Abstract
Epistaxis due to ruptured internal carotid artery (ICA) aneurysm embedded within a pituitary adenoma (PA) has seldom been reported in the literature. Here we want to elaborate the incidence, mechanisms, clinical manifestations, and treatment strategy for this condition. The first survived case of a patient with epistaxis and pituitary apoplexy due to ruptured aneurysm embedded within PA was reported and the literature was reviewed. A 53-year-old male patient presented to our institution with sudden onset epistaxis and progressive vision loss. Neurological examination revealed bilateral ptosis and dilated unresponsive pupils. A CT scan showed a large mass in the pituitary fossa with bony erosion. MRI revealed a large pituitary tumor and abnormal signal intensity in the tumor. No aneurysm was noted during the pre-operative MR angiography. Abundant arterial bleeding suddenly occurred during urgent transsphenoidal surgery. Digital subtraction angiography confirmed the presence of a 14 mm unexpected saccular aneurysm of right ICA in the cavernous sinus with the dome protruding into the sella turcica. Balloon test occlusion of the right ICA was undertaken and permanent occlusion was performed. The patient recovered well and received bromocriptine and thyroid hormone replacement therapy during the follow-up period. At 14-month followup, the patient had no neurological deficits, no features of ischaemia relating to the right ICA therapeutic occlusion. Our case indicated that epistaxis and pituitary apoplexy could be due to the rupture of an ICA aneurysm embedded in a PA. Clinical suspicion should remain high when evaluating any case of epistaxis and pituitary apoplexy. Optimal treatment should take into consideration individual features of the tumor, aneurysm, and patient. Making the correct diagnosis as well as identifying an appropriate management strategy is critical in the care of such patients.
Keywords: Cerebral aneurysm, epistaxis, pituitary apoplexy, pituitary adenoma
Introduction
Pituitary adenoma (PA) with coexisting intracranial aneurysm (IA) is not uncommon [1-3]. Co-incidental aneurysms are reported almost seven times more frequently in patients with pituitary adenomas than in patients with other types of brain tumors [3]. However, the great majority of these aneurysms are located outside the tumor itself [4]. The presence of an ICA aneurysm embedded within a PA located inside the sella turcica has rarely been reported. Furthermore, epistaxis due to a ruptured ICA aneurysm embedded within a PA has seldom been reported in the literature [5]. Here we report the first survived case of a patient with epistaxis and pituitary apoplexy due to ruptured aneurysm embedded within PA. We also review the incidence, mechanisms, clinical manifestations, and treatment strategy for this condition.
Case report
History and examination
A 53-year-old male patient presented to our institution with sudden onset of epistaxis and progressive vision loss. The patient had one episode of epistaxis during which he lost approximately 200 mL of blood 2 days prior to admission. The nasal cavity was packed and bleeding ceased. Sudden deterioration in the both side vision, accompanied by severe headache and vomiting, was noted 1 day prior to admission.
On admission, physical examination revealed that the patient was alert and cooperative. There was an absence of nuchal rigidity. Neurological examination revealed bilateral ptosis and dilated unresponsive pupils. He had no perception of light and lost all extraocular movements in the right eye. In the left eye, abducens nerve function was preserved but light perception as well as oculomotor and trochlear nerve function was completely impaired. The remainder of his neurological exam revealed no focal deficits.
Cranial imaging studies and laboratory data
An emergency head CT disclosed a large soft-tissue mass that arose from the sella and occupied the entire sphenoid sinus and extended into both cavernous sinuses. There was local bony destruction of the sella turcica, clivus and adjacent petrous portions of the temporal bones. The bright mass within the sella was consistent with pituitary apoplexy (Figure 1).
Figure 1.
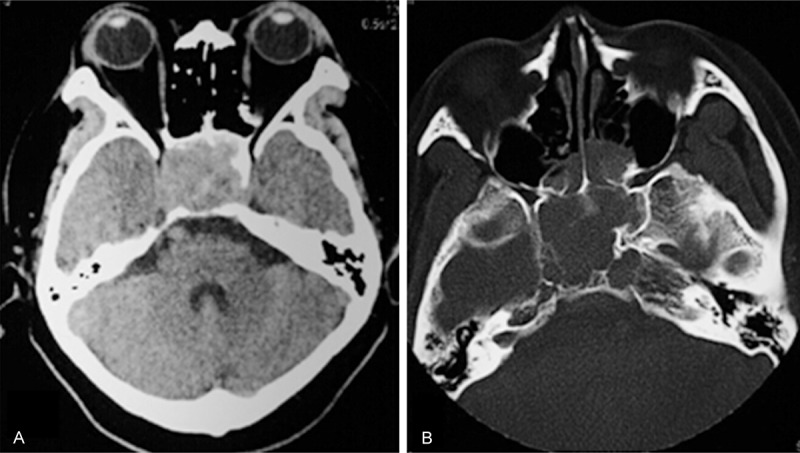
Pre-operative cranial CT disclosed (A) a large soft-tissue mass that arose from the sella and occupied all of the sphenoid sinus, together with (B) bony destruction of the sella turcica, clivus and adjacent petrous temporal bones.
MRI of the brain revealed a large pituitary tumor that extended superiorly in the suprasellar cistern to elevate the optic chiasm and extended inferiorly to fill the sphenoid sinus (Figure 2). The mass was isointense to the brain parenchyma on T1-weighted MRI, relatively hypointense on T2-weighted MRI, and showed significant contrast enhancement after injection of gadolinium. The maximum diameter of the tumor was 4.2×3.9×3.5 cm. There was abnormal signal intensity consistent with acute hemorrhage in the tumor. Furthermore, an aneurysm-like flow-void was not observed adjacent to the right ICA in the sella turcica (Figure 2).
Figure 2.
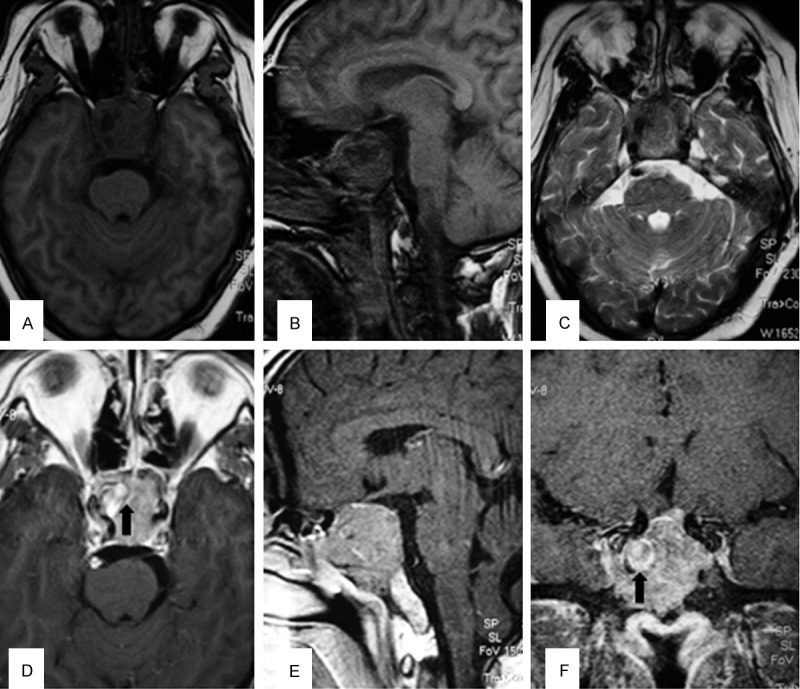
MRI of the brain revealed a large pituitary tumor that extended superiorly in the suprasellar cistern to elevate the optic chiasm and extended inferiorly to fill the sphenoid sinus. (A, B) The mass was isointense to brain parenchyma on T1-weighted MRI, (C) relatively hypointense on T2-weighted MRI. (D-F) Showed significant contrast enhancement after injection of gadolinium. There was abnormal signal intensity consistent with acute hemorrhage in the tumor (black arrow).
Preoperative laboratory examination showed no surgical contraindications. The pituitary function tests were as follows: PRL 276 ng/mL (normal 2.8-29.2 ng/mL), FT3 2.25 pg/mL (normal 2.3-4.2 ng/mL), FT4 1.71 ng/dL (normal 0.89-1.8 ng/dL). FSH, LH2, TSH, COR, GH, ACTH were within normal limits.
Management
Urgent decompression of visual pathways and/or pituitary macroadenoma resection should be preferentially arranged as this has been shown to optimize the chances of neurological recovery [6]. In order to exclude coexisiting intrasellar or intracranial aneurysms, a head MRA was arranged before emergent optic nerve decompression via the transsphenoidal route. However, no aneurysms were discovered in either the right or left internal carotid and vertebral arteries (Figure 3).
Figure 3.
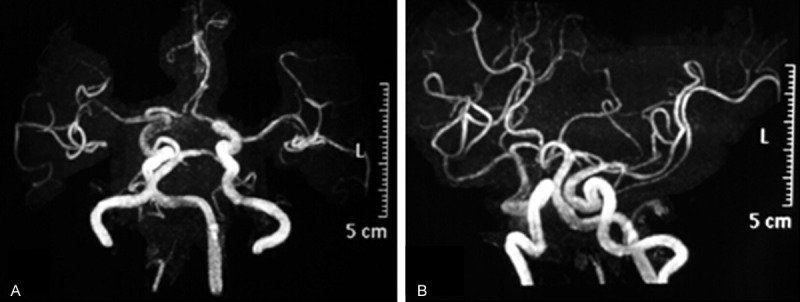
No aneurysm was found in bilateral ICA and vertebral artery in the pre-operative MRA.
Urgent transsphenoidal surgery was performed to decompress the visual pathways in order to preserve the patient’s visual acuity. Old clotted blood was seen in the meatus nasi superior. It was determined that bleeding did not originate from the nasal cavity, as the nasal mucosa was intact. After widening the ostium of sphenoidal sinus, the tumor and hematoma in the sphenoidal sinus were excavated under high pressure. No clear demarcation between the adenoma and blood clot was found. A mixture of dark red, large clot and fragile adenomatous tissue within the tumor capsule was evacuated with forceps and curette. When removal of the adenama and hematoma in the right, the hematoma extruded, a significant arterial bleeding suddenly occurred. Large caliber suction could not provide a clear surgical field and thus surgery could not proceed. Bleeding was finally stopped using Surgicel and Gelfoam and the nasal cavity was packed. The total estimated blood loss was 1.0 L. The patient recovered from anesthesia after receiving a transfusion of blood and plasma substitute.
As we suspected an ICA injury, digital subtraction angiography (DSA) was performed with exposure of both carotid arteries in the neck to occlude the vessel if required immediately after the surgery. Unexpectedly, DSA confirmed the presence of a 14 mm saccular aneurysm on the right ICA in the cavernous sinus with its dome protruding into the sella turcica (Figure 4). Due to difficulties in identifying the neck of the aneurysm, we inspected the collateral circulation to the brain by performing a balloon occlusion test. An angiogram showed good collateral flow through both the anterior and posterior communicating arteries. Balloon occlusion of the right ICA was performed under local anesthesia for thirty minutes, during which the patient did not develop any neurological symptoms. Thus, permanent occlusion was performed using two detachable 8×20 mm Gold Valve balloons (Nycomed, Ingenor, France) deployed in the petrous RICA and distal cervical RICA (Figure 4). In postoperative reevaluation, the preoperative MRI disclosed that the RICA protruded a small abnormal signal into the intratumoral hematoma, which might indicate a ruptured aneurysm (Figure 2). No new neurological deficit developed after this procedure.
Figure 4.
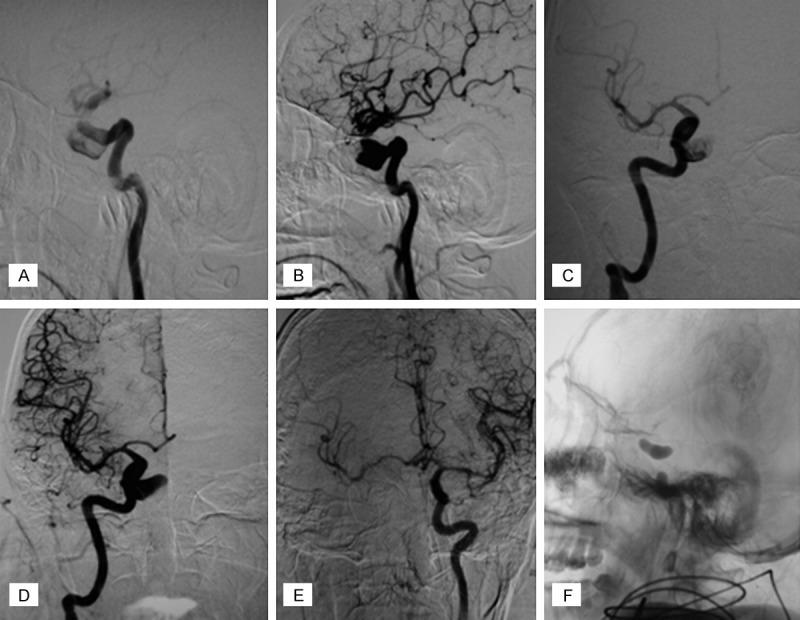
DSA confirmed the presence of an 14 mm diameter unexpected saccular aneurysm of right ICA in the cavernous sinus with the dome protruding into the sella turcica. Anteroposterior view (A, B), lateral view (C, D) Balloon test occlusion of the right ICA was undertaken under local anesthesia for thirty minutes (E), permanent occlusion was performed using two detachable 8 mm*20 mm Gold Valve balloons (Nycomed, Ingenor, France) deployed in the petrous RICA and distal cervical RICA (F).
Postoperative course and pathological findings
The patient’s symptoms of elevated intracranial pressure including headache and vomiting resolved within 24 hours post-occlusion. CT scan of the head, performed 10 hours after surgery, demonstrated no evidence of hematoma or infarction in the brain. Nasal packing was removed the next day without any further bleeding or CSF leakage. The histopathologic examination confirmed a prolactinoma with apoplexy. Treatment options were again discussed with the patient including surgical resection of the tumor, bromocriptine therapy and radiotherapy. The patient refused surgery and was discharged on bromocriptine and thyroid hormone replacement therapy during the follow-up period. Partial recovery of left eye vision was noted within 7 days post-op and visual acuity, visual fields, and extra-ocular movements completely recovered three months after surgery. Six month postoperative MRI and MRA demonstrated complete obliteration of the aneurysm, only 1.3 cm of the intrasellar remnant adenoma, and no signs of optic nerve compression (Figure 5). Serum prolactin level fell to 28 ng/mL. The patient underwent adjuvant gamma-knife radiotherapy for the residual intrasellar portion of the adenoma. At 14-month follow-up, the patient had no neurological deficits. There were no features of ischemia relating to the therapeutic right ICA occlusion.
Figure 5.
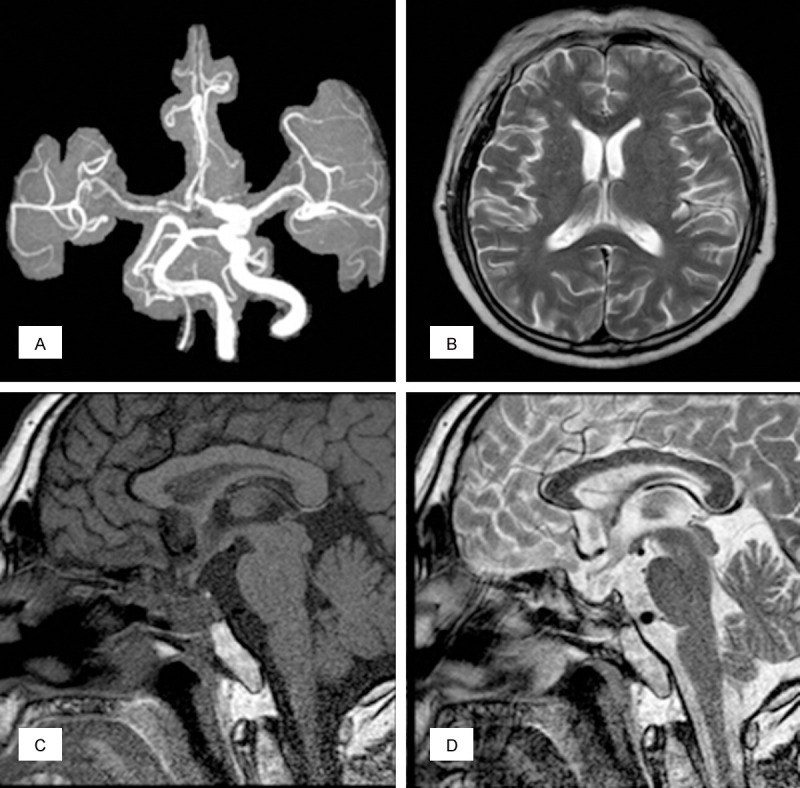
Six month postoperative MRI and MRA demonstrated complete obliteration of the aneurysm (A). There were no features of ischemia relating to the therapeutic right ICA occlusion (B). Only 1.3 cm of the intrasellar remnant adenoma, and no signs of optic nerve compression (C, D).
Discussion
The incidence
The presence of an ICA aneurysm completely embedded within a PA is a special type of pituitary adenoma coexisting with IA [7]. Based on the tumor and aneurysm location, PA with coexisting IA can be classified as non-adjacent, adjacent, intra-adenoma types [23]. In nonadjacent types, the aneurysm is located apart from the adenoma such that they are not in direct contact and are separated by other tissues; in adjacent types, pituitary adenomas and aneurysms are in close or partial contact, and the capsule of PA is intact; in intra-adenoma types, the aneurysm body is partially or completely embedded in the PA. The great majority of intracranial aneurysms that coexist with a PA are located outside of the tumor itself [5]. Aneurysms of the cavernous or supraclinoid carotid that encroach on the PA as in the present case are very rare.
On review of the current literature, only 13 cases of intra-adenomatous aneurysm have been reported [3,5-16]. Among them, 10 unruptured aneurysms were diagnosed incidentally during the preoperative workup of the PA. To our knowledge, there have been only two cases of pituitary apoplexy secondary to a ruptured aneurysm [7,8] and 1 case of epistaxis caused by aneurysmal bleeding into a PA has been reported [5]. Imamura et al reported the only case of fatal epistaxis caused by rupture of an intracavernous aneurysm with a coexisting PA. Thus our patient is the first reported non-fatal case of epistaxis due to a ruptured PA-embedded aneurysm.
The mechanism
Several mechanisms of aneurysm formation associated with PA have been proposed, including local circulatory stress, endocrine effect, mechanical effect, and direct invasion [5-7]. It is possible that one or more mechanisms play a role in aneurysm formation at locations. Whether the PA contributes to aneurysm formation is unclear [8]. Co-incidental aneurysms are reported almost seven times more frequently in patients with pituitary adenomas than in patients with other brain tumors [3]. Although aneurysms are associated with all types of pituitary tumors, the frequency of this association with growth hormone-secreting adenomas is the highest [11]. In the context of growth-hormone secreting tumors, arteriosclerotic and degenerative changes in the arterial wall as a result of ensuing hypertension and diabetes mellitus could also be relevant, and may explain the higher aneurysm frequency of associated with this group of adenomas [7]. However, among the 13 reported cases of PA-embedded aneurysm, the most commonly detected endocrinopathies were hyperprolactinemia (6/13, 46.15%) and growth-hormone secreting tumors (3/13, 23.08%).
ICA-cavernous aneurysms rarely rupture because the aneurysn is surrounded by hard tissues such as the dura and other bony structures [5]. In the present case, the bony structures of the sella turcica, clivus and adjacent petrous portion temporal bones were widely eroded and destroyed by the tumor. The right ICA was completely embedded in the tumor, which arose from the sella and extended into both cavernous sinuses. The aneurysm had apparently grown as the wall around the aneurysm had become less resistant. The tumor may also have invaded and weakened the aneurysmal wall, causing growth and rupture of the aneurysm.
Clinical manifestations
With widespread use of MRI, diagnosis of PA with or without coexisting aneurysm should be routine [17]. MRI should provide pre-operative assessment to investigate the tumor and the anatomic relationships of the sellar region and to rule out a coexisting intra- or juxtasellar aneurysm [18]. If there is abnormal signal in the PA or an unusual flow void signal, clinicians should be suspicious of a coincidental aneurysm within the adenoma. Teng et al. demonstrated that the finding of flow voids is 100% specific for aneurysms, the sensitivity of flow voids on noncontrast T1-weighted imaging, postcontrast T1-weighted imaging, and T2-weighted imaging was 88%, 22%, and 88%, respectively [19]. Furthermore, another study found that only 12 of 15 giant aneurysms (80%) showed signs of intraluminal blood flow [20]. These results indicate that misdiagnosis of such coexistence can be possible. In our case, the axial and coronal MR images presented an abnormal signal and no flow void in the medial portion of the right ICA (Figure 2). We suspected that the presence of an unusual signal suggested a vascular component from the ICA. In order to exclude coexistant intrasellar or intracranial aneurysms, an emergent head MRA was arranged before emergent optic nerve decompression via the transsphenoidal route. However, no aneurysm was found in bilateral ICA and vertebral artery. A study examining diagnosis of IAs with MRA revealed that pooled sensitivity of MRA was 95% (95% confidence interval, 89%-98%), and pooled specificity was 89% (95% confidence interval, 80%-95%) [17]. Our case indicated that epistaxis and pituitary apoplexy can be due to the rupture of a tumor-embedded ICA aneurysm. This association should be kept in mind when evaluating any case of an epistaxis and pituitary apoplexy. Further workup such as CTA or DSA is required when an aneurysm is suspected.
The ICA-cavernous aneurysms embedded within pituitary adenomas are usually asymptomatic and diagnosed incidentally [10-12]. The clinical presentation in the present case was unusual in that the rupture of an aneurysm caused a hemorrhage into the adenoma, resulting in an acute clinical syndrome of epistaxis and pituitary apoplexy. Once an aneurysm ruptures into the adenoma the clinical implications may be very serious. As the pressure within the tumor increases, the optic nerve, optic chiasm and CN III, IV, and VI nerve are compressed and their functions are completely impaired in the short term. If the bleeding bursts through the tumor capsule, epistaxis may occur. The mortality in patients with epistaxis caused by intrasellar aneurysmal bleeding is up to 22.2%. Of seven patients not receiving treatment, 5 (71.4%) died [24].
Treatment plan
Management strategies for PA-embedded aneurysm include simultaneous microsurgical treatment of the aneurysm and the adenoma through the pterional [9,21] or supraorbital keyhole approach [22], two stage procedures combined with transsphenoidal or transcranial microsurgery, medical treatment, and gamma knife radiosurgery after endovascular techniques [3,7,10-14]. The optimal treatment method should take into consideration individual features of the tumor, the aneurysm, and the patient [3]. Currently, no standard treatment protocol exists and treatment is planned on a case basis. However, treatment of each lesion becomes more challenging when the aneurysm lies inside the PA [12]. In general, treatment of the aneurysm takes priority over treatment of the PA. With the advances in endovascular materials, the embolization method achieves better treatment results than microsurgical approach for aneurysms located in a difficult region, particularly in locations such as the cavernous carotid artery or the paraclinoid segment [14].
In our case, epistaxis and pituitary apoplexy presenting in this manner required urgent surgical decompression of the visual pathways in order to preserve the patient’s visual acuity, although we did not detect the tumor-embedded aneurysm until abundant arterial bleeding occurred during the transsphenoidal procedure. Hemostasis was achieved by packing with Surgicel and Gelfoam. Our case was unprecedented in that MRI and MRA did not diagnose the ruptured aneurysm (although in retrospect it did show an unusual signal), and the aneurysm was diagnosed only after severe intraoperative hemorrhage occurred. Angiography after the surgery revealed an intracavernous carotid artery aneurysm embedded within the PA. Fortunately, the aneurysm was trapped successfully by endovascular techniques because the patient tolerated well a previous balloon occlusion test. Our treatment strategy in this case included initial urgent transsphenoidal decompression of the visual pathways, endovascular trapping of the cerebral aneurysm, followed by bromocriptine and thyroid hormone replacement therapy, with adjuvant gamma-knife radiotherapy for the remnant adenoma.
Conclusions
Our case indicated that epistaxis and pituitary apoplexy can be due to the rupture of an ICA aneurysm embedded in a tumor. This association should be kept in mind when evaluating any case of an epistaxis and pituitary apoplexy. The optimal treatment method should take into consideration individual features of the tumor, the aneurysm, and the patient. Regardless of the method, treatment of the aneurysm before PA is the safest approach.
Disclosure of conflict of interest
None.
References
- 1.Oh MC, Kim EH, Kim SH. Coexistence of intracranial aneurysm in 800 patients with surgically confirmed pituitary adenoma. J Neurosurg. 2012;116:942–947. doi: 10.3171/2011.12.JNS11875. [DOI] [PubMed] [Google Scholar]
- 2.Oshino S, Nishino A, Suzuki T, Arita H, Tateishi A, Matsumoto K, Shimokawa T, Kinoshita M, Yoshimine T, Saitoh Y. Prevalence of cerebral aneurysm in patients with acromegaly. Pituitary. 2013;16:195–201. doi: 10.1007/s11102-012-0404-x. [DOI] [PubMed] [Google Scholar]
- 3.Sade B, Mohr G, Tampieri D, Rizzo A. Intrasellar aneurysm and a growth hormone-secreting pituitary macroadenoma. Case report. J Neurosurg. 2014;100:557–559. doi: 10.3171/jns.2004.100.3.0557. [DOI] [PubMed] [Google Scholar]
- 4.Almeida Silva JM, Campos RR, Souza RR, Sette Dos Santos ME, Aguiar GB. Spontaneous subarachnoid haemorrhage from rupture of an anterior communicating artery aneurysm in a patient with pituitary macroadenoma. Neurocirugia (Astur) 2014;25:81–85. doi: 10.1016/j.neucir.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Imamura J, Okuzono T, Okuzono Y. Fatal epistaxis caused by rupture of an intratumoral aneurysm enclosed by a large prolactinomacase report. Neurol Med Chir (Tokyo) 1998;38:654–656. doi: 10.2176/nmc.38.654. [DOI] [PubMed] [Google Scholar]
- 6.Soni A, De Silva SR, Allen K, Byrne JV, Cudlip S, Wass JA. A case of macroprolactinoma encasing an ICA aneurysm, presenting as pituitary apoplexy. Pituitary. 2008;11:307–311. doi: 10.1007/s11102-007-0063-5. [DOI] [PubMed] [Google Scholar]
- 7.Chuang CC, Chen YL, Pai PC. A giant intracavernous carotid artery aneurysm embedded in pituitary macroadenoma presenting with pituitary apoplexy. Cerebrovasc Dis. 2006;21:142–144. doi: 10.1159/000090450. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki H, Muramatsu M, Murao K, Kawaguchi K, Shimizu T. Pituitary apoplexy caused by ruptured internal carotid artery aneurysm. Stroke. 2001;32:567–569. doi: 10.1161/01.str.32.2.567. [DOI] [PubMed] [Google Scholar]
- 9.Yang MY, Chen C, Shen CC. Cavernous aneurysm and pituitary adenoma: management of dual intrasellar lesions. J Clin Neurosci. 2005;12:477–481. doi: 10.1016/j.jocn.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 10.Nishijima Y, Ogawa Y, Sato K, Matsumoto Y, Tominaga T. Cushing’s disease associated with unruptured large internal carotid artery aneurysm. Case report. Neurol Med Chir (Tokyo) 2010;50:665–668. doi: 10.2176/nmc.50.665. [DOI] [PubMed] [Google Scholar]
- 11.Xia X, Ramanathan M, Orr BA, Salmasi V, Salvatori R, Reh DD, Gallia GL. Expanded endonasal endoscopic approach for resection of a growth hormone-secreting pituitary macroadenoma coexistent with a cavernous carotid artery aneurysm. J Clin Neurosci. 2012;19:1437–1441. doi: 10.1016/j.jocn.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 12.Choi HS, Kim MS, Jung YJ, Kim OL. Incidental Superior Hypophygeal Artery Aneurysm Embedded within Pituitary Adenoma. J Korean Neurosurg Soc. 2013;54:250–252. doi: 10.3340/jkns.2013.54.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seda L Jr, Cukiert A, Nogueira KC, Huayllas MK, Liberman B. Intrasellar internal carotid aneurysm coexisting with GH-secreting pituitary adenoma in an acromegalic patient. Arq Neuropsiquiatr. 2008;66:99–100. doi: 10.1590/s0004-282x2008000100026. [DOI] [PubMed] [Google Scholar]
- 14.Yu K, Herwadkar A, Kearney T, Gnanalingham KK. Pituitary adenoma and incidental superior hypophyseal aneurysm. Br J Neurosurg. 2011;25:432–433. doi: 10.3109/02688697.2010.509521. [DOI] [PubMed] [Google Scholar]
- 15.Wang CS, Yeh TC, Wu TC, Yeh CH. Pituitary macroadenoma co-existent with supraclinoid internal carotid artery cerebral aneurysm: a case report and review of the literature. Cases J. 2009;2:6459. doi: 10.4076/1757-1626-2-6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akutsu N, Hosoda K, Ohta K, Tanaka H, Taniguchi M, Kohmura E. Subarachnoid Hemorrhage Due to Rupture of an Intracavernous Carotid Artery Aneurysm Coexisting with a Prolactinoma under Cabergoline Treatment. J Neurol Surg Rep. 2014;75:73–76. doi: 10.1055/s-0033-1364166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sailer AM, Wagemans BA, Nelemans PJ, de Graaf R, van Zwam WH. Diagnosing intracranial aneurysms with MR angiography: systematic review and meta-analysis. Stroke. 2014;45:119–126. doi: 10.1161/STROKEAHA.113.003133. [DOI] [PubMed] [Google Scholar]
- 18.Cho YD, Lee JY, Kwon BJ, Kang HS, Han MH. False-positive diagnosis of cerebral aneurysms using MR angiography: location, anatomic cause, and added value of source image data. Clin Radiol. 2011;66:726–731. doi: 10.1016/j.crad.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Teng MM, Nasir Qadri SM, Luo CB, Lirng JF, Chen SS, Chang CY. MR imaging of giant intracranial aneurysm. J Clin Neurosci. 2003;10:460–464. doi: 10.1016/s0967-5868(03)00092-4. [DOI] [PubMed] [Google Scholar]
- 20.Olsen WL, Brant-Zawadzki M, Hodes J, Norman D, Newton TH. Giant intracranial aneurysms: MR imaging. Radiology. 1987;163:431–435. doi: 10.1148/radiology.163.2.3562822. [DOI] [PubMed] [Google Scholar]
- 21.Fujiwara S, Fujii K, Nishio S, Fukui M. Diagnosis and treatment of pituitary adenoma with adjacent carotid artery aneurysm. J Neurosurg Sci. 1991;35:41–46. [PubMed] [Google Scholar]
- 22.Revuelta R, Arriada-Mendicoa N, Ramirez-Alba J, Soto-Hernandez JL. Simultaneous treatment of a pituitary adenoma and an internal carotid artery aneurysm through a supraorbital keyhole approach. Minim Invasive Neurosurg. 2002;45:109–111. doi: 10.1055/s-2002-32488. [DOI] [PubMed] [Google Scholar]
- 23.Sasagawa Y, Tachibana O, Shiraga S, Takata H, Akai T, Iizuka H. [A clinical feature and therapeutic strategy in pituitary adenomas associated with intracranial aneurysms] . No Shinkei Geka. 2012;40:15–21. [PubMed] [Google Scholar]
- 24.Lehmann P, Saliou G, Page C, Balut A, Le Gars D, Vallée JN. Epistaxis revealing the rupture of a carotid aneurysm of the cavernous sinus extending into the sphenoid: treatment using an uncovered stent and coils. Review of literature. Eur Arch Otorhinolaryngol. 2009;266:767–772. doi: 10.1007/s00405-008-0743-4. [DOI] [PubMed] [Google Scholar]


