Abstract
To explore the expressions level of Livin, Survivin and Caspase-3 in prostatic cancer and the relationship among the 3 proteins and the clinicopathological features as well as the correlation among them. Totally, 43 paraffin-embedded prostate cancer tissues obtained from patients who were performed with rectal prostate biopsy or excision and 17 paraffin-embedded prostatic hyperplasia tissues were collected. All the specimens were confirmed by pathology. Immunohistochemistry SP method was used to detect the expressions of Livin, Survivin and Caspase-3 in prostatic cancer compared to hyperplasia tissues. The positive expression rates of both Livin and Survivin in prostatic cancer tissue were higher than those in prostatic hyperplasia tissue (93.02% vs. 64.70%, P < 0.05; 83.72% vs. 35.29%, P < 0.01). However, the positive expression rate of Caspase-3 in prostatic cancer tissue was obviously lower than that in prostatic hyperplasia tissue (25.58% vs. 58.82%, P < 0.01). Both Livin and Survivin expressions in prostatic cancer tissue were related to pathological grading (Gleason scores) (X2 = 14.000, P = 0.001), but not related to preoperative PSA, clinical stages and distant metastasis (P > 0.05). Capsase-3 expression in prostatic cancer tissue was related to pathological grading (Gleason scores) (X 2 = 14.000, P = 0.001) and clinical stages (X 2 = 4.896, P = 0.027), but not related to preoperative PSA and distant metastasis (P > 0.05). In prostatic cancer tissue, Livin expression had no correlation with Survivin expression (r = 0.127, P = 0.419 > 0.05), but negatively correlated with Caspase-3 expression (r = -0.497, P = 0.001). Survivin expression was negatively correlated with Caspase-3 expression (r = -0.354, P = 0.020). Livin, Survivin and Caspase-3 are closely related to the occurrence and development of prostatic cancer and which are expected to become new targets for diagnosis and treatment in future.
Keywords: Prostatic cancer, livin, survivin, caspase-3, correlation
Introduction
Prostatic cancer is a disease of serious impacting the life equality of the elderly and ranks the second of case fatality rate in males in Europe and the United States, even all over the world, second only to lung cancer [1,2]. In recent years, with the changes of environment, high fat diet and population aging, the morbidity of prostatic cancer is increasing year by year in China and ranks the third of male urogenital system tumors, which is gradually close to the European and American. A study in the United State shows that the morbidity of prostatic cancer is 12%~46%, which is found in a study of general autopsy of the male elderly over 50 years old that the morbidity has been increasing with the patients get older. Patients with prostatic cancer have no obvious symptoms in early stage, but when cancer tissue increases to a certain extent which leads to suppression of the urethra, and the abnormal urination. For example, dysuria and hematuresis occur in a small number of patients, while the distant metastasis such as mostly bone metastasis is found in a large number of patients. It is in advanced stage that patients feel lower back pain, which with poor therapeutic effect and unfavorable prognosis.
Apoptosis regulatory proteins are playing important role in the occurrence and development of tumors. At present, it is believed that gene directly control the occurrence and development of tumor cell apoptosis and other gene expressions. Through signal transduction, gene products activation is influenced by extracellular factors and indirectly regulates cell apoptosis finally. Cell apoptosis is regulated by cysteinyl aspartate-specific proyease, caspases. Inhibitory of apoptosis proteins (IAPs) family is presently the only endogenous Caspases inhibitory factors which can directly inhibit end effectors including Caspases-3 and Caspases-7. The family consists of 8 members, namely, HIAP-1, HIAP-2, NIAP, XIAP, ILP-2, Bruce, Survivin and Livin [3,4]. Liven is often over-expression in some malignant tumors tissues and makes cell apoptosis being restrained, so it is closely related to the occurrence of malignant tumors [5]. Survivin has function of antiapoptosis, which can regulate cell cycle and participate in angiogenesis. Survivin is seldom expression in normal differentiated mature tissues except from thymus gland and gonad, but is highly expressed in most malignant tumors, so it can be regarded as tumor associated factors.
Prostatic cancer belongs to a genetic disease. At present, studies on the regulatory methods and new targets for it were to explore in gene level at home and abroad. Therefore, in this study, Immunohistochemical staining method was used to detect the expressions of Livin, Survivin and Caspase-3 and explore the relationship among the 3 proteins, furthermore, the clinicopathological features and the correlation between Livin and Survivin, Livin and Caspase-3, Survivin and Caspase-3 were also investigated. In hoping to provide the optimal markers for the diagnosis and prognosis of prostatic cancer and the scientific experimental basis were applied for the effective targets in the treatment of prostatic cancer.
Materials and methods
Specimens collection
Totally 43 paraffin-embedded prostate cancer specimens obtaining from patients performed with rectal prostate biopsy or excision were collected from the Second Hospital of Hebei Medical University from Jan., 2013 to Sept, 2014. All specimens were confirmed by histopathology and have complete clinical and pathological data. All patients were not treated with radiotherapy, chemotherapy and endocrinotherapy. Patients aged 55 to 81years old, with a mean age of 72.6 years. The average volume of prostate was 51.24 cm3. The average value of prostate-specific antigen (PSA) was 20.55 ng/mL, including 4 cases with PSA < 4 ng/mL, 16 with 4~10 ng/mL and 19 with PSA > 10 ng/mL. Pathological grading was judged by Gleason points-scoring system, including 4 cases in G1 (Gleason 2~4 points, well differentiated), 10 cases in G2 (Gleason 5~6 points, medium differentiated), 29 cases in G3 (Gleason 7~10 points, poorly differentiated or undifferentiated). According to TNM staging system of American Joint Committee on Cancer (AJCC) in 2002, there were 2 cases in stage I, 17 cases in stage II, 12 cases in stage III and 12 cases in stage IV.
A total of 17 paraffin-embedded prostatic hyperplasia specimens obtaining from patients performed with excision and confirmed by pathology were selected as control. Patients aged 52 to 81 years old with the mean age of 71.9 years. The average volume of prostate was 59.06 cm3 and the average value of PSA was 4.59 ng/mL.
Key reagents
Rabbit anti-human Livin polyclonal (aa264-280) antibody and rabbit anti-human Caspase-3 polyclonal antibody were purchased from Beijing Bo Orson Biological Technology Co., LTD and Santa Cruz Biotechnology, Inc. Rabbit anti-human Survivin polyclonal antibody, SP kit and DAB chromogenic reagent kit were bought from Wuhan Boster Biological Technology, Ltd.
Immunohistochemistry
All specimens were fixed by 10% formal in and the paraffin-embedded tissue sections (4 µm thick) were stained with rabbit anti-human Livin polyclonal antibody, rabbit anti-human Caspase-3 polyclonal antibody and rabbit anti-human Survivin polyclonal antibody respectively, which were incubated overnight after being deparaffinized in xylene and rehydrated in ethanol at 50°C. To perform heat-induced antigen retrieval, the sections were placed in 10 mM citrate buffer (pH 6.0) and heated to a boil. Endogenous peroxidase function was quenched using peroxidase blocking solution and the sections were incubated with rabbit anti-Human Livin polyclonal antibody (1:100), rabbit anti-human Caspase-3 polyclonal antibody (1:100) and rabbit anti-human Survivin polyclonal antibody (1:100), overnight at 4°C. After PBS washing, the sections were incubated with streptavidin horse radish peroxide (SA-HRP)-conjugated goat anti rabbit secondary antibodies for 30 min and treated with DAB chromogen substrate buffer for time periods determined by the response of antigen with antibody. The sections were counterstained with hematoxylin for 4-5 min, washed, dehydrated in ethanol and xylene and then mounted on slides.
Evaluation criterion
Double blind was used for reading sections by two pathologists. Cells in which Livin, Survivin and Caspase-3 showed yellow-brown in cytoplasm or cell nucleus were considered to be positive. Staining intensity and number of positive cells were used to conduct semi-quantitative analysis. The expression was graded according to following staining intensity scores: 0 (no expression), 1+ (weak), 2+ (moderate) and 3+ (strong) scores stand for colorless, light yellow, clay bank and brown, respectively; and percentage of positive stained tumor cells: 0 < 10%, 1 (10%~50%), 2 (51%-75%), 3 (> 75%). Five visual fields in each slice were selected and the proportion of positive cells in 100 cells under microscope (×400) was deemed as the percentage of positive cells. The product of integral of staining intensity and positive cells proportion was used for evaluating the positive degree: 0~1, 2~4, 5~8 and 9~12 points refers to negative (-), weak positive (+), positive (++) and strong positive (+++).
Statistical data analysis
SPSS15.0 software package was applied for data analysis. Comparison of sample rate was analyzed by chi-square test. Correlation between Livin and Survivin, Livin and Caspase-3, Survivin and Caspase-3 was analyzed by Spearman rank correlation. A value of P < 0.05 was considered to be statistically significant.
Results
Expressions of livin, survivin and caspase-3 in prostatic hyperplasia and prostatic cancer tissues
The positive expression rates of Livin, Survivin and Caspase-3 in prostatic cancer and prostatic hyperplasia tissues were 93.02% (40/43) and 64.70% (11/17); 83.72% (36/43) and 35.29% (6/17), respectively, the both differences were significant (P < 0.05 and P < 0.01). The positive expression rate of Caspase-3 in prostatic cancer tissue was 25.58% (10/43), which obviously lower than that [58.82% (10/17)] in prostatic hyperplasia tissue (P < 0.01).
Positive staining of Livin, Survivin and Caspase-3 presented yellow-brown color. Livin, Survivin and Caspase-3 were positively expressed in cytoplasm and nucleus (Figures 1, 2, 3, 4, 5 and 6).
Figure 1.
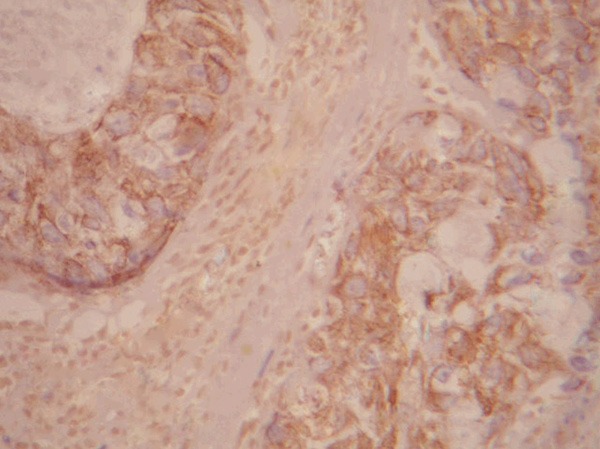
Positive expression of livin in prostatic cancer tissue (×400).
Figure 2.
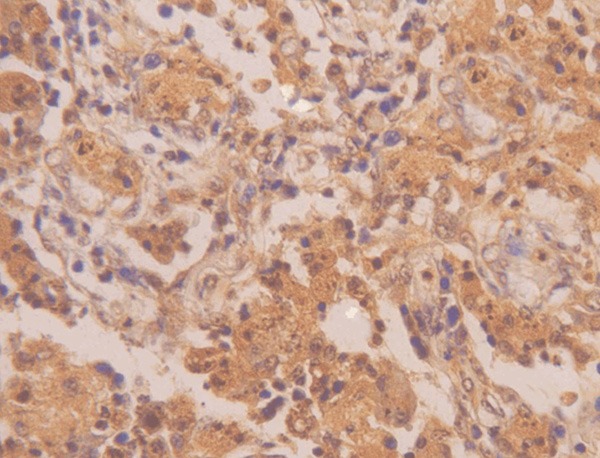
Positive expression of livin in prostatic hyperplasia tissue (×400).
Figure 3.
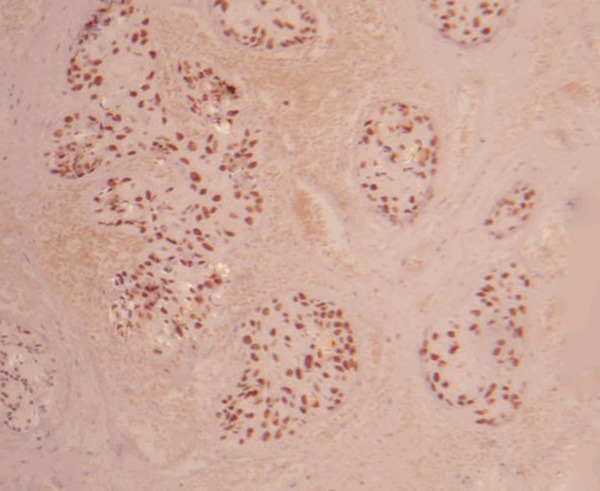
Positive expression of survivin in prostatic cancer tissue (×400).
Figure 4.
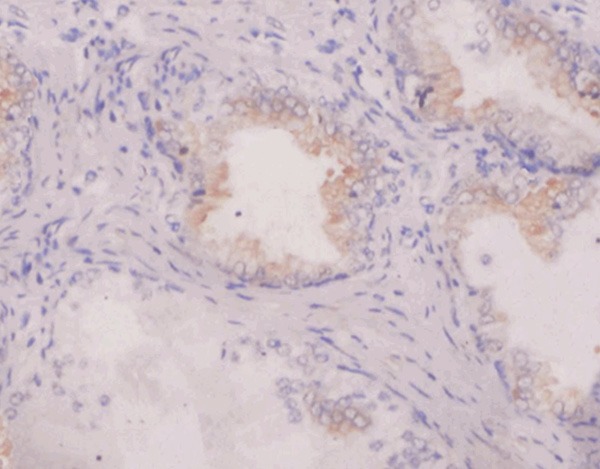
Positive expression of survivin in prostatic hyperplasia tissue (×400).
Figure 5.
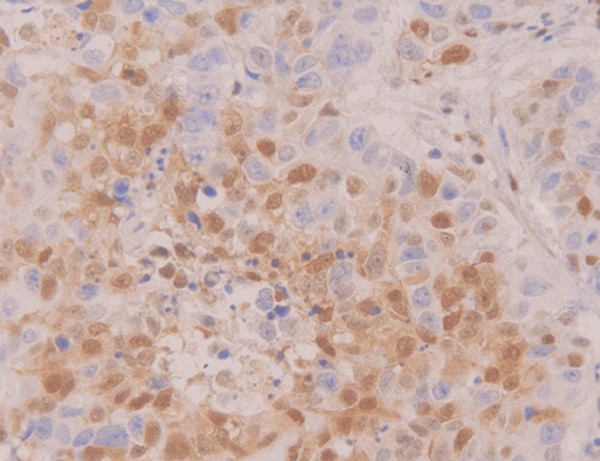
Positive expression of caspase-3 in prostatic cancer tissue (×400).
Figure 6.
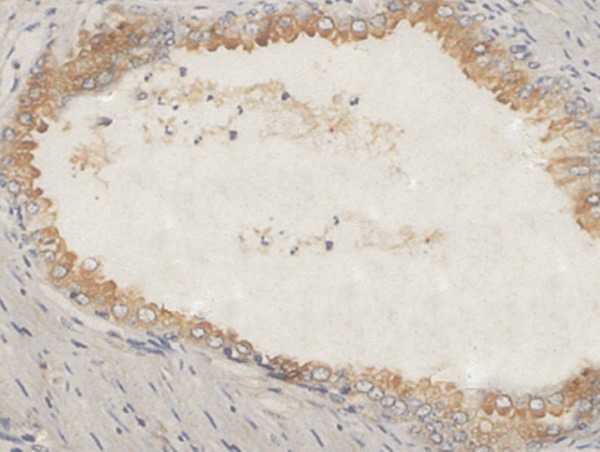
Positive expression of caspase-3 in prostatic hyperplasia tissue (×400).
Relationship among livin, survivin, caspase-3 expressions and clinicopathological features of prostatic cancer
The expression of both Livin and Survivin in prostatic cancer tissue was related to pathological grading (Gleason scores) (X 2 = 14.000, P = 0.001), but not related to preoperative PSA, clinical stages and distant metastasis is (P > 0.05). Capsase-3 expression in prostatic cancer tissue was related to pathological grading (Gleason scores) (X 2 = 14.000, P = 0.001) and clinical stages (X 2 = 4.896, P = 0.027), but not related to preoperative PSA and whether distant metastasis is or not (P > 0.05, Table 1).
Table 1.
Relationship among livin, survivin, caspase-3 expressions and clinicopathological features [n (%)]
| n | Livin | P | Survivin | P | Caspase-3 | P | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Positive | Negative | Positive | Negative | Positive | Negative | |||||
| PSA | ||||||||||
| < 4 ng/mL | 8 (18.60) | 8 (100.00) | 0 (0.00) | 0.536 | 7 (87.50) | 1 (12.50) | 0.477 | 1 (12.50) | 7 (87.50) | 0.317 |
| 4~10 ng/mL | 16 (37.21) | 14 (87.50) | 2 (12.50) | 12 (75.00) | 4 (25.00) | 5 (31.25) | 11 (68.75) | |||
| > 10 ng/mL | 19 (44.19) | 18 (94.74) | 1 (5.26) | 17 (89.47) | 2 (10.53) | 4 (21.05) | 15 (78.95) | |||
| Clinical staging | ||||||||||
| I+II | 19 (44.19) | 18 (94.74) | 1 (2.33) | 1.000 | 15 (78.95) | 4 (21.05) | 0.451 | 7 (36.84) | 12 (63.16) | 0.027 |
| III+IV | 24 (55.81) | 22 (91.67) | 2 (8.33) | 21 (87.50) | 3 (12.50) | 3 (12.5) | 21 (87.50) | |||
| Pathological grading | ||||||||||
| G1 (2~4) | 4 (9.30) | 4 (100.00) | 0 (0.00) | 0.001 | 4 (100.00) | 0 (0.00) | 0.001 | 0 (0.00) | 4 (100.00) | 0.001 |
| G2 (5~6) | 10 (23.26) | 9 (90.00) | 1 (10.00) | 9 (90.00) | 1 (10.00) | 4 (40.00) | 6 (60.00) | |||
| G3 (7~10) | 29 (67.44) | 27 (93.10) | 2 (6.90) | 23 (79.31) | 6 (20.69) | 6 (20.69) | 23 (79.31) | |||
| Distant metastasis | ||||||||||
| Yes | 13 (30.23) | 13 (100.00) | 0 (0.00) | 0.237 | 11 (84.62) | 2 (15.38) | 0.917 | 3 (23.08) | 10 (76.92) | 0.985 |
| No | 30 (69.77) | 27 (90.00) | 3 (10.00) | 25 (83.33) | 5 (16.67) | 7 (23.33) | 23 (76.67) | |||
Pairwise comparison between livin and survivin, livin and caspase-3, survivin and caspase-3 in prostatic cancer
In prostatic cancer tissue, Livin expression had no correlation with Survivin expression (r = 0.127, P = 0.419 > 0.05), but negatively correlated with Caspase-3 expression (r = -0.497, P = 0.001). Survivin expression was negatively correlated with Caspase-3 expression (r = -0.354, P = 0.020).
Discussion
It is believed that the occurrence and development of tumors are molecular events, which are involving in multi-stage process at the multi-gene and multi-molecular levels. The occurrence of tumors, on one hand, is caused by the indefinite cell proliferation which is due to the disorder of cell cycle and out of control in cell division, on the other hand, it is the result of inadequate apoptosis. When the induction of cell apoptosis happens, apoptotic genes are activated by signal transduction pathway, which leading to programmed cell death. Once the apoptotic process is suppressed, cells of gene mutation survive and proliferate, which thus result in tumor genesis. In recent years, IAPs, as a kind of important anti-apoptosis factor, increasingly catch the attention of the scholars and researchers. IAPs family is a kind of highly conservative anti-apoptotic adjustment factors, with amino terminal containing baculovirus IAP repeat (BIR) domain which consists of one or up to three highly conservative amino acid residues. IAPs play a role of anti-apoptosis by directly and indirectly inhibiting members of caspases family [6,7].
Livin gene is located on chromosome 20q13.3, with the length of 4.6 kb and includes 7 exons and 6 intron. At present, relevant studies were found in the following anti-apoptosis pathways of Livin: (1) It bindings to Caspase-3, Caspase-7 and Caspase-9 directly/indirectly by regulating Caspase signal pathway to inhibit the apoptosis [8]; (2) The activation of TAK1/JNK1 signal transduction pathway inhibits the apoptosis [9]; (3) The interaction of Livin with Smac/DLABLO regulates apoptosis [10]. Livin is not expressed or low expressed in most of terminal differentiated tissues of normal adults, but highly specific overly expressed in certain malignant tumors, such as esophageal cancer, gastric cancer, liver cancer, intestinal cancer, prostatic cancer, bladder cancer, renal carcinoma, lymphadenoma, neuroblastoma and leukemia [11-17]. Survivin was firstly separated in hybridization of effector cell protease receptor-1 (EPR-1) cDNA in human genome by Ambrosini G et al in 1997. Survivin gene is located on chromosome 20q13.3, which contains 4 exons and 3 introns and a BIR domain without RING. Survivin plays a role of apoptosis by directly and indirectly binding to Caspase-3 and Caspase-7, and participates in regulating effect of cell proliferation, division and cycle and angiogenesis effect. Survivin is expressed during embryonic development, but not in terminal differentiated tissues. However, it is highly expressed in most transformed cells and human tumors. Another study was found that Caspase-3 showed a trend of decrease gradually in non-hyperplasia tissue, adenoma and cancer tissues, which indicated that the abnormal expression of Caspase-3 was closely associated with the occurrence and development of tumors [18].
In this study, the positive expression rate of Livin in prostatic cancer and prostatic hyperplasia tissues was 93.02% (40/43) and 64.70% (11/17), respectively, and the difference between them was significant. The positive expression rate of Survivin in prostatic cancer and prostatic hyperplasia tissues was 83.72% (36/43) and 35.29% (6/17), respectively, and there was significant difference between them. The positive expression rate of Caspase-3 in prostatic cancer tissue was 25.58% (10/43), obviously lower than that 58.82% (10/17) in prostatic hyperplasia tissue. Moreover, Livin expression had no correlation with Survivin expression, but negatively correlated with Caspase-3 expression. Survivin expression was negatively correlated with Caspase-3 expression. Those results indicate that Livin and Survivin which are highly expressed in prostatic cancer tissue play an important role in tumorgenesis of prostatic cancer probably by inhibition of apoptosis. Moreover, low-expresseCaspase-3 in prostatic cancer may inhibit apoptosis and promote the tumorgenesis and development of prostatic cancer.
Livin and Survivin belong to the members of IAPs family and they are resistant to apoptosis by directly acting on Caspase-3 and Capspase-7. However, they are not completely the same in such function. Whether the mechanisms of both are correlated, it is still unclear. Livin and surviving are highly expressed in prostatic cancer, more obviously than those in prostatic hyperplasia, which indicates there may be a different mechanism for roles of Livin and Survivin in tumorgenesis and development of prostatic cancer. In conclusion, Livin, Survivin and Caspase-3 are closely related to the occurrence and development of prostatic cancer and are expected to become new targets for diagnosis and treatment of prostatic cancer.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ American Cancer Society. Cancer statistics 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman KE, Chen MH, Moran BJ, Braccioforte MH, Dosoretz D, Salenius S, Katin MJ, Ross R, D’Amico AV. Prostate cancer-specific mortality and the extent of therapy in healthy elderly men with high-risk prostate cancer. Cancer. 2010;116:2590–2595. doi: 10.1002/cncr.24974. [DOI] [PubMed] [Google Scholar]
- 3.Pan H, Yin C, Van Dyke T. Apoptosis and cancer mechanisms. Cancer Surv. 1997;29:305–327. [PubMed] [Google Scholar]
- 4.Vaux DL, Korsmeyer SJ. Cell death in development. Cell. 1999;96:245–254. doi: 10.1016/s0092-8674(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 5.Chang H, Schimmer AD. Livin/melanoma inhibitor of apoptosis protein as a potential therapeutic target for the treatment of malignancy. Mol Cancer Ther. 2007;6:24–30. doi: 10.1158/1535-7163.MCT-06-0443. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Li YS. Expression and significance of Livin in prostate cancer tissue. Fujian Med J. 2013;35:80–83. [Google Scholar]
- 7.Chao XJ, Zhao L, Wang ZM, Song XX, Du LY. Expression of Livin and Survivin in gastric carcinoma and its clinical significance. Hebei Med J. 2013;35:327–329. [Google Scholar]
- 8.Huerta S, Heinzerling JH, Anguiano-Hernandez YM, Huerta-Yepez S, Lin J, Chen D, Bonavida B, Livingston EH. Modification of gene products involved in resistance to apoptosis in metastatic colon cancer cells: roles of Fas, Apaf-1, NFkappaB, IAPs, Smac/DIABLO, and AIF. J Surg Res. 2007;142:184–194. doi: 10.1016/j.jss.2006.12.551. [DOI] [PubMed] [Google Scholar]
- 9.Maas C, Verbrugge I, de Vries E, Savich G, van de Kooij LW, Tait SW, Borst J. Smac/DIABLO release from mitochondria and XIAP inhibition are essential to limit clonogenicity of Type I tumor cells after TRAIL receptor stimulation. Cell Death Differ. 2010;17:1613–1623. doi: 10.1038/cdd.2010.39. [DOI] [PubMed] [Google Scholar]
- 10.Chen YS, Li HR, Lin M, Chen G, Xie BS, Xu NL, Lin LF. Livin abrogates apoptosis of SPC-A1 cell by regulating JNKI signaling pathway. Mol Biol Rep. 2010;37:2241–2247. doi: 10.1007/s11033-009-9711-3. [DOI] [PubMed] [Google Scholar]
- 11.Dubrez-Daloz L, Dupoux A, Cartier J. IAPs: more than just inhibitors of apoptosis proteins. Cell Cycle. 2008;7:1036–1046. doi: 10.4161/cc.7.8.5783. [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Ren GS, Li F, Sun SQ. Expression of livin and vascular endothelial growth factor in different clinical stages of human esophageal carcinoma. World J Gastroenterol. 2008;14:5749–5754. doi: 10.3748/wjg.14.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Augello C, Caruso L, Maggioni M, Donadon M, Montorsi M, Santambrogio R, Torzilli G, Vaira V, Pellegrini C, Roncalli M, Coggi G, Bosari S. Inhibitors of apoptosis proteins (IAPs) expression and their prognostic significance in hepatocellular carcinoma. BMC Cancer. 2009;9:125. doi: 10.1186/1471-2407-9-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang YL, Lin SR, Chen JS, Lin SW, Yu SL, Chen HY, Yen CT, Lin CY, Lin JF, Lin KH, Jou ST, Hu CY, Chang SK, Lu MY, Chang HH, Chang WH, Lin KS, Lin DT. Expression and prognostic significance of the apoptotic genes BCL2L13, Livin, and CASP8AP2 in childhood acute lymphoblastic leukemia. Leuk Res. 2010;34:18–23. doi: 10.1016/j.leukres.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 15.Ye L, Song X, Li S, Yang D, Zhang J, Che X, Chen X, Wang J, Zhang Z. Livin-alpha promotes cell proliferation by regulating G1-S cell cycle transition in prostate cancer. Prostate. 2011;71:42–51. doi: 10.1002/pros.21220. [DOI] [PubMed] [Google Scholar]
- 16.Cheng T, Zhang JG, Cheng YH, Gao ZW, Ren XQ. Relationship between PTEN and Livin expression and malignancy of renal cell carcinomas. Asian Pac J Cancer Prev. 2012;13:2681–2685. doi: 10.7314/apjcp.2012.13.6.2681. [DOI] [PubMed] [Google Scholar]
- 17.Ibrahim L, Aladle D, Mansour A, Hammad A, Al Wakeel AA, Abd El-Hameed SA. Expression and prognostic significance of livin/BIRC7 in childhood acute lymphoblastic leukemia. Med Oncol. 2014;31:941. doi: 10.1007/s12032-014-0941-4. [DOI] [PubMed] [Google Scholar]
- 18.Hoshi T, Sasano H, Kato K, Yabuki N, Ohara S, Konno R, Asaki S, Toyota T, Tateno H, Nagura H. Immunohistochemistry of Caspase3/CPP32 in human stomach and its correlation with cell proliferation and apoptosis. Anticancer Res. 1998;18:4347–4353. [PubMed] [Google Scholar]


