DNA polymorphisms within the retinoblastoma gene. N Engl J Med. 1988 Jan 21;318(3):151–157. doi: 10.1056/NEJM198801213180305. [DOI] [PubMed] [Google Scholar]
- Yandell D. W., Campbell T. A., Dayton S. H., Petersen R., Walton D., Little J. B., McConkie-Rosell A., Buckley E. G., Dryja T. P. Oncogenic point mutations in the human retinoblastoma gene: their application to genetic counseling. N Engl J Med. 1989 Dec 21;321(25):1689–1695. doi: 10.1056/NEJM198912213212501. [DOI] [PubMed] [Google Scholar]