Abstract
MicroRNA (miRNA, miR)-155 is the most promising pro-inflammatory miRNA molecule. Lipopolysaccharide (LPS) and oxidized low-density lipoprotein (oxLDL) are the most well-known foreign antigens, initiating immune responses against infection and the development of atherosclerosis (AS), respectively. To explore whether miR-155 is involved in regulating LPS- and oxLDL-initiated inflammations, we investigated the level of miR-155 in both LPS- and oxLDL-treated RAW264.7 cells, assessed whether miR-155 induce morphologic changes of the cells and how did it regulate the production of surface markers and cytokines. The results showed that the level of miR-155 was significantly increased by LPS and was modestly increased by oxLDL. Moreover, RAW264.7 cells displayed morphological transformations from macrophage-like cells into DC-like cells when miR-155 was over-expressed. Furthermore, the gain- and loss-of-function studies demonstrated that miR-155 induced the expression of the surface markers (including MHC-II, MHC-I, CD86, and CD83) and pro-inflammatory cytokines (including interleukin (IL)-12, IL-6, and IL-1b) in both LPS- and oxLDL-treated RAW264.7 cells. Additionally, miR-155 induced the expression of CD36 in oxLDL-treated RAW264.7 cells. In conclusion, up-regulated miR-155 is able to induce morphological and phenotypic changes, and the expression of pro-inflammatory cytokines in both LPS- and oxLDL-treated RAW264.7 cells. Therefore, our study suggests that miR-155 is one important regulator involved in enhancing both LPS- and oxLDL-initiated inflammations, which is critical for the progression of immune responses as well as for the development of AS.
Keywords: MicroRNA-155, RAW264.7 cell, DC cell, morphology, surface molecule, cytokine
Introduction
The innate immune system is the first-line of defense against pathogens and is also the basis of the adaptive immune system [1]. Dendritic cells (DCs) are critical members of the innate immune system and function as the bridge that links innate and adaptive immunity. In innate immune response, immature DCs (iDCs) respond to “danger” signals by uptaking the foreign antigens and generating protective cytokines and chemokines [2]. During this process, iDCs convert to mature DCs (mDCs), which are characterized by impaired ability to uptake and process antigens but an enhanced antigen-presenting capacity [3]. As the key antigen-presenting cells (APCs), mDCs initiate adaptive immune responses by presenting antigens to naïve T cells [3]. In addition to their critical roles in immune responses, DCs are also associated with the development of atherosclerosis (AS). They can present atherosclerotic plaque specific antigens to lymphocytes, secrete cytokines and chemokines to maintain local inflammation, and also may differentiate into foam cells during the development of AS [4].
Monocytes may differentiate to macrophages or to DCs, counting on the different cytokines present [5,6]. And in certain circumstances, even macrophages may transform to DCs [7]. RAW264.7 cells, initially derived from Abelson leukemia virus-infected Balb/c mice, constitute a murine macrophage cell line [8]. Two recent studies have demonstrated that both lipopolysaccharide (LPS) and oxidized low-density lipoprotein (oxLDL) were able to induce differentiation of RAW264.7 macrophages to DC-like cells, which showed dramatic functional changes, including increased surface antigen expression and pro-inflammatory cytokine production [9,10]. But the factors that drive these changes are still unclear.
MicroRNAs (miRNAs, miRs) are a group of short, noncoding RNAs, which are post-transcriptional regulators linked to most physiological and pathological situations [11]. It has been demonstrated that miR-155 is tightly associated with the immune system at multiple levels [12]. It is generally believed to be a pro-inflammatory molecule. During CD4+T cell activation, miR-155 drives naïve CD4+T cells to skew toward Th1 and Th17 cells, which are both pro-inflammatory CD4+T cell subsets [13,14]. While in the innate immune system, miR-155 can be induced by toll-like receptor ligands and some cytokines, like LPS and tumor necrosis factor-a (TNF-α), for example [15,16]. Up-regulation of miR-155 also seems to be a sign of DCs maturation, and it is also essential for their functions [17]. But we still do not known how miR-155 regulates RAW264.7 cells differentiation and function. In this study, we observed that miR-155 was up-regulated in both LPS- and oxLDL-treated RAW264.7 cells. Moreover, up-regulated miR-155 induced morphologic and phenotypic changes, and the expression of pro-inflammatory cytokines, which are necessary for DCs maturation and their ability to activate T cells. Therefore, our study demonstrated that miR-155 is able to promote a differentiation of RAW264.7 cells into DC-like cells.
Materials and methods
Culture, stimulation, transfection, and morphological observation of RAW264.7 cells
RAW264.7 cells, which were obtained from the American Type Culture Collection (ATCC, Rockville, Md), were cultured in DMEM medium +10% fetal bovine serum (FBS) (Gibco, USA), at 37°C, 5% of CO2 in a humidified atmosphere [9]. Cells were seeded into 24-well plates (Corning, USA) at a density of 4×104 cells per 0.5 ml medium for 24 hours and at the density of 60% before further treatments.
For detection the expression of miR-155, cells were activated with different concentrations of LPS (Gibco, USA) and oxLDL (Xiesheng Biotechnologies, Beijing, China). For the gain- and loss-of-function study, the Attractene Transfection Reagent (Qiagen, Valencia, CA) was used to transfect the oligonucleotides (including pre-miR-ctrl, pre-miR-155, anti-miR-ctrl, and anti-miR-155) into RAW264.7 cells. First, 50 pmole oligonucleotides and 1.5 μl Attractene Transfection Reagent dissolved in 60 μl OMEM medium (Gibco, USA) without serum, proteins, or antibiotics, which were then mixed gently and incubated for 13 min at the room temperature. During this 13 min, 500 μl fresh DMEM medium (containing FBS and antibiotics) was given to the cells. Then, the transfection complexes were added to the cells. Six hours after transfection, cells were stimulated with LPS (1 μg/ml) or oxLDL (10 μg/ml) for indicated times. Twenty-four hours later, the morphology of RAW264.7 cells was observed under a microscope (400×).
Flow cytometry
Harvested RAW264.7 cells were incubated with fluorescein-conjugated antibodies for 30 min, washed with washing buffer, then resuspended in 200 μl washing buffer and analyzed by BD FACSCaliburTM Flow Cytometer (San Jose, CA, USA). The antibodies were obtained from ebioscience (San Diego, CA, USA), including PE-cy7-conjugated anti-mouse MHC-II, APC-conjugated anti-mouse MHC-I, PE-conjugated anti-mouse CD86, FITC-conjugated anti-mouse CD80, PE-cy7-conjugated anti-mouse CD83, and APC-conjugated anti-mouse CD36.
Quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR)
Total RNA was extracted from prepared RAW264.7 cells with TRIzol reagents (invitrogen, USA) according to the manufacturer’s protocol. RNA purity was acceptable when OD260/OD280 ratio was between 1.8 and 2.0. Reverse Transcription System and SYBR Green PCR Master Mix Kit (#A3500 and #4309155, Applied Biosystems, Foster City, CA, USA) were used for RT and PCR, respectively, and the reagent mixes were prepared following the manufacturer’s protocol. For detection the expression of miR-155, the RT reagent mixes were incubated at 16°C for 30 min, 42°C for 42 min, and 95°C for 5 min, and for detection the mRNA levels of the cytokines (including interleukin (IL)-12p35, IL-6, IL-1b, and TNF-α), the RT reagent mixes were incubated at 37°C for 15 min and 85°C for 5 s. Then, the cDNA samples were stored at -20°C until used for qRT-PCR. QRT-PCR reactions (20 μl) were performed in the following parameters: 2 min at 95°C, followed by 40 cycles of 15 s at 95°C and 60 s at 60°C. All targets were measured in triplicates. MiR-155 and cytokine levels were normalized to U6 and β-actin levels, respectively. The primers for miR-155 and U6 detection assays were purchased from Ribobio (Guangzhou, China). The sequences of PCR primers for the cytokines detection assays were as follows:
β-actin F: 5’ GAAGGACTCCTATGTGGGTGACG 3’; R: 5’ GATCTTCTCCATGTCGTCCCAGT 3’. IL-6 F: 5’ CCTTCTTGGGACTGATGCTG 3’; R: 5’ AGGTCGGTTGGGAGTGGTAT 3’. IL-12p35 F: 5’ CTTGCCCTCCTAAACCACCTC 3’; R: 5’ TCGGGACTGGCTAAGACACC 3’. IL-1b F: 5’ AGAGCATCCAGCGTCAAATC 3’; R: 5’ TCATCTCGGAGCCTGTAGTG 3’. TNF-α F: 5’ CACCACGCTCTTCCGTCTACTG 3’; R: 5’ GGGCTACAGGCTTGTCACTC 3’.
Enzyme-linked immunosorbent assay (ELISA)
Culture supernatant of RAW264.7 cells was collected after proper treatment. IL-12p70, IL-6, IL-1b, and TNF-α levels were detected by cytokine-specific ELISA kits according to manufacturer’s instructions (ebioscience, San Diego, CA, USA), respectively. The minimal detectable concentrations were 10 pg/ml for IL-12p70, 0.21 pg/ml for IL-6, 1.2 pg/ml for IL-1b, and 4 pg/ml for TNF-α, respectively. As TNF-α produced in quite high levels, so samples were diluted by Sample Diluent for 3 times before detection the level of TNF-α. All samples were measured in triplicates. Serial diluted (1:2) mouse IL-12, IL-6, IL-1b, and TNF-α standards were used to prepare the standard curves, respectively.
Statistical analysis
All results are shown as mean ± SD. Statistical significance was determined by performing ANOVAs for four comparisons and Student’s t test for two comparisons. While p values <0.05 were considered to be statistically significant and p values <0.01 were considered to be highly statistically significant.
Results
miR-155 expression in RAW264.7 cells can be induced both by LPS and oxLDL
Previous studies demonstrated that miR-155 was greatly up-regulated during the maturation of immune cells, including macrophages, DCs, and T cells [16,18,19], while both LPS and oxLDL can induce RAW264.7 cell maturation. So we first investigated whether miR-155 expression in RAW264.7 cells could be induced by LPS and oxLDL. We stimulated RAW264.7 cells with LPS and oxLDL with different times and concentrations, and then the cells were harvested and the miR-155 levels were detected by qRT-PCR. The time course study showed that the expression of miR-155 increased about 50-fold (P=0.000), 200-fold (P=0.002), and 100-fold (P=0.019) when RAW264.7 cells were treated with 1 μg/ml LPS for 6, 24, and 48 hours, respectively, which suggested that it maximally expressed in 24 hours and began to decline in 48 hours (Figure 1A). MiR-155 expression can also be induced by oxLDL, but at much lower levels. Its expression increased about 2-fold (P=0.038 and 0.017, respectively) after RAW264.7 cells were treated with oxLDL for 6 and 24 hours, but it declined to almost normal levels 48 hours later (P>0.05) (Figure 1B). However, a 10-fold up-regulation of the concentrations of LPS and oxLDL did not induce much higher levels of miR-155. So our study shows that both LPS and oxLDL are able to induce miR-155 expression in RAW264.7 cells, and the most suitable concentration is 1 μg/mL and 10 μg/mL, respectively.
Figure 1.
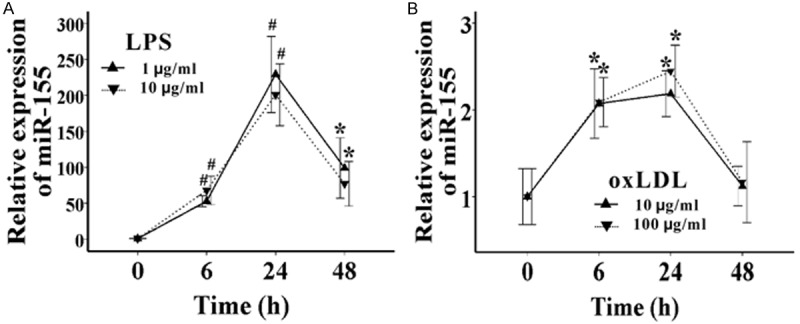
Both LPS and oxLDL can induce the expression of miR-155 in RAW264.7 cells. A. The expression of miR-155 was detected by qRT-PCR, after RAW264.7 cells were treated with 1 μg/mL or 10 μg/mL LPS for 6, 24, and 48 hours, respectively. B. The expression of miR-155 was detected by qRT-PCR, after RAW264.7 cells were treated with 10 μg/mL or 100 μg/mL oxLDL for 6, 24, and 48 hours, respectively. Data represent six independent experiments. All results are shown as mean ± SD. *P<0.05, #P<0.01.
miR-155 promotes morphologic changes in both LPS- and oxLDL-treated RAW264.7 cells
To assess whether miR-155 may cause morphological changes in RAW264.7 cells, naïve cells were transfected with pre-miR-ctrl or pre-miR-155, and then cells were either treated with 1 μg/ml LPS or 10 μg/ml oxLDL, or they were not treated. First, after 24 hours of treatment, we found that LPS- and oxLDL-treated RAW264.7 cells displayed a morphological transformation from macrophage-like cells into DC-like cells, which acquired a larger cell size, nuclear indentation, and relatively obvious cytoplasm with increased granularity, especially in LPS-treated cells. Moreover, when miR-155 was overexpressed in LPS- and oxLDL-treated RAW264.7 cells, a DC-like morphology was further induced. We observed that both the degree of morphological transformation of the cell (Figure 2A) and the number of cells with transformed morphology (Figure 2B) were enhanced by pre-miR-155. However, no morphological changes were observed in naïve RAW264.7 cells when miR-155 was overexpressed (Figure 2). So we conclude that miR-155 is critical for LPS- and oxLDL-mediated morphological transformation of macrophage-like cells into DC-like cells.
Figure 2.
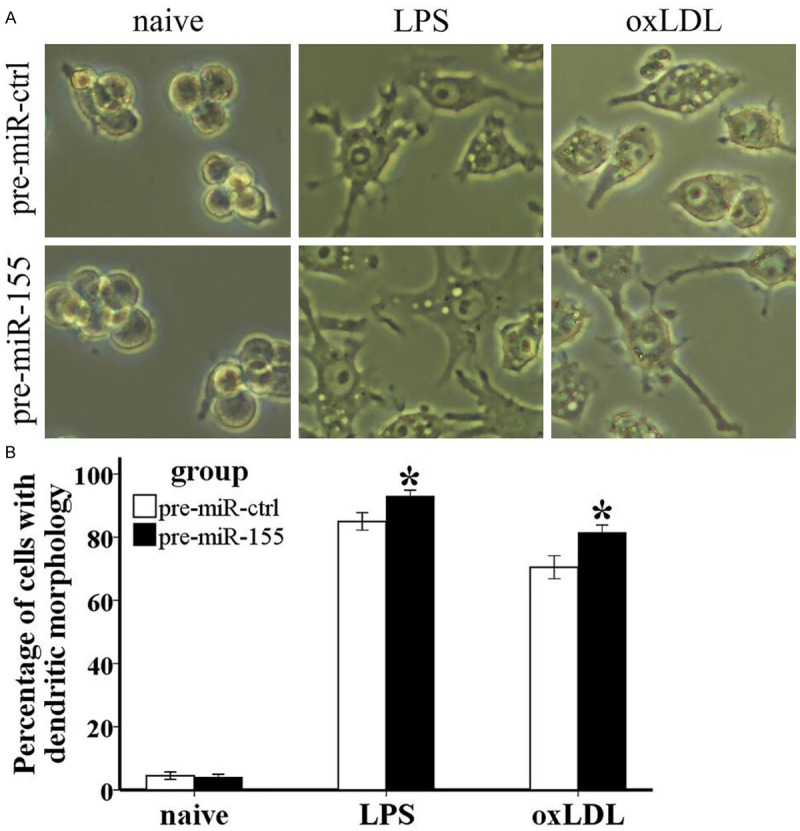
MiR-155 enhances morphologic changes in both LPS- and oxLDL-treated RAW264.7 cells. Pre-miR-ctrl, pre-miR-155, anti-miR-ctrl, and anti-miR-155 were transfected into RAW264.7 cells, which were not treated or treated with 1 μg/ml LPS or 10 μg/ml oxLDL for 24 hours, respectively. A. Cells were observed under a microscope and pictures were taken with a camera at 400×. B. Cells with dendritic morphology in the view field were calculated. Data represent six independent experiments. All results are shown as mean ± SD. *P<0.05, #P<0.01.
miR-155 induces phenotypic changes in both LPS- and oxLDL-treated RAW264.7 cells
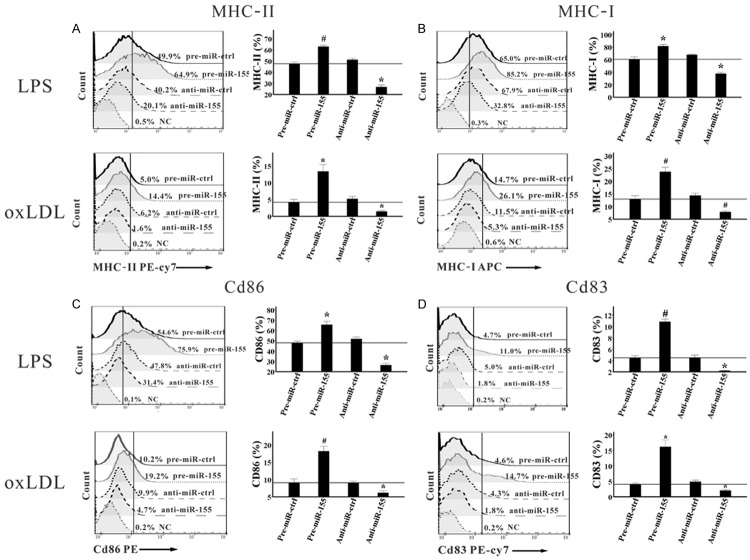
According to previous studies, both LPS and oxLDL could significantly induce phenotypic changes in RAW264.7 cells, including MHC molecules, costimulatory molecules, and adhesion molecules [9,10], this was also confirmed in our study (data are not shown). In order to clarify whether miR-155 regulates the expression of these molecules, miR-155 was overexpressed and inhibited in RAW264.7 cells, which were then treated with 1 μg/ml LPS or 10 μg/ml oxLDL for indicated times, and the levels of these molecules were detected by flow cytometry. Twenty-four hours after treatment with LPS, we observed that the proportions of MHC-II+, MHC-I+, CD86+, and CD83+RAW264.7 cells were significantly higher in the pre-miR-155 group than in the pre-miR-ctrl group. In contrast, their proportions were much lower in the anti-miR-155 group than in the anti-miR-ctrl group (Figure 3). Furthermore, when we treated RAW264.7 cells with oxLDL for 24 hours, their proportions were also obviously up-regulated by pre-miR-155, while they were substantially reduced by anti-miR-155, compared with the controls (Figure 3). However, no significant changes in their proportions were observed when the cells were treated for 6 or 48 hours, either in LPS- or oxLDL-treated RAW264.7 cells.
Figure 3.
MiR-155 induces the expression of surface molecules in both LPS- and in oxLDL-treated RAW264.7 cells. Pre-miR-ctrl, pre-miR-155, anti-miR-ctrl, and anti-miR-155 were transfected into RAW264.7 cells, which were then activated by 1 μg/ml LPS and 10 μg/ml oxLDL for 24 hours, respectively. Then cells were harvested, the proportions of MHC-II+ (A), MHC-I+ (B), CD86+ (C), and CD83+ (D) cells were determined by flow cytometry. Representative FACS pictures from a single case are shown in the left, and the collective results of six independent experiments are shown in the right as histograms. All results are shown as mean ± SD. *P<0.05, #P<0.01.
We also analyzed changes in the mean fluorescence intensities (MFIs) of these surface molecules, and the results were completely consistent with the changes in their positive cell fractions, except for the expression of CD36 in oxLDL-treated RAW264.7 cells. The proportions of CD36+ cells were almost 100% in all oxLDL-treated groups. But the MFIs of CD36 were 1.5- and 1.3-fold higher (P=0.000 and P=0.001, respectively) in the pre-miR-155 group than in pre-miR-ctrl group, while they were 29% and 26% lower (P=0.002 and P=0.007, respectively) in the anti-miR-155 group than in the anti-miR-ctrl group, when the RAW264.7 cells were treated with oxLDL for 24 and 48 hours, respectively (Figure 4). However, the level of CD80 showed no significant differences among the 4 groups in the whole study.
Figure 4.
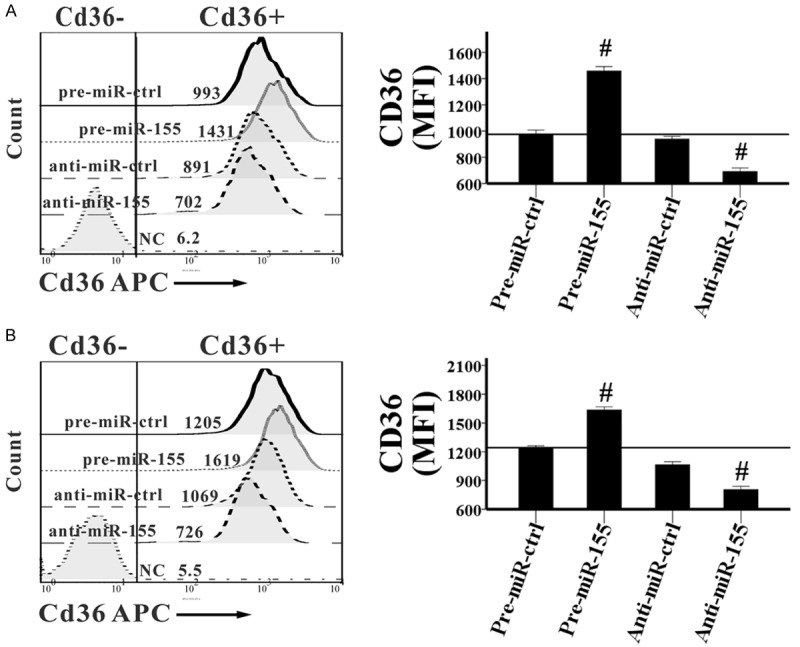
MiR-155 induces the expression of CD36 in oxLDL-treated RAW264.7 cells. Pre-miR-ctrl, pre-miR-155, anti-miR-ctrl, and anti-miR-155 were transfected into RAW264.7 cells, which were then activated by 10 μg/ml oxLDL for 24 (A) or 48 (B) hours. Then cells were harvested, the MFI of CD36 expressed in cell surface was determined by flow cytometry. Representative FACS pictures from a single case are shown in the left, and the collective results of six independent experiments are shown in the right as histograms. All results are shown as mean ± SD. #P<0.01.
In the same time, we detected the expression of these surface markers in naïve RAW264.7 cells when miR-155 was overexpressed or inhibited. However, no significant changes in their expression were observed, which indicated that miR-155 alone could not induce the phenotypic changes in naïve RAW264.7 cells (data are not shown).
In summary, our study showed that miR-155 induced MHC-II, MHC-I, CD86, and CD83 expression in LPS-treated RAW264.7 cells and induced MHC-II, MHC-I, CD86, CD83, and CD36 expression in oxLDL-treated RAW264.7 cells.
miR-155 accelerates pro-inflammatory cytokine expression in both LPS- and oxLDL-treated RAW264.7 cells
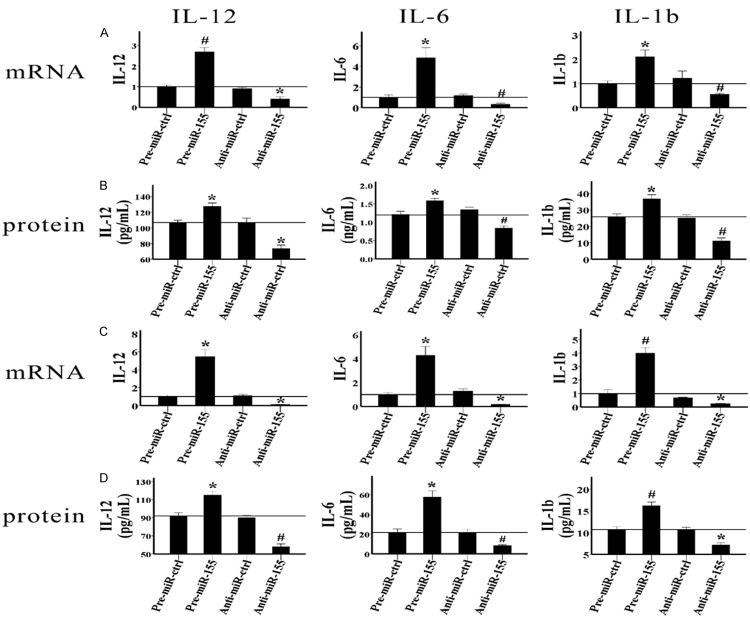
IL-12, IL-6, IL-1b, and TNF-α are important pro-inflammatory cytokines of the innate immune system. The levels of these cytokines indicate the directions of immune responses. So next, we analyzed how miR-155 regulates the production of these cytokines in LPS- and oxLDL-activated RAW264.7 cells. We cultured RAW264.7 cells with 1 μg/ml LPS or 10 μg/ml oxLDL for indicated times after the oligonucleotides were transfected, and then the mRNA levels of these cytokines and their protein levels in a cell culture supernatant were detected by qRT-PCR and ELISA, respectively. As shown in Figure 4A and 4B, 24 hours after the RAW264.7 cells were treated with LPS, both the mRNA and protein levels of IL-12 and IL-6 dramatically increased in the pre-miR-155 group compared with those in the pre-miR-ctrl group, and they significantly decreased in the anti-miR-155 group compared with those in the anti-miR-ctrl group. Forty-eight hours later, IL-1b production was also induced by miR-155. The results indicated that both the mRNA expression and protein production of IL-1b were obviously higher in the pre-miR-155 group than in the pre-miR-ctrl group, and they were much lower in the anti-miR-155 group than in the anti-miR-ctrl group (Figure 5A and 5B). Moreover, miR-155 also induced the expression of IL-12, IL-6, and IL-1b in oxLDL-activated RAW264.7 cells. Twenty-four hours after the RAW264.7 cells were treated with oxLDL, both the mRNA and protein levels of IL-12, IL-6, and IL-1b were induced by pre-miR-155 and were inhibited by anti-miR-155 (Figure 5C and 5D). However, miR-155 did not regulate the expression of TNF-α in either LPS- or oxLDL-treated RAW264.7 cells (data are not shown). To evaluate whether miR-155 regulates the expression of these cytokines in naïve RAW264.7 cells, we detected the mRNA levels of these cytokines in RAW264.7 cells when miR-155 was overexpressed or inhibited. The results demonstrated that none of these cytokines were regulated by miR-155. In conclusion, our study suggested that miR-155 could enhance the production of IL-12, IL-6, and IL-1b in LPS- and oxLDL-treated RAW264.7 cells, but not in naïve RAW264.7 cells.
Figure 5.
MiR-155 induces the expression of IL-12, IL-6, and IL-1b in both LPS- and oxLDL-treated RAW264.7 cells. Pre-miR-ctrl, pre-miR-155, anti-miR-ctrl, and anti-miR-155 were transfected into RAW264.7 cells. A. The mRNA levels of IL-12, IL-6, and IL-1b in the cells were detected by qRT-PCR after the cells were activated by 1 μg/ml LPS for 24 or 48 hours. B. The protein levels of these cytokines in the cell culture supernatant were detected by ELISA after the cells were activated by 1 μg/ml LPS for 24 or 48 hours. C. The mRNA levels of IL-12, IL-6, and IL-1b in the cells were detected by qRT-PCR after the cells were activated by 10 μg/ml oxLDL for 24 hours. D. The protein levels of these cytokines in the cell culture supernatant were detected by ELISA after the cells were activated by 10 μg/ml oxLDL for 24 hours. Data represent six independent experiments. All results are shown as mean ± SD. *P<0.05, #P<0.01.
Discussion
LPS and oxLDL are two factors that can induce the differentiation of monocytes to DCs as well as the differentiation of RAW264.7 cells to DC-like cells [5,6,20,21]. DCs stimulated by LPS in vitro mimic they exerting immunostimulatory capacity after encountering antigens in vivo, while oxLDL-pulsed DCs show up-regulation of scavenger-receptors, the induction of maturation, and differentiation, as well as the capacity to activate a specific T cell response, which is involved in stimulating atherosclerotic plaque development [22,23]. We observed that both LPS and oxLDL could induce the expression of miR-155 in RAW264.7 cells, and up-regulated miR-155 is able to enhance morphological changes, the production of surface molecules and pro-inflammatory cytokines in the cells, which suggests that miR-155 induction is associated with DC maturation and also might be involved in AS development. However, our study illustrated that miR-155 was expressed in naïve RAW264.7 cells at quite a low level, and it could not induce morphological and phenotypic changes or cytokine expression in these cells. This indicates that miR-155 is involved in inducing macrophage differentiation into DC-like cells when a “danger” signal shows up, but it does not regulate macrophage function in the normal surroundings.
The ability to stimulate T cell proliferation and differentiation is a major DC function [3]. Studies showed that miR-155 deficiency in DCs impaired their ability to induce T cell proliferation and differentiation [24]. But the mechanisms involved in this are still unclear. We observed that miR-155 enhanced the expression of phenotypic molecules, including MHC-II, MHC-I, CD86, and CD83, and also the expression of pro-inflammatory cytokines, including IL-12, IL-6, and IL-1b. These data might explain how miR-155 regulates DC maturation and DCs’ ability to induce T cell activation and differentiation. CD83 is the most specific cell surface marker for mDCs. Our data showed that miR-155 induced the expression of CD83 in RAW264.7 cells, which suggests that miR-155 can induce RAW264.7 cells to convert to mDCs. MDCs are the professional APCs, which initiate T cells to activate and differentiate by presenting antigens to them. This procedure requires at least two signals: MHC-II and MHC-I provide the peptide antigens to TCR, while CD80 and CD86 provide costimulatory signals to their receptors in T cells [25]. We observed that miR-155 up-regulated the expression of MHC-II, MHC-I, and CD86 in LPS-treated RAW264.7 cells, which indicated that miR-155 expression is highly associated with the antigen-presenting ability of DCs and the activation of T cells. Cytokine production is one important way that immune cells exert their functions. IL-12 is a key cytokine responsible for DC maturation and for polarizing naïve CD4+T cells toward the Th1 phenotype, while IL-6 and IL-1b secreted by DCs promote Th17 differentiation and maintenance [26]. So up-regulation of the expression of IL-12, IL-6, and IL-1b in DCs by miR-155 not only indicates the important role of miR-155 in inducing DC maturation, but it also might be an indirect way of inducing the differentiation of Th1 and Th17 cells. In conclusion, LPS can induce the expression of miR-155 in RAW264.7 cells, and up-regulated miR-155 not only can promote RAW264.7 cells showing a DC-like phenotype but also might enhance their ability to stimulate T cell activation and differentiation.
AS is a chronic inflammatory disease. It initiates from the response of cells such as DCs, macrophages, T cells, and endothelial cells in the arterial wall to modified lipoproteins (e.g., oxLDL) [27]. Nazari-Jahantigh et al. [28] demonstrated that miR-155 was up-regulated in advanced stenotic plaques and that miR-155 deficiency reduced plaque size. Here we found that miR-155 enhanced the production of phenotypic molecules and pro-inflammatory cytokines in oxLDL-pulsed RAW264.7 cells, which provides more evidence for the important role of miR-155 in regulating the development of AS. MiR-155 enhanced the ability of oxLDL to induce the production of DC phenotypic molecules, including CD36, MHC-II, MHC-I, CD86, and CD83. CD83 and CD36 are mature markers of DCs. CD36 levels also reflect the oxLDL uptake capacity of DCs. Through binding to CD36, oxLDL is able to activate DCs and enhance cytokine production [4], whereas the expression of MHC-II, CD86, and CD83 in DCs is necessary for efficient T cell stimulation in local atherosclerotic lesions [29,30]. IL-12, IL-6, and IL-1b are all pro-inflammatory cytokines as well as pro-atherosclerotic cytokines. IL-12 is important for recruiting T cells into the plaque and differentiating them toward Th1 [31]. It is also able to enhance LDL oxidation by activating monocytes and modifying the production of chemokines by vascular smooth muscle cells [32]. IL-6 is locally produced by plaque-infiltrating macrophages and DCs. It can trigger the release of chemokine lig and 2 (CCL2) by endothelial cells, which exacerbates artery wall inflammation and induces atherosclerotic plaque development by recruiting more immune cells [33,34]. IL-1b also plays important roles in improving atheroma formation and stability. Bhaskar et al. [35] demonstrated that the antibody targeting IL-1b could inhibit the secretion of pro-atherogenic cytokines and matrix metalloproteinases (MMPs), including IL-6, IL-8, CCL2, TNF-α, MMP-3, and MMP-9. So up-regulation the release of IL-12, IL-6, and IL-1b by miR-155 in oxLDL-treated RAW264.7 cells indicates the pro-atherosclerotic character of miR-155. Therefore, miR-155 must be one of the most essential factors involved in regulating the development of AS.
Using TargetScan and miRBase prediction algorithms, we obtained several targets of miR-155 that contribute to regulating the innate immune response, including suppressors of cytokine signaling (SOCS1), Src homology 2 domain-containing inositol-5-phosphatase 1 (SHIP1), c-FOS, transforming growth factor-β-activated kinase-1-binding protein 2 (TAB2), Fas-associated death domain protein (FADD), mothers against decapentaplegic homolog 2 (SMAD2), and PU.1. Among them, SOCS1, SHIP1, and c-FOS are potential transcriptional factors involved in governing RAW264.7 cells differentiating into DC-like cells. SOCS1 is a feedback inhibitor of TLR4 signaling. Knockout of SOCS1 in DCs instructs the expression of MHC-II and costimulatory molecules and the secretion of pro-inflammatory cytokines, including IFN-γ, IL-6, IL-12, and TNF-α [36]. Zhang et al. [37] demonstrated that let-7i, another miRNA, induces DC maturation (increased expression of the surface costimulatory molecules and pro-inflammatory cytokines) via directly inhibiting the expression of SOCS1. SOCS1 also is a confirmed target of miR-155 involved in regulating immune response. So it is probable that both miR-155 and let-7i can regulate DC maturation through targeting SOCS1. SHIP1 is another important negative regulator that regulates innate immune cell proliferation, differentiation, and function. It negatively regulates the combination of TLR4 and MyD88 and inhibits the activation of NF-κB and MAPK [38]. SHIP1 knockout of DCs shows an up-regulated expression of pro-inflammatory cytokines such as IL-6 and TNF-α [38]. However, Antignano et al. [39] found that SHIP1-/- DCs display increased expression of MHC-II and IL-12, enhanced ability to induce T cell expansion and differentiation to Th1. c-FOS is a component of the activator protein-1 signaling pathway. Koga et al. [40] demonstrated that over-expression of c-Fos suppressed LPS-induced production of IL-6, IL-12, and TNF-α. Dunand-Sauthier et al. [41] validated that c-Fos is the direct target of miR-155 and that miR-155 regulates DC maturation through inhibiting the expression of c-Fos. Therefore, SOCS1, SHIP1, or c-FOS might be the direct targets of miR-155 involved in inducing LPS- and oxLDL-treated RAW264.7 cell differentiation into DC-like cells. However, it is possible that other potential targets of miR-155 may govern this process, as this process was controlled by multiple signalling pathways, and a single miRNA is known to target multiple mRNAs [42]. So, further studies are necessary to reveal the entire “targetome” of miR-155 involved in this process.
Besides miR-155 and let-7i, miR-221, miR-142-3p, and miR-148 family (miR-148a, miR-148b, and miR-152) are other potential miRNAs that involve in regulating DC maturation [43-45]. MiR-221 contributed to iDC homeostasis, while its expression decreased during DC maturation [43]. MiR-142-3p was able to induce IL-6 expression in both iDCs and mDCs, but it could not improve the ability of DCs to stimulate T cells [44]. MiR-148 family suppressed DC function by inhibiting the expression of MHC-II and the secretion of pro-inflammatory cytokines, although they were up-regulated upon DC activation by LPS [45]. Collectively, these miRNAs play different roles in DC maturation and function.
In summary, our study showed that both LPS and oxLDL induced the expression of miR-155 in RAW264.7 cells. And up-regulated miR-155 facilitated the ability of LPS and oxLDL in transforming RAW264.7 cells into DC-like cells by inducing morphological and phenotypic changes and pro-inflammatory cytokine expression. These results indicate that miR-155 is a pro-inflammatory as well as a pro-atherosclerotic factor.
Acknowledgements
The work described in this article was supported by the National Natural Science Foundation of China (No. 81000085).
Disclosure of conflict of interest
None.
References
- 1.Rasmussen SB, Reinert LS, Paludan SR. Innate recognition of intracellular pathogens: detection and activation of the first line of defense. APMIS. 2009;117:323–337. doi: 10.1111/j.1600-0463.2009.02456.x. [DOI] [PubMed] [Google Scholar]
- 2.Mercer J, Greber UF. Virus interactions with endocytic pathways in macrophages and dendritic cells. Trends Microbiol. 2013;21:380–388. doi: 10.1016/j.tim.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 4.Koltsova EK, Ley K. How dendritic cells shape atherosclerosis. Trends Immunol. 2011;32:540–547. doi: 10.1016/j.it.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 6.Chomarat P, Banchereau J, Davoust J, Palucka AK. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. 2000;1:510–514. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- 7.Bobryshev YV, Lord RS. Vascular-associated lymphoid tissue (VALT) involvement in aortic aneurysm. Atherosclerosis. 2001;154:15–21. doi: 10.1016/s0021-9150(00)00441-x. [DOI] [PubMed] [Google Scholar]
- 8.Raschke WC, Baird S, Ralph P, Nakoinz I. Functional macrophage cell lines transformed by Abelson leukemia virus. Cell. 1978;15:261–267. doi: 10.1016/0092-8674(78)90101-0. [DOI] [PubMed] [Google Scholar]
- 9.Saxena RK, Vallyathan V, Lewis DM. Evidence for lipopolysaccharide-induced differentiation of RAW264.7 murine macrophage cell line into dendritic like cells. J Biosci. 2003;8:129–134. doi: 10.1007/BF02970143. [DOI] [PubMed] [Google Scholar]
- 10.Shen LH, Zhou L, Wang BY, Pu J, Hu LH, Chai DJ, Wang L, Zeng JZ, He B. Oxidized low-density lipoprotein induces differentiation of RAW264.7 murine macrophage cell line into dendritic-like cells. Atherosclerosis. 2008;199:257–264. doi: 10.1016/j.atherosclerosis.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Sayed D, Abdellatif M. MicroRNAs in development and disease. Physiol Rev. 2011;91:827–887. doi: 10.1152/physrev.00006.2010. [DOI] [PubMed] [Google Scholar]
- 12.Vigorito E, Kohlhaas S, Lu D, Leyland R. MiR-155: an ancient regulator of the immune system. Immunol Rev. 2013;253:146–157. doi: 10.1111/imr.12057. [DOI] [PubMed] [Google Scholar]
- 13.Thai TH, Calado DP, Casola S, Ansel KM, Xiao C, Xue Y, Murphy A, Frendewey D, Valenzuela D, Kutok JL, Schmidt-Supprian M, Rajewsky N, Yancopoulos G, Rao A, Rajewsky K. Regulation of the germinal center response by microRNA-155. Science. 2007;316:604–608. doi: 10.1126/science.1141229. [DOI] [PubMed] [Google Scholar]
- 14.Yao R, Ma YL, Liang W, Li HH, Ma ZJ, Yu X, Liao YH. MicroRNA-155 modulates Treg and Th17 cells differentiation and Th17 cell function by targeting SOCS1. PLoS One. 2012;7:e46082. doi: 10.1371/journal.pone.0046082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci U S A. 2007;104:1604–1609. doi: 10.1073/pnas.0610731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruggiero T, Trabucchi M, De Santa F, Zupo S, Harfe BD, McManus MT, Rosenfeld MG, Briata P, Gherzi R. LPS induces KH-type splicing regulatory protein-dependent processing of microRNA-155 precursors in macrophages. FASEB J. 2009;23:2898–2908. doi: 10.1096/fj.09-131342. [DOI] [PubMed] [Google Scholar]
- 17.Turner ML, Schnorfeil FM, Brocker T. MicroRNAs regulate dendritic cell differentiation and function. J Immunol. 2011;187:3911–3917. doi: 10.4049/jimmunol.1101137. [DOI] [PubMed] [Google Scholar]
- 18.Jin P, Han TH, Ren J, Saunders S, Wang E, Marincola FM, Stroncek DF. Molecular signatures of maturing dendritic cells: implications for testing the quality of dendritic cell therapies. J Transl Med. 2010;8:4. doi: 10.1186/1479-5876-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fassi Fehri L, Koch M, Belogolova E, Khalil H, Bolz C, Kalali B, Mollenkopf HJ, Beigier-Bompadre M, Karlas A, Schneider T, Churin Y, Gerhard M, Meyer TF. Helicobacter pylori induces miR-155 in T cells in a cAMP-Foxp3-dependent manner. PLoS One. 2010;5:e9500. doi: 10.1371/journal.pone.0009500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gabriele L, Borghi P, Rozera C, Sestili P, Andreotti M, Guarini A, Montefusco E, Foà R, Belardelli F. IFN-alpha promotes the rapid differentiation of monocytes from patients with chronic myeloid leukemia into activated dendritic cells tuned to undergo full maturation after LPS treatment. Blood. 2004;103:980–987. doi: 10.1182/blood-2003-03-0981. [DOI] [PubMed] [Google Scholar]
- 21.Perrin-Cocon L, Coutant F, Agaugué S, Deforges S, André P, Lotteau V. Oxidized low-density lipoprotein promotes mature dendritic cell transition from differentiating monocyte. J Immunol. 2001;167:3785–3791. doi: 10.4049/jimmunol.167.7.3785. [DOI] [PubMed] [Google Scholar]
- 22.Nickel T, Schmauss D, Hanssen H, Sicic Z, Krebs B, Jankl S, Summo C, Fraunberger P, Walli AK, Pfeiler S, Weis M. oxLDL uptake by dendritic cells induces upregulation of scavenger-receptors, maturation and differentiation. Atherosclerosis. 2009;205:442–450. doi: 10.1016/j.atherosclerosis.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Habets KL, van Puijvelde GH, van Duivenvoorde LM, van Wanrooij EJ, de Vos P, Tervaert JW, van Berkel TJ, Toes RE, Kuiper J. Vaccination using oxidized low-density lipoprotein-pulsed dendritic cells reduces atherosclerosis in LDL receptor-deficient mice. Cardiovasc Res. 2010;85:622–630. doi: 10.1093/cvr/cvp338. [DOI] [PubMed] [Google Scholar]
- 24.Thai TH, Calado DP, Casola S, Ansel KM, Xiao C, Xue Y, Murphy A, Frendewey D, Valenzuela D, Kutok JL, Schmidt-Supprian M, Rajewsky N, Yancopoulos G, Rao A, Rajewsky K. Regulation of the germinal center response by microRNA-155. Science. 2007;316:604–608. doi: 10.1126/science.1141229. [DOI] [PubMed] [Google Scholar]
- 25.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 26.Espinosa V, Rivera A. Cytokines and the regulation of fungus-specific CD4 T cell differentiation. Cytokine. 2012;58:100–106. doi: 10.1016/j.cyto.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan J, Watanabe T. Inflammatory reactions in the pathogenesis of atherosclerosis. J Atheroscler Thromb. 2003;10:63–71. doi: 10.5551/jat.10.63. [DOI] [PubMed] [Google Scholar]
- 28.Nazari-Jahantigh M, Wei Y, Noels H, Akhtar S, Zhou Z, Koenen RR, Heyll K, Gremse F, Kiessling F, Grommes J, Weber C, Schober A. MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. J Clin Invest. 2012;122:4190–4202. doi: 10.1172/JCI61716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dopheide JF, Sester U, Schlitt A, Horstick G, Rupprecht HJ, Münzel T, Blankenberg S. Monocyte-derived dendritic cells of patients with coronary artery disease show an increased expression of costimulatory molecules CD40, CD80 and CD86 in vitro. Coron Artery Dis. 2007;18:523–531. doi: 10.1097/MCA.0b013e3282eff1ad. [DOI] [PubMed] [Google Scholar]
- 30.Bobryshev YV. Dendritic cells and their role in atherogenesis. Lab Invest. 2010;90:970–984. doi: 10.1038/labinvest.2010.94. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Niessner A, Nakajima T, Ma-Krupa W, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM. Interleukin 12 induces T-cell recruitment into the atherosclerotic plaque. Circ Res. 2006;12:5245–5231. doi: 10.1161/01.RES.0000204452.46568.57. [DOI] [PubMed] [Google Scholar]
- 32.Folcik VA, Aamir R, Cathcart MK. Cytokine modulation of LDL oxidation by activated human monocytes. Arterioscler Thromb Vasc Biol. 1997;12:1954–1961. doi: 10.1161/01.atv.17.10.1954. [DOI] [PubMed] [Google Scholar]
- 33.Maier W, Altwegg LA, Corti R, Gay S, Hersberger M, Maly FE, Sütsch G, Roffi M, Neidhart M, Eberli FR, Tanner FC, Gobbi S, von Eckardstein A, Lüscher TF. Inflammatory markers at the site of ruptured plaque in acute myocardial infarction: locally increased interleukin-6 and serum amyloid A but decreased C-reactive protein. Circulation. 2005;12:1355–1361. doi: 10.1161/01.CIR.0000158479.58589.0A. [DOI] [PubMed] [Google Scholar]
- 34.Rott D, Zhu J, Zhou YF, Burnett MS, Zalles-Ganley A, Epstein SE. IL-6 is produced by splenocytes derived from CMV-infected mice in response to CMV antigens, and induces MCP-1 production by endothelial cells: a new mechanistic paradigm for infection-induced atherogenesis. Atherosclerosis. 2003;170:223–228. doi: 10.1016/s0021-9150(03)00295-8. [DOI] [PubMed] [Google Scholar]
- 35.Bhaskar V, Yin J, Mirza AM, Phan D, Vanegas S, Issafras H, Michelson K, Hunter JJ, Kantak SS. Monoclonal antibodies targeting IL-1 beta reduce biomarkers of atherosclerosis in vitro and inhibit atherosclerotic plaque formation in Apolipoprotein E-deficient mice. Atherosclerosis. 2011;216:313–320. doi: 10.1016/j.atherosclerosis.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 36.Evel-Kabler K, Song XT, Aldrich M, Huang XF, Chen SY. SOCS1 restricts dendritic cells’ ability to break self tolerance and induce antitumor immunity by regulating IL-12 production and signaling. J Clin Invest. 2006;116:90–100. doi: 10.1172/JCI26169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang M, Liu F, Jia H, Zhang Q, Yin L, Liu W, Li H, Yu B, Wu J. Inhibition of MicroRNA let-7i Depresses Maturation and Functional State of Dendritic Cells in Response to Lipopolysaccharide Stimulation via Targeting Suppressor of Cytokine Signaling 1. J Immunol. 2011;187:1674–1683. doi: 10.4049/jimmunol.1001937. [DOI] [PubMed] [Google Scholar]
- 38.An H, Xu H, Zhang M, Zhou J, Feng T, Qian C, Qi R, Cao X. Src homology 2 domain-containing inositol-5-phosphatase 1 (SHIP1) negatively regulates TLR4-mediated LPS response primarily through a phosphatase activity- and PI-3K-independent mechanism. Blood. 2005;105:4685–4692. doi: 10.1182/blood-2005-01-0191. [DOI] [PubMed] [Google Scholar]
- 39.Antignano F, Ibaraki M, Kim C, Ruschmann J, Zhang A, Helgason CD, Krystal G. SHIP is required for dendritic cell maturation. J Immunol. 2010;184:2805–2813. doi: 10.4049/jimmunol.0903170. [DOI] [PubMed] [Google Scholar]
- 40.Koga K, Takaesu G, Yoshida R, Nakaya M, Kobayashi T, Kinjyo I, Yoshimura A. Cyclicadenosine monophosphate suppresses the transcription of proinflammatory cytokines via the phosphorylatedc-Fos protein. Immunity. 2009;30:372–383. doi: 10.1016/j.immuni.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 41.Dunand-Sauthier I, Santiago-Raber ML, Capponi L, Vejnar CE, Schaad O, Irla M, Seguín-Estévez Q, Descombes P, Zdobnov EM, Acha-Orbea H, Reith W. Silencing of c-Fos expressionby microRNA-155 is critical for dendritic cell maturation and function. Blood. 2011;117:4490–4500. doi: 10.1182/blood-2010-09-308064. [DOI] [PubMed] [Google Scholar]
- 42.Alshalalfa M. MicroRNA Response Elements-Mediated miRNA-miRNA Interactions in Prostate Cancer. Adv Bioinformatics. 2012;2012:839837. doi: 10.1155/2012/839837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu C, Huang X, Zhang X, Roensch K, Cao Q, Nakayama KI, Blazar BR, Zeng Y, Zhou X. MiR-221 and miR-155 regulate human dendritic cell development, apoptosis, and IL-12 production through targeting of p27kip1, KPC1, and SOCS-1. Blood. 2011;117:4293–4303. doi: 10.1182/blood-2010-12-322503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun Y, Varambally S, Maher CA, Cao Q, Chockley P, Toubai T, Malter C, Nieves E, Tawara I, Wang Y, Ward PA, Chinnaiyan A, Reddy P. Targeting of microRNA-142-3p in dendritic cells regulates endotoxin-induced mortality. Blood. 2011;117:6172–683. doi: 10.1182/blood-2010-12-325647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu X, Zhan Z, Xu L, Ma F, Li D, Guo Z, Li N, Cao X. MicroRNA-148/152 impair innate response and antigen presentation of TLR-triggered dendritic cells by targeting CaMKIIa. J Immunol. 2010;185:7244–7251. doi: 10.4049/jimmunol.1001573. [DOI] [PubMed] [Google Scholar]