Abstract
Primary splenic angiosarcoma is an extremely rare and aggressive neoplasm. The prognosis of this disease is dismal, and the mean survival is less than 6 months after the diagnosis. This neoplasm typically presents with abdominal pain, splenomegaly, weight loss, and spontaneous splenic rupture. Fever is a very rare presentation of splenic angiosarcoma. Here we report the case of a 64-year-old man who presented with fever and anemia. A laparoscopic splenectomy was performed and revealed splenic angiosarcoma. The postoperative course was uneventful and the patient received 5 cycles of adjuvant chemotherapy with ifosfamide plus epirubicin. He remained disease free at 9 months after surgery. This is the first case of splenic angiosarcoma with fever as the initial presentation that was treated with laparoscopic splenectomy to be reported in the English literature.
Keywords: Splenic angiosarcoma, fever, anemia, splenectomy, disease free
Introduction
Primary splenic angiosarcoma is a very rare and aggressive neoplasm with a high metastasis rate and a poor prognosis. Primary splenic angiosarcoma was first described by T. Langerhans in 1879. Only approximately 200 cases have been reported thus far. The outcome of splenic angiosarcoma is typically dismal; only 20% of patients survive for 6 months [1]. The clinical presentation of this disease is variable. Abdominal pain, abdominal masses, weight loss, and spontaneous splenic ruptures are common symptoms. Herein, we report a case of primary splenic angiosarcoma that presented with fever. To our knowledge, this is the first reported case of splenic angiosarcoma to undergo laparoscopic splenectomy and the second reported case in which fever was the first manifestation [2]. The study was reviewed and approved by the Union Hospital Institutional Review Board. Written informed consent prior to study enrollment was obtained from the patient.
Case report
A 64-year-old man was initially admitted to our hospital with a fever peaked at 40.5°C and vomiting for 2 weeks. His past medical history was notable for hypertension and chronic bronchitis. His vitality was limited; he had anorexia and had lost 4 kg of weight during the 2 weeks prior to presentation. The patient’s urine volume was below normal. Physical examination revealed a palpable spleen approximately 2 cm below the left costal margin and no hepatomegaly. The complete blood cell count values were as follows: Hct 25.7%, RBC 2.97 × 1012/L, Hb 83 g/L, platelets 274 × 109/L, WBC 6.93 × 109/L (neutrophils 74.7%, lymphocytes 14.6%, and monocytes 8.7%). The results of liver function tests and the coagulation profiles were within the normal ranges. The C-reactive protein level (38.9 mg/L, normal value < 8.0) and erythrocyte sedimentation rate (83 mm/h, normal value < 15) were both elevated. Tumor markers, including CEA, CA19-9, CA15-3, CA-125, AFP, ferritin, SCC and PSA were measured, and only ferritin was found to be abnormal (371.6 μg/l). ECG revealed a sinus rhythm and occasional premature atrial contraction. A chest X-ray revealed chronic bronchitis and pleural disease of the right lower lung field. Abdominal ultrasonography revealed an enlarged spleen filled with irregular nodules. These findings were further confirmed with an abdominal enhanced computed tomography scan (Figure 1). A bone marrow biopsy revealed left-shift neutrophilia suggestive of a leukemoid reaction.
Figure 1.

Abdominal CT scan showing multiple irregular heterogeneous masses in the spleen.
The patient was diagnosed with an occupied lesion of the spleen prior to surgery and underwent laparoscopic splenectomy under general anesthesia on May 22, 2014. He was positioned in the supine position and a five-port method was employed. The splenic artery was dissociated and ligated with a Hem-o-lok clip to diminish the risk of severe hemorrhage. The ligaments surrounding the spleen were divided using a harmonic scalpel. The splenic hilum was sectioned with a linear laparoscopic vascular stapler. During the procedure, the patient did not receive a blood transfusion. The operating time was 80 minutes. The resected spleen was placed in a retrieval bag and removed through an enlarged port site incision.
After the operation, the spleen weighed 460 g, measured 13.5 cm × 10.5 cm × 7 cm, and exhibited a nodular appearance and areas of necrosis and hemorrhagic tissue (Figure 2). The final pathological diagnosis was angiosarcoma originating from the spleen (Figure 3). Immunohistochemical staining was positive for CD31 (Figure 4), CD34 (Figure 5), and factor VIII (Figure 6) and negative for PCK, EMA, S-100, CD30, LCA, AFP, SMA and Desmin. The Ki-67 proliferation index was approximately 20%. The patient exhibited an uneventful post-operative course and was discharged 10 days after the operation. Later the patient was transferred to the oncology department and subsequently received 5 cycles of post-operative adjuvant chemotherapy with ifosfamide (1800 mg/m2) plus epirubicin (60 mg/m2). He was followed up for 9 months and no relapse or metastasis were discovered.
Figure 2.
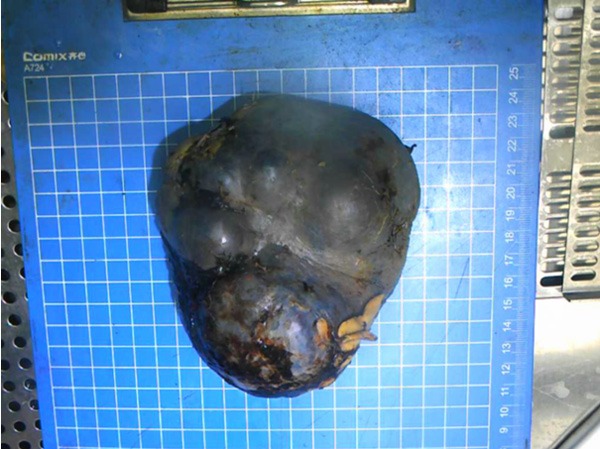
Excised specimen of the enlarged spleen with a nodular appearance.
Figure 3.

Microscopic view of the splenic angiosarcoma. The neoplasm was primarily composed of spindle cells that had replaced the normal red and white pulp in the spleen. Atypical vascular spaces lined by hypertrophied tumor cells were notable (H&E × 100).
Figure 4.
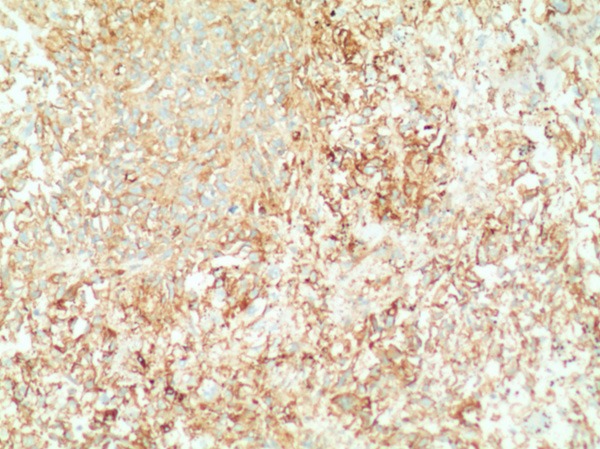
Immunohistochemical staining of the specimen revealing strongly diffuse positivity for CD31 (× 100).
Figure 5.

Specimen exhibiting focal positivity for CD34 (× 100).
Figure 6.

Specimen exhibiting diffuse positivity for factor VIII (× 100).
Discussion
Primary splenic angiosarcoma is an extremely rare and aggressive malignant neoplasm that arises from splenic vascular endothelium and has an annual incidence of 0.14-0.23 cases per million persons [1,3-5]. Since Langhans reported the first case of splenic angiosarcoma in 1879, only approximately 200 cases have been reported in the literature worldwide, and these reports are primarily case reports [2,6,7]. This neoplasm exhibits a slight male predominance and can develop at any age. The mean patient age at presentation is 59 years old [1].
Currently, the pathogenesis of splenic angiosarcoma remains unknown. Some authors have hypothesized that splenic angiosarcomas develop from previously existing benign counterparts, such as hemangiomas and hemangioendotheliomas [8-10]. However, our patient had no such previously existing pathologies. Similarly, no causative factors for splenic angiosarcomas were identified in two large case studies. The clinical presentation of this disease is variable. Left upper abdominal pain is the predominant presenting symptom and occurs in more than 80% of cases. Thirteen to thirty-two percent of patients present with acute abdominal pain due to spontaneous splenic rupture. Other complaints include fatigue, anorexia, weight loss, dyspnea and gastrointestinal bleeding [1,11-13].
Laboratory findings include cytopenia, leukocytosis, thrombocytosis and an elevated erythrocyte sedimentation rate. Thus far, tumor markers have been found to remain normal in the majority of patients. Although our patient exhibited elevated ferritin, the sign has no specificity. Imaging examinations are helpful for establishing diagnoses of splenomegaly and splenic neoplasm, although they lack diagnostic accuracy. The most common ultrasound findings are splenomegaly with one or more ill-defined solid and/or cystic masses. CT scans may reveal an enlarged spleen with hypo- or hyper-attenuating areas on nonenhanced scans. Areas of hyper-attenuation are likely to reflect acute hemorrhage. Enhanced CTs reveal splenic masses with varying degrees of enhancement. Liver metastasis and lymph node enlargement may also be detected [14]. MRI results depend on the extents of hemorrhage and necrosis within the tumor. Areas that exhibit hyper-attenuation are related to acute hemorrhage or tumor necrosis. Areas that exhibit hypo-attenuation likely reflect chronic hemorrhage or fibrosis within the tumor [14-16].
Patients with splenic angiosarcomas exhibit irregular spleen enlargement. The cut surface of the tumor is typically described as nodular, necrotic, and hemorrhagic. Pathological examination is the gold standard for the diagnosis of splenic angiosarcoma. Preoperative biopsies are contraindicated in splenic angiosarcoma due to the high risks of hemorrhage and dissemination [17]. Thus, a definitive diagnosis of splenic angiosarcoma is generally possible only after splenectomy. Microscopically, splenic angiosarcomas consist of disorganized anastomosing vascular channels lined with atypical endothelial cells with significantly irregular hyperchromatic nuclei. Immunohistochemically, the tumor cells exhibit a strong positivity for at least one of the endothelial markers (CD31, CD34, and factor VIII) and may also display histiocytic differentiation markers, such as lysozyme and/or CD68 [3,18].
Splenectomy remains the mainstay of treatment for splenic angiosarcoma. The selection of the surgical approach depends on the patients’ age, underlying disease, tumor size, site, and metastasis of the tumor. Early diagnosis via splenectomy prior to rupture can result in a more favorable survival rate. Montemayor et al. [19] found that patients with splenic angiosarcoma exhibit longer survival time if splenectomies are performed prior to rupture rather than after rupture (14.4 vs. 4.4 months, respectively). Therefore, the prevention of intraoperative tumor rupture is crucial. In our case, we utilized an incision protection sleeve, and the specimen was placed into a specimen retrieval bag to prevent peritoneal tumor dissemination and wound seeding during the operation. Laparoscopic splenectomy was first reported in 1991 [20]. Since that time, several studies have demonstrated the advantages of laparoscopic splenectomy over the open approach, which include decreased surgical trauma and blood loss, shorter hospital stays, faster recoveries, and better cosmetic outcomes [21-24]. Laparoscopic splenectomy has gradually become the gold standard for the removal of the spleen even in malignant splenic disorders. This case report highlights the value of laparoscopic splenectomy as an effective and safe procedure for diagnosis and treatment. Nevertheless, due to the rarity of splenic angiosarcoma, there is no standard chemotherapy regimen. Our patient received chemotherapy with epirubicin and ifosfamide, which is often used as the first-line therapy for soft tissue sarcomas [25].
The prognosis of splenic angiosarcoma is typically dismal; only 20% of patients survive for 6 months, and this low survival rate is primarily a consequence of the high incidence of metastasis. In previous studies, metastases occurred in 69%-100% of cases [1,3,4]. Neuhauser et al. [1] reported a study of 28 patients with primary splenic angiosarcomas. In this study, the common metastatic sites were the liver (n=24), lungs (n=21), lymph nodes (n=15), bones (n=12), soft tissues (n=7), gastrointestinal tract (n=6), brain (n=6), and adrenal glands (n=4). Furthermore, only 2 patients were alive at the final follow-up; one exhibited evidence of disease at 8 years, and the other exhibit no evidence of disease at 10 years.
Conclusion
In summary, primary splenic angiosarcoma is a very rare neoplasm with a high rate of metastasis and a poor prognosis. Primary splenic angiosarcomas may cause pyrexia of unknown origin, and this symptom should be given sufficient attention. Primary splenic angiosarcoma is very difficult to diagnose preoperatively, and a definitive diagnosis requires splenectomy which is both diagnostic and therapeutic. Ultimately, early detection and prompt splenectomy prior to splenic rupture followed by chemotherapy may be the most effective treatment regimen.
Disclosure of conflict of interest
None.
References
- 1.Neuhauser TS, Derringer GA, Thompson LD, Fanburg-Smith JC, Miettinen M, Saaristo A, Abbondanzo SL. Splenic angiosarcoma: a clinicopathologic and immunophenotypic study of 28 cases. Mod Pathol. 2000;13:978–987. doi: 10.1038/modpathol.3880178. [DOI] [PubMed] [Google Scholar]
- 2.Shukla M, Basu S, Shukla VK, Kumar M. Fever, anemia, and splenomegaly: A rare presentation of splenic angiosarcoma. Indian J Med Paediatr Oncol. 2011;32:230–232. doi: 10.4103/0971-5851.95148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falk S, Krishnan J, Meis JM. Primary angiosarcoma of the spleen. A clinicopathologic study of 40 cases. Am J Surg Pathol. 1993;17:959–970. doi: 10.1097/00000478-199310000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Kren L, Kaur P, Goncharuk VN, Dolezel Z, Krenova Z. Primary angiosarcoma of the spleen in a child. Med Pediatr Oncol. 2003;40:411–412. doi: 10.1002/mpo.10140. [DOI] [PubMed] [Google Scholar]
- 5.Hamid KS, Rodriguez JA, Lairmore TC. Primary splenic angiosarcoma. JSLS. 2010;14:431–435. doi: 10.4293/108680810X12924466006521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manouras A, Giannopoulos P, Toufektzian L, Markogiannakis H, Lagoudianakis EE, Papadima A, Papanikolaou D, Filis K, Kekis P. Splenic rupture as the presenting manifestation of primary splenic angiosarcoma in a teenage woman: a case report. J Med Case Rep. 2008;2:133. doi: 10.1186/1752-1947-2-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hai SA, Genato R, Gressel I, Khan P. Primary splenic angiosarcoma: case report and literature review. J Natl Med Assoc. 2000;92:143–146. [PMC free article] [PubMed] [Google Scholar]
- 8.Alt B, Hafez GR, Trigg M, Shahidi NT, Gilbert EF. Angiosarcoma of the liver and spleen in an infant. Pediatr Pathol. 1985;4:331–339. doi: 10.3109/15513818509026906. [DOI] [PubMed] [Google Scholar]
- 9.Sordillo EM, Sordillo PP, Hajdu SI. Primary hemangiosarcoma of the spleen: report of four cases. Med Pediatr Oncol. 1981;9:319–324. doi: 10.1002/mpo.2950090403. [DOI] [PubMed] [Google Scholar]
- 10.Plotnik AN, Schweder P, Tsui A, Kavar B. Splenic angiosarcoma metastasis to the brain. J Clin Neurosci. 2008;15:927–929. doi: 10.1016/j.jocn.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 11.Hsu JT, Chen HM, Lin CY, Yeh CN, Hwang TL, Jan YY, Chen MF. Primary angiosarcoma of the spleen. J Surg Oncol. 2005;92:312–316. doi: 10.1002/jso.20419. [DOI] [PubMed] [Google Scholar]
- 12.Valbuena JR, Levenback C, Mansfield P, Liu J. Angiosarcoma of the spleen clinically presenting as metastatic ovarian cancer. A case report and review of the literature. Ann Diagn Pathol. 2005;9:289–292. doi: 10.1016/j.anndiagpath.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Liu Z, Du X, Li H, Wang Z, Shen Z, Yao Y, Zhao H. Primary splenic angiosarcoma. Vasa. 2012;41:57–62. doi: 10.1024/0301-1526/a000164. [DOI] [PubMed] [Google Scholar]
- 14.Thompson WM, Levy AD, Aguilera NS, Gorospe L, Abbott RM. Angiosarcoma of the spleen: imaging characteristics in 12 patients. Radiology. 2005;235:106–115. doi: 10.1148/radiol.2351040308. [DOI] [PubMed] [Google Scholar]
- 15.Ha HK, Kim HH, Kim BK, Han JK, Choi BI. Primary angiosarcoma of the spleen. CT and MR imaging. Acta Radiol. 1994;35:455–458. [PubMed] [Google Scholar]
- 16.Karakas HM, Demir M, Ozyilmaz F, Cakir B. Primary angiosarcoma of the spleen: in vivo and in vitro MRI findings. Clin Imaging. 2001;25:192–196. doi: 10.1016/s0899-7071(01)00286-8. [DOI] [PubMed] [Google Scholar]
- 17.Delacruz V, Jorda M, Gomez-Fernandez C, Benedetto P, Ganjei P. Fine-needle aspiration diagnosis of angiosarcoma of the spleen: a case report and review of the literature. Arch Pathol Lab Med. 2005;129:1054–1056. doi: 10.5858/2005-129-1054-FADOAO. [DOI] [PubMed] [Google Scholar]
- 18.Young RJ, Brown NJ, Reed MW, Hughes D, Woll PJ. Angiosarcoma. Lancet Oncol. 2010;11:983–991. doi: 10.1016/S1470-2045(10)70023-1. [DOI] [PubMed] [Google Scholar]
- 19.Montemayor P, Caggiano V. Primary hemangiosarcoma of the spleen associated with leukocytosis and abnormal spleen scan. Int Surg. 1980;65:369–373. [PubMed] [Google Scholar]
- 20.Delaitre B, Maignien B. [Splenectomy by the laparoscopic approach. Report of a case] Presse Med. 1991;20:2263. [PubMed] [Google Scholar]
- 21.Targarona EM, Espert JJ, Balague C, Piulachs J, Artigas V, Trias M. Splenomegaly should not be considered a contraindication for laparoscopic splenectomy. Ann Surg. 1998;228:35–39. doi: 10.1097/00000658-199807000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terrosu G, Donini A, Baccarani U, Vianello V, Anania G, Zala F, Pasqualucci A, Bresadola F. Laparoscopic versus open splenectomy in the management of splenomegaly: our preliminary experience. Surgery. 1998;124:839–843. [PubMed] [Google Scholar]
- 23.Smith L, Luna G, Merg AR, McNevin MS, Moore MR, Bax TW. Laparoscopic splenectomy for treatment of splenomegaly. Am J Surg. 2004;187:618–620. doi: 10.1016/j.amjsurg.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Silecchia G, Boru CE, Fantini A, Raparelli L, Greco F, Rizzello M, Pecchia A, Fabiano P, Basso N. Laparoscopic splenectomy in the management of benign and malignant hematologic diseases. JSLS. 2006;10:199–205. [PMC free article] [PubMed] [Google Scholar]
- 25.Rupolo M, Berretta M, Buonadonna A, Stefanovski P, Bearz A, Bertola G, Canzonieri V, Morassut S, Frustaci S. Metastatic angiosarcoma of the spleen. A case report and treatment approach. Tumori. 2001;87:439–443. doi: 10.1177/030089160108700617. [DOI] [PubMed] [Google Scholar]


