Abstract
Background: Patients with gastric cancer (GC) commonly exhibit a hypercoagulable state that results in significant morbidity and mortality. Recent studies have shown that neutrophil extracellular traps (NETs) trigger coagulation through an intrinsic pathway and contribute to thrombus initiation and progression. In this study, we aimed to determine the procoagulant activity (PCA) of NETs in patients with GC. Methods: NET formation and their PCAs were assessed in 48 patients with GC and 36 healthy controls using immunofluorescence microscopy of neutrophil markers and extracellular DNA as well as a modified capture ELISA technique, and thrombin-antithrombin complex and clot (fibrin) spectroscopic detection, respectively. Results: Here we showed that neutrophils isolated from patients with GC displayed significantly enhanced NET formation compared with those from healthy controls; furthermore, plasma or platelets obtained from patients with GC induced control neutrophils to release NETs. In addition, NETs released by GC neutrophils significantly increased the potency of control plasma to generate thrombin and fibrin. Notably, these procoagulant effects were dramatically attenuated by application of DNase I. We further found that spontaneous NET formation in patients with GC was significantly higher than that in controls, increased with tumor- node-metastasis stage elevation, and positively correlated with thrombin-antithrombin complex levels and D-dimers. Additionally, the effect of DNase I on cell-free plasma generation of fibrin was dependent on the concentration of NET formation. Conclusion: These results suggest that GC creates a systemic environment that primes neutrophils to release procoagulant NETs. Thus, targeting NETs might improve the coagulopathy of patients with GC.
Keywords: Stomach neoplasm, prothrombotic state, neutrophils, NETs, cell-free DNA
Introduction
A hypercoagulable state is a common complication and a major contributor to morbidity and mortality in patients with gastric cancer as the activation of coagulation promotes tumor growth and metastasis and increases the risk of venous thrombosis, which is the second leading cause of death in these patients [1-10]. On the other hand, thromboprophylaxis, the prophylactic employment of anticoagulation therapies, in patients with cancer has a strong potential to limit the risk of thrombosis, translating into improved patient survival [11,12]. However, anticoagulant use in these patients remains a significant challenge owing to the unequal distribution of venous thrombosis incidence among patients and the high risk of bleeding related with anticoagulant treatment [5-7,13,14]. Therefore, a better understanding of the pathogenesis of the hypercoagulable state in patients with cancer is urgently needed.
Recently, neutrophil extracellular traps (NETs), generated from activated neutrophils and composed of cell-free DNA, histones, and granular and cytoplasmic neutrophil proteins, have been shown to offer a novel mechanism for promoting coagulation and thrombosis and are viewed as a link between interfile inflammation and thrombosis [14-18]. Cell-free DNA promotes coagulation activation through an intrinsic pathway [18] and histones induce platelet and erythrocyte activation, which triggers coagulation [19,20]. Notably, inflammation is a hallmark of cancer [21,22], and activated neutrophils play a vital role in the thrombosis associated with cancer in mouse [23,24]. Furthermore, Demers et al. recently found that NET formation increased and consequently induced the prothrombotic state in mice with cancer [24]; in addition, NET formation has been found to induce organ dysfunction associated with cancer in a very recent animal study [25]. In this context, we hypothesized that NET formation increases and then contributes to a hypercoagulable state in patients with gastric cancer. Accordingly, we assessed the ability of the circulation environment of patients with gastric cancer to prime neutrophils to release NETs, and investigated the contribution of NETs to coagulation in these patients. The current results led us to believe that gastric cancer primes neutrophils to release NETs, which, in turn, play a role in coagulation activation in patients with gastric cancer.
Patients and methods
Patients
In this prospective study, pretreatment blood samples were collected from 48 consecutive patients with gastric cancer admitted to the Department of Gastrointestinal Surgery in the Second Affiliated Hospital of Harbin Medical University of China, between April 2015 and August 2015. Histological classification and pathologic tumor-node-metastasis (TNM) staging were assessed according to the 7th American Joint Committee on Cancer (AJCC). Numbers of neutrophils and D-dimers were determined in the hospital’s routine laboratories. Thirty-six healthy volunteers were recruited from among the hospital staff as healthy controls. Exclusion criteria were age < 18 years, active or chronic infection, cardiovascular disease, diabetes, liver or renal dysfunction, other coexisting cancer, thromboembolic complications, platelets and/or blood coagulation disorders, and administration of anticoagulant and/or antiplatelet treatment. Informed consent was obtained from each patient and control. The study was approved by the Ethics Committee of Harbin Medical University and conducted in accordance with the principles enshrined in the Declaration of Helsinki.
Preparation of platelet rich plasma (PRP), platelet free plasma (PFP), platelets, and neutrophils
Fresh whole venous blood samples were collected with a 21-gauge needle into 3.2% sodium citrate the morning after overnight fasting, and centrifuged (10 min, 150 g) at room temperature to obtain PRP [17]. PFP was prepared with two serial centrifugations (2500 g for 15 min, twice) and stored in aliquots at -80°C until used, as previously described [26,27]. Platelets were isolated immediately from PRP with centrifugation (10 min, 600 g), and then washed and resuspended in HEPES buffer, and used for the in vitro study [17]. Neutrophils were obtained through density gradient centrifugation with Percoll according to the manufacturer’s instructions, followed by hypotonic lysis as described previously [24]. Neutrophil purity (> 98%) was assessed by Wright-Giemsa staining and viability (> 98%) by Trypan blue stain.
In-vitro NET formation
Purified neutrophils (1 × 106) isolated from patients with GC or healthy controls were subsequently incubated for 3 hours at 37°C in 5% CO2. For in vitro studies, neutrophils from control individuals (n = 5) were treated with 6% plasma isolated from patients (n = 48) or from control individuals (n = 36) or with PBS, or treated with platelets derived from patients with GC (n = 10) or from control individuals in a ratio of 1:50 for 3 hours (n = 10). Then, the supernatants were collected by centrifugation (10 min, 1500 g) [18], and cell-free DNA (CFDNA) was quantified with fluorescence quantification, as described previously [28,29]. Briefly, 50 μl samples were mixed with 50 μl SytoxGreen (final concentration 2 M; Invitrogen, Carlsbad, CA, USA) to label the DNA. Fluorescence was recorded with a Spectramax microplate fluorometer (TECAN Infinite M 200, Tecan, San Jose, CA, USA) at 485 nm excitation and 538 nm emission. DNA concentrations were calculated based on a standard curve of known concentrations of DNA (Invitrogen).
Immunofluorescence
To further visualize NET formation, neutrophils with and without stimulation were seeded into 24 wells (coated with poly-L-lysine) at 37°C in the presence of 5% CO2 for 3 hours. Cells were fixed with 4% paraformaldehyde and stained using a mouse anti-myeloperoxidase (MPO) mAb (1/100, Wanleibio, Shenyang, China) and an IgG1 anti-CD19 mAb (1/200, DAKO, Glostrup, Denmark) was utilized as an isotype control. A polyclonal rabbit anti-mouse Alexa fluor 647 (1/100 dilution, ZSGB-BIO, Beijing, China) were utilized as secondary antibodies. SytoxGreen was used for DNA counterstaining. Visualization was performed in a Nikon ECLIPSE Ti microscope (Tokyo, Japan). Data were expressed as the percentage of NET-forming cells in relation to the total number of cells [17].
Measurement of NET procoagulant activity using the thrombin and fibrin generation test
To assess NET procoagulant activity, we performed a thrombin and fibrin generation test. Thrombin generation was assessed using the thrombin-antithrombin complex (TAT), as previously described [17]. Briefly, 80 l plasma obtained from patients or controls or control plasma stimulated in vitro with NET structures (in a final concentration of 20%) was incubated with PBS (5 l), or DNase I (400 µg/ml, 5 l) for 30 minutes at 37°C in a 96-well plate [18]. Clotting was initiated by addition of CaCl2 (15 l; 0.1 M). The reaction was performed for 5 minutes at 37°C. To stop the reaction, samples were immediately transferred in ice. TAT was measured according to manufacturer instructions (BlueGene, Shanghai, China). To further assess the NET procoagulant role, we performed a fibrin generation test as previously described [31-33]. Fibrin (clot) formation was continually monitored by measuring the optical density (405 nm) of the plasma on a Spectramax microplate reader at 37°C for 1 hour.
Quantification of autonomous NET formation in patients with GC
To quantify NET formation in patients with GC, we measured the amount of circulating myeloperoxidase (MPO)-DNA complex, a well-established marker of NET formation, using a modified capture ELISA technique as previously described [17,33,34]. In addition, nucleosome (Roche Diagnostics GmbH, Mannheim, Germany) and neutrophil elastase (NE) (BlueGene, Shanghai, China) were measured using ELISA kits according to the manufacturers’ instructions, and CFDNA was tested as described above.
Statistical analysis
Continuous variables were presented as means ± standard deviation (SD). A T test was used for quantitative data, the least significant difference (LSD) method was used for multiple comparisons, the Kal-Wallis test for ordered variables, and Spearman’s rank correlation analysis for the correlation between continuous variables. Paired t-tests were performed for paired sample analyses. Statistical significance was set as P-values of 0.05 or less. SAS9.2 software (SAS Institute Inc., Cary, NC, USA) was used for statistical analysis.
Results
Characteristics of patients and healthy controls
Forty-eight patients with GC, including 7 at stage I, 9 stage II, 25 stage III, and 7 at stage IV along with 36 healthy controls were enrolled in this study. The characteristics of patients and healthy subjects are summarized in Table 1. There were no significant differences in the means of age, sex, and neutrophil counts between patients and healthy controls. However, plasma TAT and D-dimer levels in patients with GC were obviously higher than those in controls (713 ± 328 pg/ml, n = 48, vs. 383 ± 60 pg/ml, n = 36; 359 ± 296 ng/ml, n = 48, vs. 92 ± 54 ng/ml, n = 36, P < 0.0001 for both) (Table 1), suggesting a hypercoagulable state in the patients with GC.
Table 1.
Clinical and demographic characteristics of study subjects
| Variable | CTR | GC | P value |
|---|---|---|---|
| Sex (M/F) | 24/12 | 35/13 | 0.541 |
| Age (years) | 56.1 ± 9.2 | 55.9 ± 9.6 | 0.2066 |
| Neutrophils (109/l) | 3.46 ± 1.04 | 3.94 ± 1.31 | 0.68 |
| TAT (pg/ml) | 383 ± 60 | 714 ± 328 | < .0001 |
| D-dimers (ng/ml) | 92 ± 54 | 359 ± 296 | < .0001 |
| MPO-DNA (OD 405) | 0.076 ± 0.024 | 0.518 ± 0.507 | < .0001 |
| DNA (mg/ml) | 0.063 ± 0.027 | 0.463 ± 0.604 | < .0001 |
| Nucleosome (OD 405) | 0.128 ± 0.045 | 0.747 ± 0.62 | < .0001 |
| NE (ng/ml) | 1.76 ± 0.27 | 2.99 ± 1.04 | < .0001 |
| TNM stage | |||
| I | 7 | - | |
| II | 9 | - | |
| III | 25 | - | |
| IV | 7 | - | |
| Differentiation | |||
| Well | - | 3 | - |
| Moderate | - | 9 | - |
| Poor | - | 31 | - |
CTR, healthy controls; GC, gastric cancer; TAT, thrombin-antithrombin complex; OD, optic density; MPO, myeloperoxidase; NE, neutrophil elastase; TNM, tumor-node-metastasis.
GC primes neutrophils to form NETs
Since NETs play a potential role in linking sterile inflammation and venous thrombosis and contribute to coagulation activation [15-17], we assessed the potency of neutrophils isolated from patients with GC to form NETs. We observed that the number of ex vivo NET releasing neutrophils from patients with GC was significantly higher than that from control individuals, as demonstrated by NET formation in cells (7.1%, n = 48, vs. 3.4%, n = 36, P < 0.0001) (MPO/DNA counterstaining, Figure 1A-C) and extracellular DNA levels in isolated NET structures (1.92 ± 1.04, μg/ml, n = 48, vs. 0.49 ± 0.03, n = 36, P < 0.0001) (Figure 1D). To further illustrate whether the circulation environment of GC induces neutrophils to release NETs, we investigated the effects of plasma and platelets from patients with GC on neutrophil NET release in vitro. Following GC plasma or platelet stimulation, significant increases in NET formation in control neutrophils was observed compared to the baseline levels (Figure 2). However, the effect was not significant when compared to control neutrophils stimulated with healthy individual plasma or platelets (Figure 2).
Figure 1.
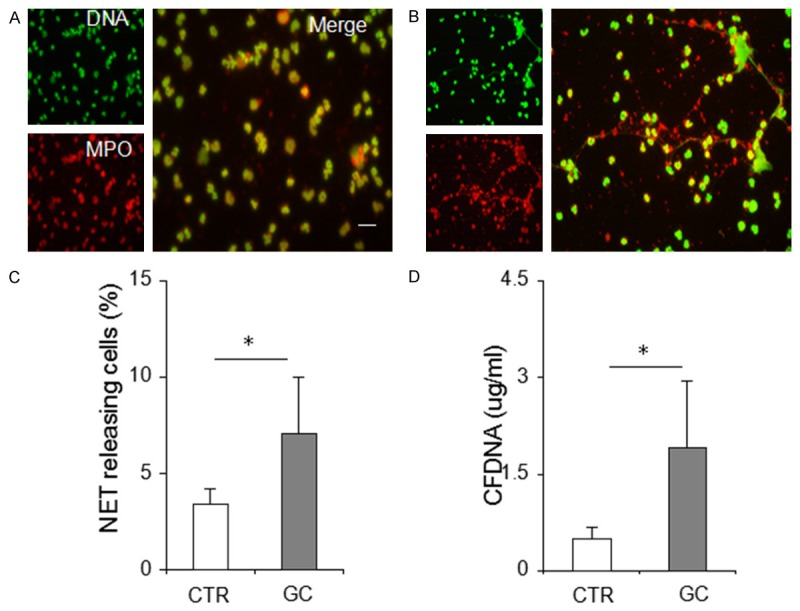
Enhanced neutrophil extracellular trap (NET) formation in neutrophils derived from patients with GC. (A) and (B). Representative microphotographs displaying NETs of neutrophils obtained from healthy controls and from patients with GC. Magnification, × 200. Scale bars, 20 µm. (C) The percentage of extracellular trap-releasing neutrophils and (D) extracellular DNA levels significantly increase in patients with GC compared with healthy controls. *indicates P < 0.001. Results are expressed as means ± standard deviation. CTR, healthy control (n = 36); GC, gastric cancer (n = 48), MPO, myeloperoxidase; CFDNA, cell-free DNA.
Figure 2.
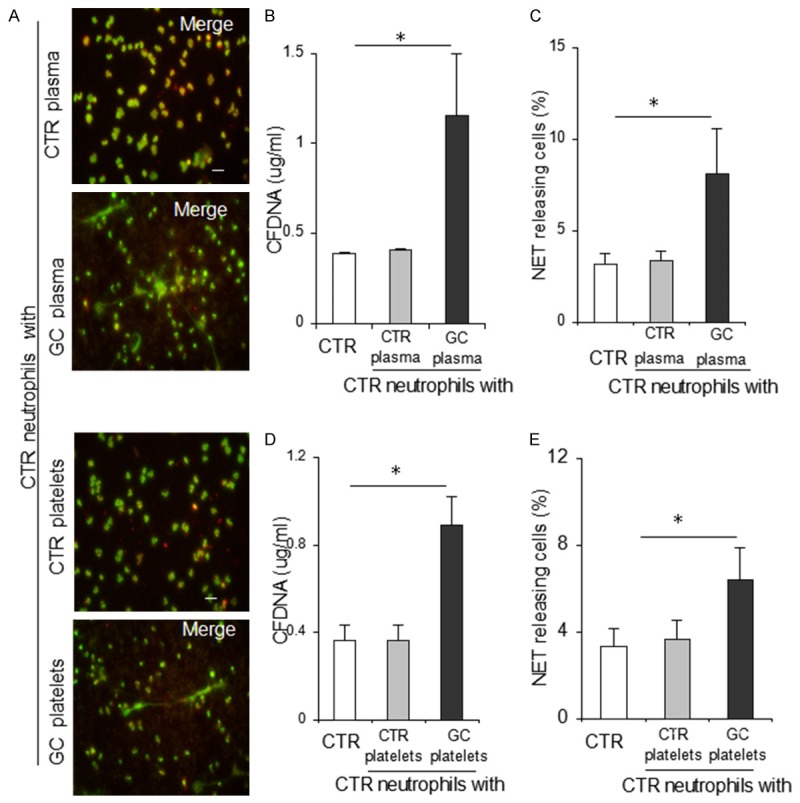
The microenvironment in patients with GC primes control neutrophils to release neutrophil extracellular traps. A. Representative microphotographs showing neutrophil extracellular trap generation in control neutrophils treated with plasma and platelets obtained from control individuals or patients with GC, respectively. Original magnification: × 200. Scale bar: 20 μm. B-E. Percentage of extracellular trap-releasing neutrophils and extracellular DNA levels were respectively used for illustrating that the potency of plasma and platelets derived from patients with GC to induce control neutrophils (n = 5) to generate NETs were significantly higher than those from healthy controls. *P < 0.001. Results are expressed as means ± standard deviation. Plasma was respectively derived from 48 patients with GC and 36 healthy controls and platelets were respectively derived from 10 patients and 10 healthy controls. CTR, healthy control; GC, gastric cancer; CFDNA, cell-free DNA.
Procoagulant activity of NETs derived from patients with GC
To study the procoagulant activity of NETs in patients with GC, thrombin levels and the potency of fibrin generation were assessed in control plasma treated with NETs. We observed significantly increased TAT levels after incubation of control plasma with NETs released by neutrophils isolated from GC compared with baseline (621 ± 164 pg/ml, vs. 380 ± 40 pg/ml, n = 48, P < 0.0001) (Figure 3A). However, TAT levels were not significantly increased after incubation of control plasma with NETs released by neutrophils isolated from healthy individuals (Figure 3A). Similarly, NETs released by neutrophils isolated from GC significantly increased the potency of fibrin generation in control plasma, as the time to peak and the peak turbidity of fibrin generation were significantly shorter and higher, respectively, than those in the baseline (Figure 3B, 3C). The effects of NETs released by neutrophils derived from controls on fibrin formation were not significant. To further certify a NET procoagulant role, we also performed an inhibition assay with DNase I. The results showed that treatment with DNase I significantly reduced the procoagulant role of NETs released by neutrophils derived from patients with GC (Figure 3D-F).
Figure 3.
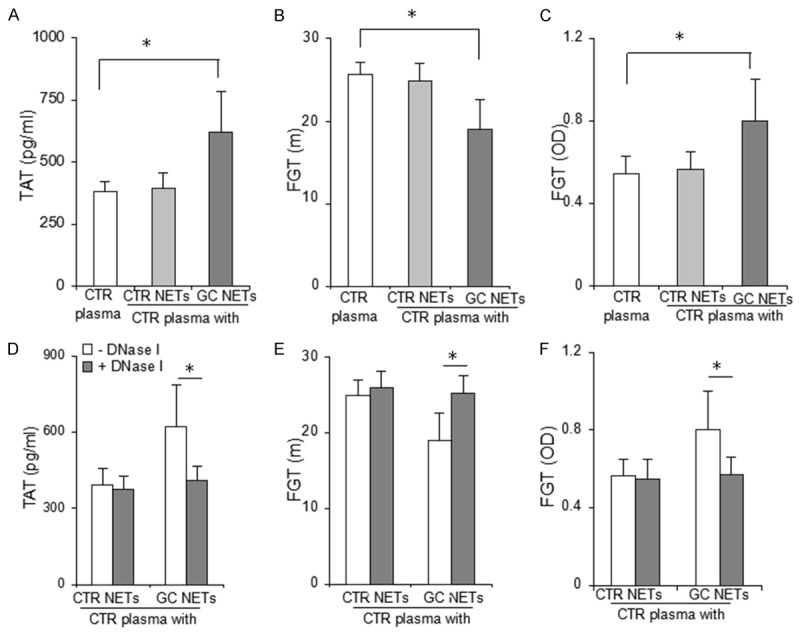
Procoagulant activity of NETs released by neutrophils derived from patients with GC. A. The inclusion of 20% NETs released by GC neutrophils significantly increased the amount of control plasma-generated TAT complex after recalcification. B, C. The inclusion of 20% NETs released by GC neutrophils significantly shortened the time to peak and increased the peak turbidity of control plasma generation of fibrin. D-F. DNase I significantly reduced the effect of NETs released by neutrophils derived from patients with GC on control plasma generation of thrombin and fibrin. *P < 0.001. Results are expressed as means ± standard deviation. CTR plasma, plasma derived from healthy controls (n = 5); CTR NETs, NETs released by healthy control neutrophils (n = 36); GC NETs, NETs released by neutrophils derived from patients with gastric cancer (n = 48); FGT, fibrin generation test.
Increased NET formation in patients with GC
To further certify autonomous NET formation in patients with GC, we assessed the levels of circulating MPO-DNA complex, a well-established marker of NET formation [17,33,34]. We found that the amount of circulating MPO-DNA complex in patients with GC was significantly higher than that in healthy controls (OD 405 value, 0.518 ± 0.507, n = 48, vs. 0.076 ± 0.024, n = 36, P < 0.001) (Table 1). In addition, circulating MPO-DNA complex was significantly elevated with increased TNM stage (Figure 4) and positively correlated with TAT and D-dimers (Table 2). To further confirm NET formation in these patients, CFDNA, nucleosomes, and NE, all of which are utilized in NET production, were evaluated. We found that they all significantly increased in patients with GC compared with healthy controls and also increased in parallel with TNM stage elevation (Figure 4), and were positively correlated with TAT and D-dimer levels (Table 2). In contrast, NET formation was not significantly correlated with tumor differentiation (not shown).
Figure 4.
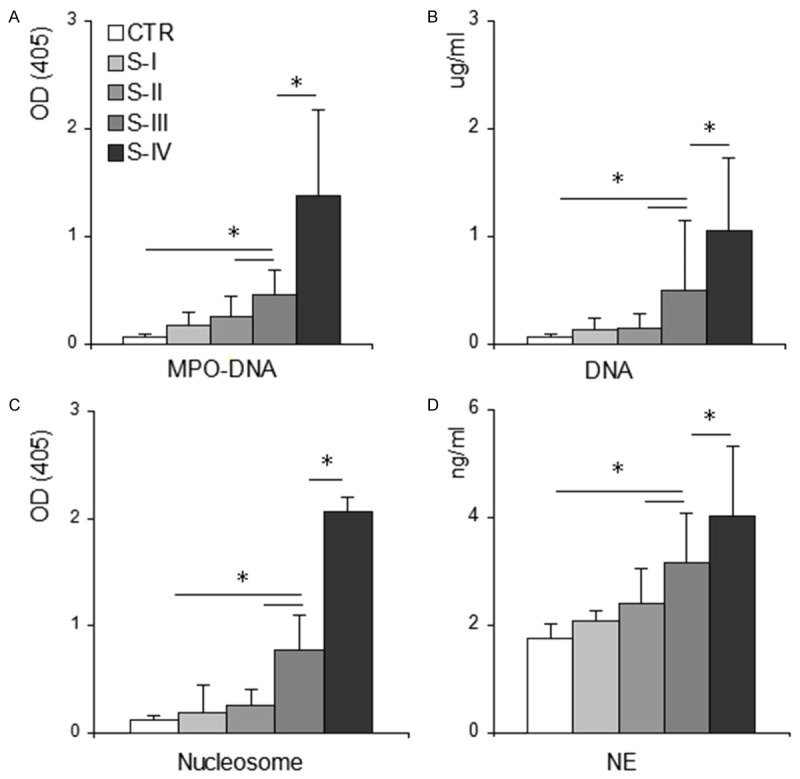
Significant increase of NET formation in patients with GC. Myeloperoxidase-deoxyribonucleic acid complex (MPO-DNA), cell-free nucleosomes, and neutrophil elastase were measured with ELISA using plasma samples from healthy donors (CTR, n = 36), individuals with GC including patients in stage I (S-I, n = 7), stage II (S-II, n = 9), stage III (S-III, n = 25) and stage IV (S-IV, n = 7) cancer. Plasma CFDNA was quantified with fluorescent quantification. (A) MPO-DNA complex, (B) CFDNA, (C) nucleosomes, and (D) NE in patients with GC were increased with disease progression. Data are expressed as means ± standard deviation. *P < 0.001. GC, gastric cancer; OD, optical density; MPO, myeloperoxidase; CFDNA, cell-free DNA; NE, neutrophil elastase.
Table 2.
Correlation between neutrophil extracellular trap components and a hypercoagulable state in gastric cancer
| Variable | TAT (pg/mL) | D-dimers (ng/ml) | ||
|---|---|---|---|---|
|
| ||||
| r | P | r | P | |
| CFDNA | 0.602 | < .0001 | 0.674 | <. 0001 |
| MPO-DNA (OD 405) | 0.625 | < .0001 | 0.49 | <. 0001 |
| Nucleosomes (OD 405) | 0.473 | < .0001 | 0.597 | < .0001 |
| NE (ng/mL) | 0.812 | < .0001 | 0.363 | 0.011 |
TAT, thrombin-antithrombin complex; CFDNA, cell-free deoxyribonucleic acid; MPO, myeloperoxidase; OD, optic density; NE, neutrophil elastase.
NET contribution to fibrin generation in plasma from patients with GC
To further certify that NETs contribute to the hypercoagulability in patients with GC, we investigated the role of NETs in the potency of autonomous plasma to generate fibrin. We found that the potency of GC plasma to generate fibrin significantly increased in patients with stage III/IV cancer compared with that of plasma from healthy controls (Figure 5A, 5B). Furthermore, treatment with DNase I significantly prolonged the time to peak and reduced peak turbidity in autonomous plasma derived from patients with GC and stage III/IV cancer (Figure 5C, 5D), whereas, the effect in patients with stage I/II cancer or healthy controls was not significant (Figure 5C, 5D). These data strongly suggest that NETs contribute to coagulation in patients with GC.
Figure 5.
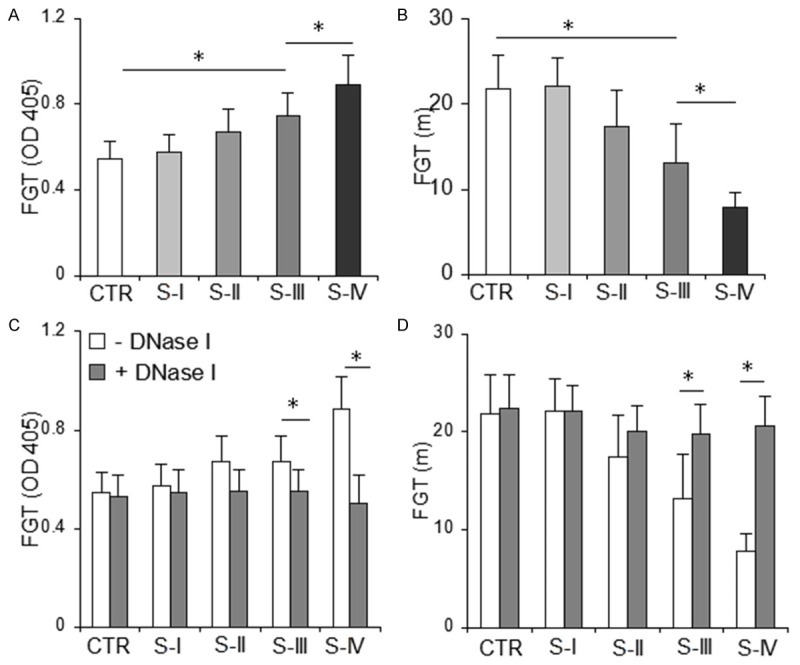
DNase I significantly inhibits fibrin generation in autonomous plasma from patients with stage III/IV GC. Autonomous plasma was incubated without or with DNase I, and fibrin formation was continually monitored after addition of CaCl2. A, B. The time to peak and peak turbidity of autonomous plasma derived from stage III/IV patients were significantly shorter and higher, respectively, than those from healthy controls. C, D. DNase I significantly prolonged the time to peak and reduced peak turbidity in patients with stage III/IV cancer. Each panel consists of the healthy controls (CTR, n = 36), patients in stage I (S-I, n = 7), stage II (S-II n = 9), stage III (S-III, n = 25) and stage IV (S-IV, n = 7) cancer. Data are represented as means ± SD. *P < 0.001. GC, gastric cancer; FGT, fibrin generation test.
Discussion
Hypercoagulability contributes to a significantly increased morbidity and mortality in patients with cancer [8-10] and activated neutrophils play an important role in the thrombosis associated with cancer in animal models [23,24]. Here, our results suggest that neutrophils derived from patients with GC display an enhanced ability to release NETs, which promote thrombin generation and then convert fibrinogen to fibrin. In addition, plasma or platelets derived from patients with GC stimulate control neutrophils to release NETs in vitro. We also identified that autonomous NET formation increased and contributed to the hypercoagulable state in patients with GC, whereas the procoagulant effect could be attenuated with DNase I dismantling of the NET structure. Furthermore, NET formation increased concomitant with TNM stage elevation and positively correlated with TAT and D-dimers levels.
NETs released from activated neutrophils were originally recognized as a host defense mechanism [35]. However, NET formation has also been shown to increase during sterile inflammation [17,33,34,36-38]. In this study, we found that the ability of neutrophils isolated from patients with GC to extrude the DNA/MPO complex was significantly higher than that of neutrophils isolated from healthy controls, and that plasma or platelets isolated from these patients could induce control neutrophils to release NETs. Furthermore, autonomous NET formation in patients with GC was significantly higher than that in healthy controls. These data strongly support our assertion that NETs are spontaneously formed in patients with GC, which extends previous reports indicating that NETs form in patients with cancer plus thrombotic microangiopathies or thrombosis [28,39].
In the present study, we found that plasma or platelets obtained from GC are able to induce control neutrophils to release NET in vitro. Inflammation is a hallmark in cancer [21,22] and significant increase of cytokines such as IL-8 or TNF-a, has been observed in patients with GC [40,41]. Additionally, we have previously found that platelet activation increases in patients with GC, certified by increased phosphatidylserine-positive platelets and platelet-derived microparticles (unpublished). All these data suggest that GC induces a systemic environment to prime neutrophils to release NETs, and further supports previous opinion that cytokine and inflammatory factors as well as activated platelet-neutrophil interactions induce NET release [17,33,34,38].
In this study, we also found that NETs released by neutrophils derived from GC significantly increased the potency of control plasma to generate thrombin and fibrin. Furthermore, the potency of plasma isolated from patients with stage III/IV GC to generate fibrin was attenuated with DNase I dismantling of NET structure. These results were consistent with and extend previous reports that NETs trigger coagulation activation [18]. In addition, research has also shown that cell-free histones, an important component of NETs, can cause activation of platelets and erythrocytes, which itself results in an increase in thrombin generation [19,20]. Thus, NETs play at least a partial role in the coagulopathy of patients with GC.
Finally, we found that NET formation was significantly elevated in parallel with TNM stage increase. These data suggested that increased tumor burden caused enhanced NET formation. However, a recent study showed that NETs could trap circulating cancer cells and contribute to the homing of metastasis in mice with infections [42], and could modulate the immune response in Ewing sarcoma [43], and in addition that NE could promote tumor growth and metastasis in vivo and in vitro [44]. We speculate that NET formation and tumor progression might therefore create a vicious circle in patients with GC. Further research is needed to certify the role of NETs in cancer development.
In conclusion, hypercoagulability is a common aggravating factor that facilitates tumor progression and increases the risk of thrombosis [8-10]. In this study, we found that NETs contributed to the hypercoagulable state in patients with stage III/IV GC, and that the effect could be inhibited with DNase 1 treatment. Recent animal studies have shown that targeting NETs could alleviate a prothrombotic state, inhibit cancer metastasis, and ameliorate the organ dysfunction associated with cancer [24,25,42]. Accordingly, targeting NETs might attenuate the hypercoagulable state correlated with increased NET formation, which might in turn translate into a relative good prognosis for patients with GC.
Acknowledgements
We thank Dong Wang and Yan Xia for excellent technical assistance and editorial assistance from Editage. This work was supported by a Ministry of Education, “Chunhui Plan” research project (Z2010001).
Disclosure of conflict of interest
None.
References
- 1.Di Micco P, Romano M, Niglio A, Nozzolillo P, Federico A, Petronella P, Nunziata L, Di Micco B, Torella R. Alteration of haemostasis in nonmetastatic gastric cancer. Dig Liver Dis. 2001;33:546–50. doi: 10.1016/s1590-8658(01)80105-5. [DOI] [PubMed] [Google Scholar]
- 2.Fidan E, Kavgaci H, Orem A, Yilmaz M, Yildiz B, Fidan S, Akcan B, Ozdemir F, Aydin F. Thrombin activatable fibrinolysis inhibitor and thrombinantithrombin-III-complex levels in patients with gastric cancer. Tumour Biol. 2012;33:1519–25. doi: 10.1007/s13277-012-0403-6. [DOI] [PubMed] [Google Scholar]
- 3.Kwon HC, Oh SY, Lee S, Kim SH, Han JY, Koh RY, Kim MC, Kim HJ. Plasma levels of prothrombin fragment F1+2, D-dimer and prothrombin time correlate with clinical stage and lymph node metastasis in operable gastric cancer patients. Jpn J Clin Oncol. 2008;38:2–7. doi: 10.1093/jjco/hym157. [DOI] [PubMed] [Google Scholar]
- 4.Young A, Chapman O, Connor C, Poole C, Rose P, Kakkar AK. Thrombosis and cancer. Nat Rev Clin Oncol. 2012;9:437–49. doi: 10.1038/nrclinonc.2012.106. [DOI] [PubMed] [Google Scholar]
- 5.Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122:1712–23. doi: 10.1182/blood-2013-04-460121. [DOI] [PubMed] [Google Scholar]
- 6.Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166:458–64. doi: 10.1001/archinte.166.4.458. [DOI] [PubMed] [Google Scholar]
- 7.Lee KW, Bang SM, Kim S, Lee HJ, Shin DY, Koh Y, Lee YG, Cha Y, Kim YJ, Kim JH, Park DJ, Kim HH, Oh D, Lee JS. The incidence, risk factors and prognostic implications of venous thromboembolism in patients with gastric cancer. J Thromb Haemost. 2010;8:540–7. doi: 10.1111/j.1538-7836.2009.03731.x. [DOI] [PubMed] [Google Scholar]
- 8.Nierodzik ML, Karpatkin S. Thrombin induces tumor growth, metastasis, and angiogenesis: Evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell. 2006;10:355–62. doi: 10.1016/j.ccr.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Lip GY, Chin BS, Blann AD. Cancer and the prothrombotic state. Lancet Oncol. 2002;3:27–34. doi: 10.1016/s1470-2045(01)00619-2. [DOI] [PubMed] [Google Scholar]
- 10.Prandoni P, Falanga A, Piccioli A. Cancer and venous thromboembolism. Lancet Oncol. 2005;6:401–10. doi: 10.1016/S1470-2045(05)70207-2. [DOI] [PubMed] [Google Scholar]
- 11.Lee AY, Rickles FR, Julian JA, Gent M, Baker RI, Bowden C, Kakkar AK, Prins M, Levine MN. Randomized comparison of low molecular weight heparin and coumarin derivatives on the survival of patients with cancer and venous thromboembolism. J. Clin. Oncol. 2005;23:2123–9. doi: 10.1200/JCO.2005.03.133. [DOI] [PubMed] [Google Scholar]
- 12.Klerk CP, Smorenburg SM, Otten HM, Lensing AW, Prins MH, Piovella F, Prandoni P, Bos MM, Richel DJ, van Tienhoven G, Büller HR. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J. Clin. Oncol. 2005;23:2130–5. doi: 10.1200/JCO.2005.03.134. [DOI] [PubMed] [Google Scholar]
- 13.Akl EA, Schünemann HJ. Routine heparin for patients with cancer? One answer, more questions. N Engl J Med. 2012;366:661–2. doi: 10.1056/NEJMe1113672. [DOI] [PubMed] [Google Scholar]
- 14.Prandoni P, Lensing AW, Piccioli A, Bernardi E, Simioni P, Girolami B, Marchiori A, Sabbion P, Prins MH, Noventa F, Girolami A. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484–8. doi: 10.1182/blood-2002-01-0108. [DOI] [PubMed] [Google Scholar]
- 15.Martinod K, Demers M, Fuchs TA, Wong SL, Brill A, Gallant M, Hu J, Wang Y, Wagner DD. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci U S A. 2013;110:8674–79. doi: 10.1073/pnas.1301059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD Jr, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107:15880–5. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stakos DA, Kambas K, Konstantinidis T, Mitroulis I, Apostolidou E, Arelaki S, Tsironidou V, Giatromanolaki A, Skendros P, Konstantinides S, Ritis K. Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardialinfarction. Eur Heart J. 2015;36:1405–14. doi: 10.1093/eurheartj/ehv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gould TJ, Vu TT, Swystun LL, Dwivedi DJ, Mai SH, Weitz JI, Liaw PC. Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms. Arterioscler Thromb Vasc Biol. 2014;34:1977–84. doi: 10.1161/ATVBAHA.114.304114. [DOI] [PubMed] [Google Scholar]
- 19.Semeraro F, Ammollo CT, Morrissey JH, Dale GL, Friese P, Esmon NL, Esmon CT. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood. 2011;118:1952–61. doi: 10.1182/blood-2011-03-343061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Semeraro F, Ammollo CT, Esmon NL, Esmon CT. Histones induce phosphatidylserine exposure and a procoagulant phenotype in human red blood cells. J Thromb Haemost. 2014;12:1697–02. doi: 10.1111/jth.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15:e493–503. doi: 10.1016/S1470-2045(14)70263-3. [DOI] [PubMed] [Google Scholar]
- 23.Shao B, Wahrenbrock MG, Yao L, David T, Coughlin SR, Xia L, Varki A, McEver RP. Carcinoma mucins trigger reciprocal activation of platelets and neutrophils in a murine model of trousseau syndrome. Blood. 2011;118:4015–23. doi: 10.1182/blood-2011-07-368514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demers M, Krause DS, Schatzberg D, Martinod K, Voorhees JR, Fuchs TA, Scadden DT, Wagner DD. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci U S A. 2012;109:13076–81. doi: 10.1073/pnas.1200419109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cedervall J, Zhang Y, Huang H, Zhang L, Femel J, Dimberg A, Olsson AK. Neutrophil extracellular traps accumulate in peripheral blood vessels and compromise organ function in tumorbearing animals. Cancer Res. 2015;75:2653–62. doi: 10.1158/0008-5472.CAN-14-3299. [DOI] [PubMed] [Google Scholar]
- 26.Lacroix R, Judicone C, Poncelet P, Robert S, Arnaud L, Sampol J, Dignat-George F. Impact of pre-analytical parameters on the measurement of circulating microparticles: towards standardization of protocol. J Thromb Haemost. 2012;10:437–46. doi: 10.1111/j.1538-7836.2011.04610.x. [DOI] [PubMed] [Google Scholar]
- 27.Lacroix R, Judicone C, Mooberry M, Boucekine M, Key NS, Dignat-George F. The ISTH SSC Workshop. Standardization of pre-analytical variables in plasma microparticle determination: results of the international society on thrombosis and haemostasis ssc collaborative workshop. J Thromb Haemost. 2013;11:1190–93. doi: 10.1111/jth.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuchs TA, Kremer Hovinga JA, Schatzberg D, Wagner DD, Lämmle B. Circulating DNA and myeloperoxidase indicate disease activity in patients with thrombotic microangiopathies. Blood. 2012;120:1157–1164. doi: 10.1182/blood-2012-02-412197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borissoff JI, Joosen IA, Versteylen MO, Brill A, Fuchs TA, Savchenko AS, Gallant M, Martinod K, Ten Cate H, Hofstra L, Crijns HJ, Wagner DD, Kietselaer BL. Elevated levels of circulating DNA and chromatin are independently associated with severe coronary atherosclerosis and a prothrombotic state. Arterioscler Thromb Vasc Biol. 2013;33:2032–2040. doi: 10.1161/ATVBAHA.113.301627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aleman MM, Gardiner C, Harrison P, Wolberg AS. Differential contributions of monocyte- and platelet-derived microparticles towards thrombin generation and fibrin formation and stability. J Thromb Haemost. 2011;9:2251–2261. doi: 10.1111/j.1538-7836.2011.04488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berckmans RJ, Sturk A, van Tienen LM, Schaap MC, Nieuwland R. Cell-derived vesicles exposing coagulant tissue factor in saliva. Blood. 2011;117:3172–3180. doi: 10.1182/blood-2010-06-290460. [DOI] [PubMed] [Google Scholar]
- 32.van Doormaal F, Kleinjan A, Berckmans RJ, Mackman N, Manly D, Kamphuisen PW, Richel DJ, Büller HR, Sturk A, Nieuwland R. Coagulation activation and microparticle-associated coagulant activity in cancer patients. An exploratory prospective study. Thromb Haemost. 2012;108:160–165. doi: 10.1160/TH12-02-0099. [DOI] [PubMed] [Google Scholar]
- 33.Kessenbrock K, Krumbholz M, Schönermarck U, Back W, Gross WL, Werb Z, Gröne HJ, Brinkmann V, Jenne DE. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caudrillier A, Kessenbrock K, Gilliss BM, Nguyen JX, Marques MB, Monestier M, Toy P, Werb Z, Looney MR. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest. 2012;122:2661–2671. doi: 10.1172/JCI61303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–5. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 36.Wong SL, Demers M, Martinod K, Gallant M, Wang Y, Goldfine AB, Kahn CR, Wagner DD. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med. 2015;21:815–9. doi: 10.1038/nm.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schauer C, Janko C, Munoz LE, Zhao Y, Kienhöfer D, Frey B, Lell M, Manger B, Rech J, Naschberger E, Holmdahl R, Krenn V, Harrer T, Jeremic I, Bilyy R, Schett G, Hoffmann M, Herrmann M. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med. 2014;20:511–7. doi: 10.1038/nm.3547. [DOI] [PubMed] [Google Scholar]
- 38.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, Friday S, Li S, Patel RM, Subramanian V, Thompson P, Chen P, Fox DA, Pennathur S, Kaplan MJ. Nets are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. 2013;5:178ra40. doi: 10.1126/scitranslmed.3005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Montfoort ML, Stephan F, Lauw MN, Hutten BA, Van Mierlo GJ, Solati S, Middeldorp S, Meijers JC, Zeerleder S. Circulating nucleosomes and neutrophil activation as risk factors for deep vein thrombosis. Arterioscler Thromb Vasc Biol. 2013;33:147–51. doi: 10.1161/ATVBAHA.112.300498. [DOI] [PubMed] [Google Scholar]
- 40.Lu W, Pan K, Zhang L, Lin D, Miao X, You W. Genetic polymorphisms of interleukin (il)-1b, il-1rn, il-8, il-10 and tumor necrosis factor {alpha} and risk of gastric cancer in a Chinese population. Carcinogenesis. 2005;26:631–6. doi: 10.1093/carcin/bgh349. [DOI] [PubMed] [Google Scholar]
- 41.Macrì A, Versaci A, Loddo S, Scuderi G, Travagliante M, Trimarchi G, Teti D, Famulari C. Serum levels of interleukin 1 beta, interleukin 8 and tumour necrosis factor alpha as markers of gastric cancer. Biomarkers. 2006;11:184–9. doi: 10.1080/13547500600565677. [DOI] [PubMed] [Google Scholar]
- 42.Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P, Ferri L. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013;123:3446–58. doi: 10.1172/JCI67484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berger-Achituv S, Brinkmann V, Abed UA, Kühn LI, Ben-Ezra J, Elhasid R, Zychlinsky A. A proposed role for neutrophils extracellular traps in cancer immunoediting. Front Immunol. 2013;4:48–53. doi: 10.3389/fimmu.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Houghton AM, Rzymkiewicz DM, Ji H, Gregory AD, Egea EE, Metz HE, Stolz DB, Land SR, Marconcini LA, Kliment CR, Jenkins KM, Beaulieu KA, Mouded M, Frank SJ, Wong KK, Shapiro SD. Neutrophil elastase-mediated degradation of irs-1 accelerates lung tumor growth. Nat Med. 2010;16:219–23. doi: 10.1038/nm.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]


