Abstract
The use of DPP-4 inhibitors in combination with insulin has been proposed as an alternative therapeutic option for poorly controlled type 2 diabetes (T2D) patients. We thus performed a meta-analysis of randomized controlled trials (RCTs) to assess the efficacy and safety of this combination therapy in adult T2D patients. Seven eligible studies involving 3,384 participants were included for the study. The resulting data revealed that the combination therapy of DPP-4 inhibitor and insulin is associated with a modest reduction in HbA1c (-0.52%; 95% CI -0.59 to -0.44), a decrease in 2h-PPG (-1.81 mmol/l; -2.23 to -1.38), and an increase in the proportion of patients reaching the target HbA1c of ≤ 7% (RR 2.24; 95% CI 1.80 to 2.77) without increasing the risk of hypoglycemia (RR 1.04; 0.83 to 1.31) or body weight (-0.11 kg; -0.56 to 0.33), as compared with other anti-diabetic treatments. These results support that this combination therapy could serve as a potential therapeutic strategy that offers an alternative option for patients inadequately controlled on other anti-diabetic agents in clinical practice.
Keywords: DPP-4 inhibitors, insulin, type 2 diabetes, hypoglycemia, weight gain
Introduction
Type 2 diabetes (T2D) is a progressive metabolic disease characterized by the insufficient insulin secretion and/or insulin resistance with resultant hyperglycemia [1]. The first-line treatment drugs such as metformin have proved to be effective in lowering glucose levels and were widely used in newly diagnosed T2D patients [2]. However, due to the progressive nature of this disease, many patients on oral anti-diabetic drugs (OADs) failed to achieve or maintain a satisfactory glycemic control over time [3]. Insulin therapy has been the mainstay treatment for patients inadequately controlled on OADs. However, the exogenous insulin cannot regulate glucose levels as accurately as the endogenous insulin released by the functioning pancreatic islets, which in some degree limited its application in clinical settings [3-5]. Data from the National Health and Nutrition Examination Survey (NHANES) show that around 33% of US patients with T2D were on insulin monotherapy, while only a few patients were able to reach an HbA1c level of less than 7% [6]. One reason is that, although dose titration of insulin improves glycemic control, its use is often limited in clinical practice by concerns about the risks of hypoglycemia, weight gain and patients’ barriers for dosing adjustment [7,8]. Also, some insulin regimens do not target postprandial glucose levels effectively [9,10]. These observations suggest that insulin monotherapy can no longer offer a satisfactory glycemic control over time during the course of T2D progression, and patients would eventually require the combination treatment of insulin and other anti-diabetic agents. Thus, an optimal anti-diabetic therapy would be the one that can improve glycemic control without increasing the risk of hypoglycemia or causing weight gain.
Dipeptidyl peptidase-4 (DPP-4) inhibitors, also known as gliptins, are a new class of oral anti-diabetic agents that have been commonly used as the second or third-line medication in type 2 diabetes [11]. They work by blocking the action of DPP-4, which prevents the degradation of gastrointestinal incretins glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). Incretins are a group of hormones secreted by gut cells after meal, which could induce insulin secretion and inhibit glucagon release in a glucose-dependent manner [12-14]. Moreover, DPP-4 inhibitor can also slow down food digestion, decrease gastric emptying and suppress appetite [12]. Therefore, DPP-4 inhibitor can help to regulate blood glucose levels (especially the postprandial glucose excursion) with a low propensity of causing hypoglycemia or weight gain by protecting incretins from degradation.
Given the complementary action of DPP-4 inhibitor and insulin on glucose regulation, the combination of DPP-4 inhibitor with insulin was supposed to be a potential treatment strategy for poorly controlled T2D patients [15,16]. First, DPP-4 inhibitor lowers the postprandial plasma glucose (PPG) levels by decelerating gastric emptying, which best complements the action of basal insulin on fasting glucose control [14], and therefore, it offers the potential for T2D patients to achieve their target HbAc1. Second, patients on insulin treatment often manifest a significant insulin resistance and might need a higher dose, which can exacerbate weight gain and hypoglycemia, while DPP-4 inhibitor does not appear to increase the risk of hypoglycemia or promote weight gain [17,18]. As such, the combination of DPP-4 inhibitor and insulin might improve insulin sensitivity and enable insulin dosage to be reduced, which could further lower the risk of hypoglycemia and weight gain. Third, unlike other anti-diabetic agents, Linagliptin (one of the DPP-4 inhibitors) has a primarily non-renal route of elimination, rather it is mainly excreted by the bile and gut system, and therefore, it does not require dose adjustment in the condition of renal or hepatic impairment [19,20]. This is beneficial for the elderly patients and those with impaired renal function. Fourth, DPP-4 inhibitors are administered orally with acceptable side effects, which can help to improve drug compliance and increase medication adherence. Finally, data from cardiovascular safety studies show that DPP-4 inhibitors do not increase the risk of long term vascular complications in type 2 diabetes [21,22].
In light of the above rationale, a number of clinical studies were conducted to assess the efficacy and safety of this combination therapy in adult patients with type 2 diabetes. However, these studies might not be able to provide sufficient information for clinical condition on their own. We, therefore, conducted a meta-analysis of RCTs to examine the effect of this combination therapy on body weight, glycemic control and risk of hypoglycemia in adult T2D patients.
Methods
Literature search strategy and selection criteria
We searched the PubMed, Medline, Embase, Cochrane Library and ClinicalTrials.gov for relevant records published from January 1, 1990 to July 31, 2015. Both Medical Subject Heading (MeSH) terms and keywords were employed to search for potentially eligible records involving Dipeptidyl-Peptidase IV Inhibitors and insulin (Dipeptidyl-peptidase-IV inhibitor or Dipeptidyl peptidase-4 inhibitor or DPP-4 inhibitor or linagliptin or saxagliptin or sitagliptin or alogliptin or vildagliptin; insulin or glargine or detemir or degludec or NPH). The resulting records were combined with diabetes mellitus, type 2 diabetes or T2DM to identify participants with T2D. A manual search of the bibliographies of retrieved literatures was also performed to identify additional studies. We considered all potentially eligible studies for further review with no restriction on treatment history or language.
Study selection and data extraction
To identify relevant studies, two independent investigators reviewed titles, abstracts and keywords of retrieved records. Full-text articles were retrieved for further assessment if they meet the following criteria: (1) randomized trials done in adult patients with T2D; (2) compared the combination therapy of DPP-4 inhibitor and insulin with placebo or other anti-diabetic agents; (3) reported at least one clinical outcome of interest; (4) had a minimum duration of no less than 8 weeks. Studies were excluded if (1) they were observational or retrospective studies; or (2) the combination treatment of DPP-4 inhibitor and insulin was not assessed; or (3) the duration was less than 8 weeks; or (4) insufficient data were provided for calculating the pooled estimates. Two investigators extracted data from the identified studies independently and disagreements were resolved by consensus. The following information were extracted: study design of included trials (randomized, open-label, blinded, parallel-group, etc.), type of anti-diabetic treatments, trial duration, baseline characteristics (number of participants, age, male %, racial, duration of diabetes, complications, etc.) and outcomes [change in body weight, fasting plasma glucose (FPG), PPG and HbA1c; the number of participants achieving a target HbA1c of ≤ 7.0% at the endline; and the number of participants with any hypoglycemic episodes].
Quality assessment
Two independent investigators assessed the quality of included studies on the Jadad scale [23]. The value of quality ranged from 0 to 5 points with a score ≥ 3 as high quality, while low quality was designated as a score ≤ 2. Discrepancies were resolved by consensus.
Statistical analysis
Changes in body weight and glycemic control (HbA1c, FPG and PPG) were reported as continuous variables, and the pooled estimates of the between-group differences for these outcomes were calculated with fixed effects model. Effect sizes were presented as weighted mean difference (WMD) with 95 percent confidence interval (95% CI). A random effects model (DerSimonian-Laird method) was used when heterogeneity between studies was statistically significant. For categorical outcomes such as the number of participants having any episodes of hypoglycemia and those achieving an HbA1c level of ≤ 7.0%, the pooled relative risk was calculated under fixed effects model when no heterogeneity was detected. Risk of publication bias was evaluated by funnel plot and Egger’s test [24], which assesses the asymmetry of funnel plot with p-value < 0.1 to be considered of significant bias. Heterogeneity was assessed using the Chi-Square test and p-value < 0.1 was considered statistically significant [25]. STATA 11.2 statistical software was used in all statistical analysis in the current report.
Results
Description of the studies
One thousand and eleven records from electronic search with additional 5 from manual search were obtained. Title and abstract screening excluded 985 papers that did not meet the inclusion criteria, leaving 18 records for full-text screening, and finally, 7 studies were included for the analysis (Figure 1).
Figure 1.
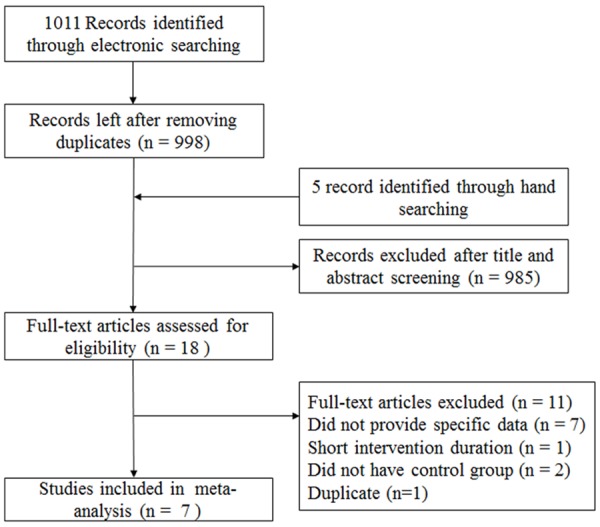
Flow chart for the selection of literatures.
The quality of included studies was further assessed as described (Table 1). All of these 7 studies reported detailed randomization method and were classified as of high quality based on the Jadad scaling system [26-32]. Of which, 6 studies were multicenter [26-28,30-32], 5 were double-blind, and 2 were open-label [28,29]. The dropout rate varied from 11.3% to 19.6% among studies. However, only one of them was not industry-funded [29].
Table 1.
Analysis for the quality of included studies
| Study | Multicenter | Sequence generation | Allocation concealment | Double blinding | With-drawn (%) | Score | ITT analysis | Industry funded |
|---|---|---|---|---|---|---|---|---|
| Hollander 2011 | Yes | Yes | Yes | No | 15.2 | 3 | Not informed | Yes |
| Barnett 2012 | Yes | Yes | Yes | Yes | 11.3 | 5 | Not informed | Yes |
| Hong 2012 | No | Yes | Yes | No | 11.4 | 3 | Yes | No |
| Vilsbol 2010 | Yes | Yes | Yes | Yes | 12.0 | 5 | Not informed | Yes |
| Yki-Jarvinen 2013 | Yes | Yes | Yes | Yes | 15.7 | 5 | No | Yes |
| Fonseca 2007 | Yes | Yes | Yes | Yes | 19.6 | 4 | Yes | Yes |
| Rosenstock 2009 | Yes | Yes | Yes | Yes | 18.2 | 5 | Yes | Yes |
The included studies were published between 2007 and 2013 with a total of 3,384 participants (Table 2). The sample size ranged from 124 to 1,261 for each individual study. The proportion of male participants was 49.1% in average with a mean age of 58 years old (ranged from 57 to 74.3 years). Half of the participants have had T2D for more than 10 years. Intervention duration was 28 weeks in average (ranged from 24 to 52 weeks). Mean level of HbA1c and weight at baseline were 8.7% (ranged from 8.3% to 9.3%) and 85.9 kg (ranged from 67.4 to 94.8 kg), respectively.
Table 2.
Results for clinical manifestations of included studies
| Intervention | Subjects | Baseline outcomes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Author | Background treatment | Study arms | Duration (wk) | No. | Male (%) | Age (y) | Disease duration (y) | BMI (kg/m2) | Weight (kg) | HbA1c (%) |
| Hollander 2011 | MET ± OADs | Determir + sitagliptin vs. sitagliptin | 26 | 217 | 54.4 | 57 | 9.8 | 31.9 | 90.7 | 8.5 |
| Barnett 2012 | Basal or premixed insulin ± MET | Saxagliptin vs. placebo | 24 | 455 | 41 | 57.3 | 12 | 32.2 | 87.0 | 8.7 |
| Hong 2012 | Basal or mixed insulin ± OADs | Sitagliptin vs. insulin | 24 | 124 | 52.4 | 59.2 | 15.9 | 25.6 | 67.4 | 9.2 |
| Vilsbol 2010 | Basal or premixed insulin ± MET | Sitagliptin vs. placebo | 24 | 641 | 50.9 | 57.8 | 12.5 | 31 | 86.9 | 8.7 |
| Yki-Jarvinen 2013 | Basal insulin ± MET ± pioglitazone | Linagliptin vs. placebo | 52 | 1261 | 52.2 | 60 | Not informed | 31 | Not informed | 8.3 |
| Fonseca 2007 | Basal or mixed insulin | Vildagliptin vs. placebo | 24 | 296 | 51.4 | 59.3 | 14.7 | 33.1 | 94.8 | 8.4 |
| Rosenstock 2009 | Basal insulin ± MET | Alogliptin vs. placebo | 26 | 390 | 41.3 | 55.4 | 12.6 | 32.5 | 88.5 | 9.3 |
Among the 5 currently available DPP-4 inhibitors, sitagliptin was assessed in 3 studies [28,29,31], while the rest of each (saxagliptin, vildagliptin, linagliptin and alogliptin) was only assessed in one study. The initial combination of DPP-4 inhibitor with insulin was evaluated only in one study [28], and DPP-4 inhibitor as add-on agents to insulin were assessed in 6 studies. Recruited patients in 4 studies used both basal and mixed insulin, while patients from other 3 studies only used basal insulin with or without other OADs [28,30,32]. Five studies compared DPP-4 inhibitors with placebo on a background of basal or mixed insulin therapy with or without other OADs; one study compared the combination treatment to basal or mixed insulin therapy with dose titration based on glucose levels [29]; and one study compared the combination treatment with sitagliptin plus metformin therapy [28].
Risk of publication bias
No publication bias was detected by Egger’s test for any outcome assessed (Figure 2). The p-value for each outcome was listed below: the changes in body weight (Figure 2A, p = 0.85), HbA1c levels (Figure 2B, p = 0.524), FPG (Figure 2C, p = 0.386) and PPG levels (Figure 2D, p = 0.7); the number of participants achieving an HbA1c of ≤ 7.0% (Figure 2E, p = 0.61), and the number of participants with any hypoglycemic episodes (Figure 2F, p = 0.924).
Figure 2.
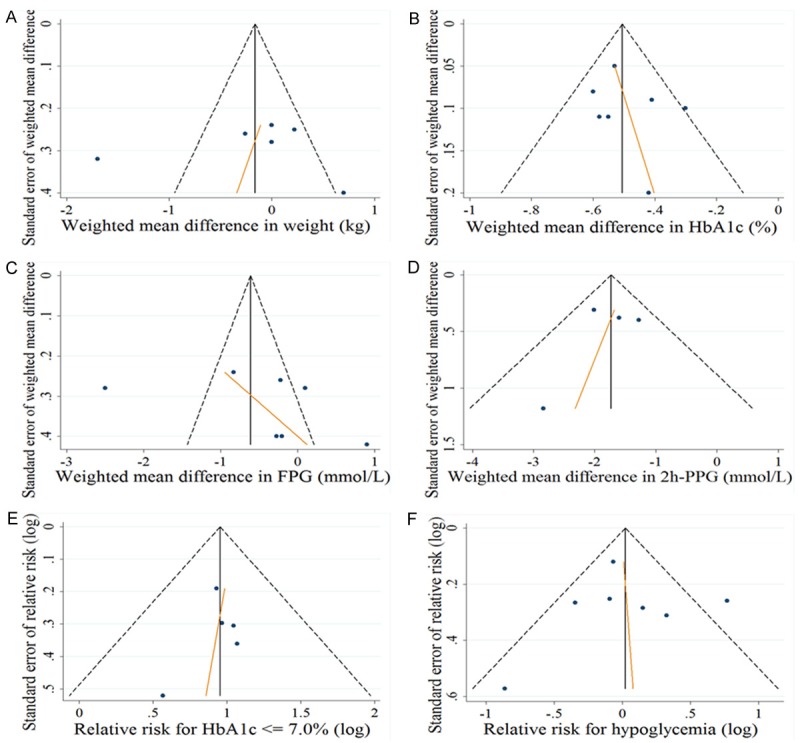
Funnel plot with Egger’s test was employed to assess the risk of publication bias for the changes in (A) body weight (kg), (B) HbA1c (%), (C) FPG (mmol/l), (D) PPG (mmol/l), (E) relative risk for HAb1c ≤ 7.0%, and (F) relative risk for hypoglycemia episodes.
The effect of intervention
Six out of 7 studies with 3,167 participants evaluated changes in body weight [26,27,29-32]. Pooled data showed no significant difference in terms of body weight change (-0.11 kg; 95% CI -0.56 to 0.33) between the DPP-4 inhibitor plus insulin therapy and other anti-diabetic treatments, but a significant heterogeneity was detected (I2 = 69.4%, P = 0.006) (Figure 3A). All seven studies involving 3,384 patients assessed the change of HbA1c levels. Our analysis revealed that the combination therapy of DDP-4 inhibitor and insulin led to a greater reduction for HbA1c level (-0.52%; -0.59 to -0.44) absent of a significant heterogeneity (I2 = 0%, P = 0.465) (Figure 3B). Pooled analysis of 7 studies that assessed the change in fasting glucose levels showed a glucose lowering tendency for the combination treatment, but did not reach a statistical significance (-0.68 mmol/l; -1.40 to 0.04) (Figure 4A). Four out of 7 studies with 1,437 patients evaluated the change of 2h-postprandial glucose levels, and pooled analysis indicated a greater reduction for PPG levels in favor of the combination treatment (-1.81 mmol/l; -2.23 to -1.38), and no significant heterogeneity was detected (I2 = 24.8%, P = 0.263) (Figure 4B) [26,28,29,31]. Five out of 7 studies with 2,698 patients assessed the proportion of patients reaching the target HbA1c (≤ 7%) [26,28,29,31,32], and compared with other treatments, the combination therapy of DPP-4 inhibitor and insulin manifested a higher propensity in achieving this goal (RR 2.24; 95% CI 1.80 to 2.77) without a significant heterogeneity (I2 = 0%, P = 0.797) (Figure 5A). Pooled analysis of 7 studies that examined the relative risk of hypoglycemia showed no significant difference between the combination therapy (RR 1.04; 0.83 to 1.31) and other anti-diabetic treatments, while a significant heterogeneity was noted (I2 = 58.5%, P = 0.025) (Figure 5B).
Figure 3.
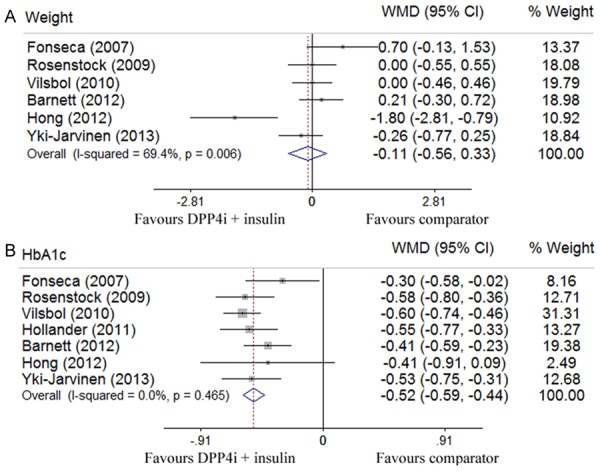
Outcomes for the comparison of body weight (A) and HbA1c (%) (B) of combination therapy (DPP-4 inhibitors and insulin) with placebo or other anti-diabetic agents in patients with type 2 diabetes by Forest plots.
Figure 4.
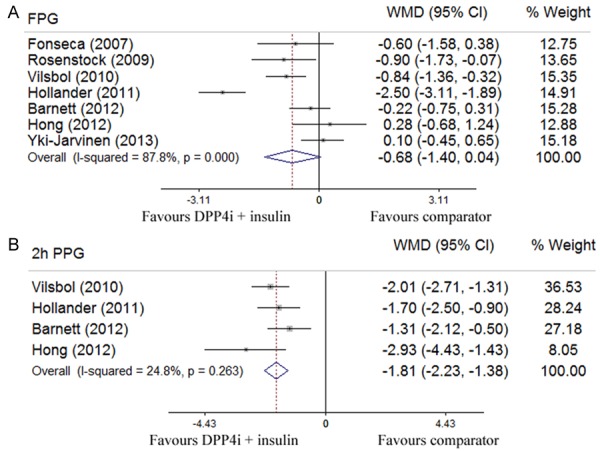
Results assessed by Forest plots for the combination treatment of DPP4 inhibitors and insulin with placebo or other anti-diabetic agents in T2D patients for FPG (mmol/l) (A) and PPG (mmol/l) (B).
Figure 5.
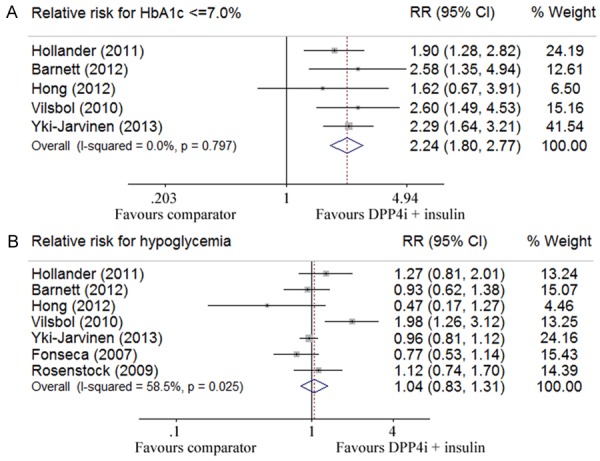
Relative risks assessed by Forest plots in terms of failing to reach HAb1c ≤ 7.0% (A), and the episodes for developing hypoglycemia (B).
Discussion
Although insulin therapy is thus far the mainstay for treatment of patients with inadequately controlled glucose levels by oral anti-diabetic drugs (OADs), while insulin therapy alone sometimes also fails to provide good results for those patients, indicating the requirement of combination therapy (i.e., insulin combined with other anti-diabetic agents) for this particular group of patients. We thus in the current report conducted a meta-analysis to assess the effect of combination therapy on body weight, glycemic control and risk of hypoglycemia in adult patients with T2D. By analysis of 7 studies involving a total of 3,384 patients, we demonstrated that the combination therapy of DPP-4 inhibitor and insulin on adult T2D patients is moderately effective in improving glycemic control without increasing the risk of hypoglycemia or causing weight gain as compared with that of other anti-diabetic treatments. Our findings were consistent across different types and sizes of trials and strongly supported the effectiveness and safety of this combination therapy in the management of type 2 diabetes.
Recent developments in pharmacology and clinical medicine have largely promoted new drug development and treatment optimization in type 2 diabetes [2,13]. However, limitations of currently available treatments have vented the achievement of glycemic goals. Particularly, some anti-diabetic agents (e.g., insulin, thiazolidinedione and sulfonylurea) may somehow manifest greater efficacy at the expense of dose-related side effect such as hypoglycemia, edema and weight gain [33,34]. More importantly, no anti-diabetic treatment has been shown to be long term effective in preventing beta cell deterioration and disease progression [35]. In this context, DPP-4 inhibitor which improves glycemic control with a favorable side-effect profile is therefore of interest. First, it is associated with a low risk of hypoglycemia [18]. An intriguing hypothesis for the underlying mechanism is that DPP-4 inhibitor restores pancreatic alpha cell function and improves its ability to sense and respond to changes in plasma glucose levels. It is believed that DPP-4 inhibitor mediates the suppression of inappropriate glucagon secretion in the presence of hyperglycemia while enhances the stimulatory effect of glucose on alpha cell during hypoglycemia [12,13]. However, hypoglycemia can still occur, especially when DPP-4 inhibitor is applied with insulin secretagogues such as sulfonylureas [36]. Therefore, once it was employed in combination, the dose of insulin secretagogues should be adjusted to minimize hypoglycemia. Second, DPP-4 inhibitor is weight-neutral overall but possesses a favorable weight profile relative to thiazolidinedione or sulfonylureas [37,38], which is an advantage when considering agents for combination treatment. Third, the elderly patients, who tend to have predominant postprandial hyperglycemia, would gain more benefits from DPP-4 inhibitor treatment due to its glucagon lowering effect [39]. Based on the above knowledge, the combination of DPP-4 inhibitor and insulin was suggested as a promising treatment strategy for improving glycemic control while attenuating insulin-related risks in T2D patients.
Several limitations for our analysis are necessary to point out. First, except one study with a trial duration of 52 weeks, the others were in short durations (less than 30 weeks), which limits the assessment of long term efficacy and safety of this combination treatment. Second, the included studies were generally of good quality, while there were still some factors that might introduce bias such as the use of open-label studies and being industry funded. Moreover, the implementation of insulin dose titration was not consistent in studies included in our meta-analysis, which rendered the interpretation of results with difficulty. Third, the included participants in this analysis were predominantly white and specific data for other races were not provided. As a result, the differential effect of this combination therapy could not be assessed by race or ethnicity. Furthermore, the ideal timing of initiating this combination therapy for dealing with type 2 diabetes is not clear. Generally, insulin and DPP-4 inhibitors were used as the second or third-line therapy in poorly controlled T2D patients, by then beta cell function has been seriously deteriorated and more intensified therapy was needed. Hence, further studies with focus to assess the efficacy of early application of this treatment are warranted. Finally, there are 5 different DPP-4 inhibitors available in the market, each of which may display different pharmacological profiles such as absorption rate, systemic half-life and mode of clearance due to variations in structure. Therefore, follow-up studies designed to assess the optimal approach for their individual application with insulin are needed for achieving a better glucose control.
In conclusion, our data indicate that the combination treatment of insulin and DPP-4 inhibitor is beneficial in terms of glycemic control. However, additional studies are warranted to establish the optimal approach for its application in clinical practice.
Acknowledgements
This work was supported by grants from the Natural Science Foundation of China (81130014, 81471046 and 81530024), and Innovative Funding for Translational Research from Tongji Hospital.
References
- 1.DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. 2004;88:787–835. ix. doi: 10.1016/j.mcna.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD) Management of hyperglycemia in type 2 diabetes: a patientcentered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281:2005–2012. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]
- 4.Korytkowski M. When oral agents fail: practical barriers to starting insulin. Int J Obes Relat Metab Disord. 2002;26(Suppl 3):S18–24. doi: 10.1038/sj.ijo.0802173. [DOI] [PubMed] [Google Scholar]
- 5.Guler S, Vaz JA, Ligthelm R. Intensification lessons with modern premixes: from clinical trial to clinical practice. Diabetes Res Clin Pract. 2008;81(Suppl 1):S23–30. doi: 10.1016/j.diabres.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 6.National Health and Nutrition Examination Survey 2005-2006 data files. Accessed 19 November 2007. [Google Scholar]
- 7.Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B American Diabetes Association; European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnett AH, Cradock S, Fisher M, Hall G, Hughes E, Middleton A. Key considerations around the risks and consequences of hypoglycaemia in people with type 2 diabetes. Int J Clin Pract. 2010;64:1121–1129. doi: 10.1111/j.1742-1241.2009.02332.x. [DOI] [PubMed] [Google Scholar]
- 9.Holman RR, Thorne KI, Farmer AJ, Davies MJ, Keenan JF, Paul S, Levy JC 4-T Study Group. Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med. 2007;357:1716–1730. doi: 10.1056/NEJMoa075392. [DOI] [PubMed] [Google Scholar]
- 10.Bretzel RG, Nuber U, Landgraf W, Owens DR, Bradley C, Linn T. Once-daily basal insulin glargine versus thrice-daily prandial insulin lispro in people with type 2 diabetes on oral hypoglycaemic agents (APOLLO): an open randomised controlled trial. Lancet. 2008;371:1073–1084. doi: 10.1016/S0140-6736(08)60485-7. [DOI] [PubMed] [Google Scholar]
- 11.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 12.Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17:819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Janardhan S, Sastry GN. Dipeptidyl peptidase IV inhibitors: a new paradigm in type 2 diabetes treatment. Curr Drug Targets. 2014;15:600–621. doi: 10.2174/1389450115666140311102638. [DOI] [PubMed] [Google Scholar]
- 14.Gerich J. Pathogenesis and management of postprandial hyperglycemia: role of incretinbased therapies. Int J Gen Med. 2013;6:877–895. doi: 10.2147/IJGM.S51665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rizos EC, Ntzani EE, Papanas N, Tsimihodimos V, Mitrogianni Z, Maltezos E, Elisaf MS. Combination therapies of DPP4 inhibitors and GLP1 analogues with insulin in type 2 diabetic patients: a systematic review. Curr Vasc Pharmacol. 2013;11:992–1000. doi: 10.2174/15701611113119990103. [DOI] [PubMed] [Google Scholar]
- 16.Goldenberg R. Insulin plus incretin agent combination therapy in type 2 diabetes: a systematic review. Curr Med Res Opin. 2014;30:431–445. doi: 10.1185/03007995.2013.852078. [DOI] [PubMed] [Google Scholar]
- 17.Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP Sitagliptin Study 024 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab. 2007;9:194–205. doi: 10.1111/j.1463-1326.2006.00704.x. [DOI] [PubMed] [Google Scholar]
- 18.Neumiller JJ, Wood L, Campbell RK. Dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes mellitus. Pharmacotherapy. 2010;30:463–484. doi: 10.1592/phco.30.5.463. [DOI] [PubMed] [Google Scholar]
- 19.Graefe-Mody U, Friedrich C, Port A, Ring A, Retlich S, Heise T, Halabi A, Woerle HJ. Effect of renal impairment on the pharmacokinetics of the dipeptidyl peptidase-4 inhibitor linagliptin(*) Diabetes Obes Metab. 2011;13:939–946. doi: 10.1111/j.1463-1326.2011.01458.x. [DOI] [PubMed] [Google Scholar]
- 20.Graefe-Mody U, Rose P, Retlich S, Ring A, Waldhauser L, Cinca R, Woerle HJ. Pharmacokinetics of linagliptin in subjects with hepatic impairment. Br J Clin Pharmacol. 2012;74:75–85. doi: 10.1111/j.1365-2125.2012.04173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drucker DJ, Goldfine AB. Cardiovascular safety and diabetes drug development. Lancet. 2011;377:977–979. doi: 10.1016/S0140-6736(10)62299-4. [DOI] [PubMed] [Google Scholar]
- 22.Kim SC, Glynn RJ, Liu J, Everett BM, Goldfine AB. Dipeptidyl peptidase-4 inhibitors do not increase the risk of cardiovascular events in type 2 diabetes: a cohort study. Acta Diabetol. 2014;51:1015–1023. doi: 10.1007/s00592-014-0663-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 24.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 26.Barnett AH, Charbonnel B, Donovan M, Fleming D, Chen R. Effect of saxagliptin as addon therapy in patients with poorly controlled type 2 diabetes on insulin alone or insulin combined with metformin. Curr Med Res Opin. 2012;28:513–523. doi: 10.1185/03007995.2012.665046. [DOI] [PubMed] [Google Scholar]
- 27.Fonseca V, Schweizer A, Albrecht D, Baron MA, Chang I, Dejager S. Addition of vildagliptin to insulin improves glycaemic control in type 2 diabetes. Diabetologia. 2007;50:1148–1155. doi: 10.1007/s00125-007-0633-0. [DOI] [PubMed] [Google Scholar]
- 28.Hollander P, Raslova K, Skjoth TV, Rastam J, Liutkus JF. Efficacy and safety of insulin detemir once daily in combination with sitagliptin and metformin: the TRANSITION randomized controlled trial. Diabetes Obes Metab. 2011;13:268–275. doi: 10.1111/j.1463-1326.2010.01351.x. [DOI] [PubMed] [Google Scholar]
- 29.Hong ES, Khang AR, Yoon JW, Kang SM, Choi SH, Park KS, Jang HC, Shin H, Walford GA, Lim S. Comparison between sitagliptin as addon therapy to insulin and insulin dose-increase therapy in uncontrolled Korean type 2 diabetes: CSI study. Diabetes Obes Metab. 2012;14:795–802. doi: 10.1111/j.1463-1326.2012.01600.x. [DOI] [PubMed] [Google Scholar]
- 30.Rosenstock J, Rendell MS, Gross JL, Fleck PR, Wilson CA, Mekki Q. Alogliptin added to insulin therapy in patients with type 2 diabetes reduces HbA(1C) without causing weight gain or increased hypoglycaemia. Diabetes Obes Metab. 2009;11:1145–1152. doi: 10.1111/j.1463-1326.2009.01124.x. [DOI] [PubMed] [Google Scholar]
- 31.Vilsboll T, Rosenstock J, Yki-Jarvinen H, Cefalu WT, Chen Y, Luo E, Musser B, Andryuk PJ, Ling Y, Kaufman KD, Amatruda JM, Engel SS, Katz L. Efficacy and safety of sitagliptin when added to insulin therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2010;12:167–177. doi: 10.1111/j.1463-1326.2009.01173.x. [DOI] [PubMed] [Google Scholar]
- 32.Yki-Jarvinen H, Rosenstock J, Duran-Garcia S, Pinnetti S, Bhattacharya S, Thiemann S, Patel S, Woerle HJ. Effects of adding linagliptin to basal insulin regimen for inadequately controlled type 2 diabetes: a ≥52-week randomized, double-blind study. Diabetes Care. 2013;36:3875–3881. doi: 10.2337/dc12-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raskin P, Rendell M, Riddle MC, Dole JF, Freed MI, Rosenstock J Rosiglitazone Clinical Trials Study Group. A randomized trial of rosiglitazone therapy in patients with inadequately controlled insulin-treated type 2 diabetes. Diabetes Care. 2001;24:1226–1232. doi: 10.2337/diacare.24.7.1226. [DOI] [PubMed] [Google Scholar]
- 34.Wulffele MG, Kooy A, Lehert P, Bets D, Ogterop JC, Borger van der Burg B, Donker AJ, Stehouwer CD. Combination of insulin and metformin in the treatment of type 2 diabetes. Diabetes Care. 2002;25:2133–2140. doi: 10.2337/diacare.25.12.2133. [DOI] [PubMed] [Google Scholar]
- 35.Wajchenberg BL. beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev. 2007;28:187–218. doi: 10.1210/10.1210/er.2006-0038. [DOI] [PubMed] [Google Scholar]
- 36.Yabe D, Seino Y. Dipeptidyl peptidase-4 inhibitors and sulfonylureas for type 2 diabetes: Friend or foe? J Diabetes Investig. 2014;5:475–477. doi: 10.1111/jdi.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seino Y, Yabe D. Glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1: Incretin actions beyond the pancreas. J Diabetes Investig. 2013;4:108–130. doi: 10.1111/jdi.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foley JE, Jordan J. Weight neutrality with the DPP-4 inhibitor, vildagliptin: mechanistic basis and clinical experience. Vasc Health Risk Manag. 2010;6:541–548. doi: 10.2147/vhrm.s10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Du YF, Ou HY, Beverly EA, Chiu CJ. Achieving glycemic control in elderly patients with type 2 diabetes: a critical comparison of current options. Clin Interv Aging. 2014;9:1963–1980. doi: 10.2147/CIA.S53482. [DOI] [PMC free article] [PubMed] [Google Scholar]


