Abstract
HOTAIR, a long noncoding RNA, regulates development and progression of tumor cells and function of normal stem cells. However, the role and the molecular mechanism of HOTAIR in the spermatozoa of patients with asthenozoospermia and oligoasthenozoospermia are still unclear. Herein, 45 healthy control, 45 asthenozoospermic patients and 45 oligoasthenozoospermic patients were enrolled. Initially, through analyzing HOTAIR expression, we observed a decreased level of HOTAIR expression in patients. Subsequently, we found that there was a positive correlation between HOTAIR expression and Nrf2 expression in patients. The low expression of HOTAIR was also observed to be associated with specific sperm function parameters, including motility and vitality. In the ejaculated spermatozoa from patients, low level of histone H4 acetylation of the Nrf2 gene promoter was observed. Finally, we found that downregulation of HOTAIR expression reduced histone H4 acetylation in Nrf2 promoter and Nrf2 expression. Therefore, this study demonstrated that HOTAIR expression was low in the spermatozoa of patients with asthenozoospermia and oligoasthenozoospermia, which resulted in down-regulation of Nrf2 expression. Our data suggested the decrease of HOTAIR expression led to ROS related defects in sperm function.
Keywords: HOTAIR, Nrf2, histone acetylation, asthenozoospermia and oligoasthenozoospermia, ROS
Introduction
Infertility is the failure of conception after more than 12 months of unprotected intercourse and becomes a global health problem [1]. In infertile couples, about 50% of cases are due to male factors [2]. Sperm abnormalities such as asthenozoospermia (a sperm concentration ≥ 15×106/ml and sperm progressive motility < 32%) and oligoasthenozoospermia (a sperm concentration < 15×106/ml and sperm progressive motility < 32%) are the most common cause of male infertility [3]. However, the molecular mechanism of sperm abnormalities still remains unclear.
Due to the high content of polyunsaturated fatty acids on their plasma membranes, spermatozoa are susceptible to oxidative damage [4]. Antioxidant genes and enzymes in spermatozoa play an important role in protection of sperm function and viability [5]. It has been confirmed that the low expression of antioxidant enzymes such as superoxide dismutases (SODs), glutathione S-transferase (GSTs) and catalase (CAT) is associated with poor sperm quality [6]. The transcription factor of Nrf2 (nuclear factor erythroid 2-related factor 2) could promote antioxidant enzymes expression by binding directly to promoters of these genes [7]. Thus, the level and activity of Nrf2 is very important for spermatozoa against oxidative damage [8]. In knockout of Nrf2 mice, the decrease of sperm concentration and motility was observed [9]. Clinical evidence also showed that the level of Nrf2 expression in spermatozoa from asthenozoospermic and oligoasthenozoospermic patients was significantly lower than that from fertile men [10]. However, litter is known about the molecular mechanism of regulation of Nrf2 expression in spermatozoa.
Long ncRNAs (lncRNAs), longer than 200 bp, play multiple roles in the regulation of many cellular processes [11]. lncRNAs regulate coding genes’ expression by epigenetic modifiers such as DNA methylation and histone modification [12,13]. More and more evidence showed that irregularities of lncRNAs in sperm could be as markers and potential therapeutic targets of male infertility [14]. HOTAIR (Hox transcript antisense intergenic RNA) is one of well-documented lncRNAs in tumor development and progression [15]. Besides, HOTAIR is capable of altering biological characteristic of liver normal stem cells [16]. In this study, we measured the level of HOTAIR in the spermatozoa from asthenozoospermic and oligoasthenozoospermic patients. We also measured whether the HOTAIR expression is associated with specific sperm function parameters. The molecular mechanisms of HOTAIR regulating Nrf2 expression were explored in depth.
Materials and methods
Ethics statement
This study was approved by the ethical committee of Taizhou People’s Hospital (Taizhou, China) and written informed consent was obtained from all participants. The experimental protocol was established according to the associated national guidelines from Nantong University of Medicine (Taizhou, China).
Subjects and semen samples
Infertile males (age between 25 to 50 years) with idiopathic asthenozoospermia (n = 45) or oligoasthenozoospermia (n = 45) from the department of reproductive medicine of Taizhou people’s Hospital affiliated of Nantong University of medicine from January 2014 to October 2014 were enrolled in the study after excluding those with varicocele, teratozoospermia and leukocytospermia. Additional exclusion criterions included abnormal semen liquefaction, reproduction tract infection, testicular injury or pathology, history of cryptorchidism, orchitis, or epididymitis, and some systemic diseases (diabetes mellitus, hypertension, hypercholesterolemia, and hypoandrogenism). Asthenozoospermia or oligoasthenozoospermia was diagnosed if semen parameters were below the cutoff levels according to the criteria of the World Health Organization [17]. All the volunteers (n = 45) matched for age and smoking status were recruited and proven to have normal semen parameters.
All the semen samples were collected from the study subjects by masturbation after 2 to 5 days of abstinence, and then allowed to liquefy at 37°C for 30 min. Liquefied semen samples were analyzed for sperm morphology, concentration and motility by computer-assisted semen analysis (CASA) according to the criteria of the 5th edition of WHO laboratory manual for the examination and processing of human semen [17]. The spermatozoa pellet was snap-frozen in liquid nitrogen and stored at -80°C for use.
Cell culture
GC1-spg cells was purchased from ATCC (ATCC Global Bioresource Center, Atlanta, USA) and cultured in DMEM supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin and 25 μg/mL ascorbic acid at 37°C and 5% CO2.
Real-time PCR
The total RNA of each spermatozoa pellet was extracted using TRIzol reagent (Invitrogen, Grand Island, NY, USA) according to the manufacturer’s instruction. Total RNA (500 ng) was reverse transcribed in a final volume of 10 μl using random primers under standard conditions for the PrimeScript RT reagent Kit (TaKaRa, Dalian, China). We used the SYBR Premix Ex Taq (TaKaRa, Dalian, China) to determine mrhl expression levels, following the manufacturer’s instructions. Results were normalized to the expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The specific primers were as follows: HOTAIR, 5’-CAGTGGGGAACTC TGACTCG-3’ (forward) and 5’-GTGCCTGGTGCTCTCTTACC-3’ (reverse); Nrf2, 5’-TTCAGCCAGCCCAGCACATC-3’ (forward); and 5’-CGTAGCCGAAGAAACC TCATTGTC-3’ (reverse); GAPDH, 5’-TGGTGAAGGTCGGTGTGAAC-3’ (forward); and 5’-CCATGTAGTTGAGGTCAATGAAGG-3’ (reverse).
Western blot analysis
Spermatozoa pellet and GC1-spg cells were lysed with ice-cold lysis buffer containing: 50 mmol/l Tris-HCl, pH 7.4; 1% NP-40; 150 mmol/l NaCl; 1 mmol/l EDTA; 1 mmol/l phenylmethylsulfonyl fluoride; and complete proteinase inhibitor mixture (one tablet per 10 ml; Roche Molecular Biochemicals, Indianapolis, IN, USA). Protein concentration in the cell lysate was quantified using the DC protein assay kit (Bio-Rad). Following protein content determination using a DC Protein Assay kit, western blot analysis was performed.
Chromatin immunoprecipitation (ChIP) assay
The spermatocytes were prepared using the ChIP assay kit (Upstate Biotechnology) following the manufacturer’s protocol. Briefly, cells were harvested and fixed in 1% (v/v) formaldehyde for 10 min at room temperature. Then, cells were lysed in SDS lysis buffer and the chromatin was sonicated. The chromatin was incubated overnight at 4°C with histone H3 and H4, and normal mouse serum (IgG) as a negative control. The sequences of PCR using primers framing the mouse Nrf2 promoter region of interest (-680 to -850) are 5’-GCGTGGTGGCTGCGCTTT-3’ (forward) and 5’-TCAGGGTGACTGCGAACAC-3’ (reverse). To examine whether low sperm motility mediated deacetylation of histone H3 and H4 at the Nrf2 gene promoter in spermatocytes, we used chromatin immunoprecipitation and quantitative real-time PCR (ChIP-qPCR) assay. For every promoter studied, a ΔCt value was calculated for each sample: ΔCt = Ct (sample)-Ct (Input). Next, a ΔΔCt value was calculated: ΔΔCt = ΔCt (sample immunoprecipitated with acetylated histone H3/H4 antibody)-ΔCt (sample immunoprecipitated with IgG). The fold difference between acetylated histone H3/H4 antibody-immunoprecipitated samples and those immunoprecipitated with IgG was calculated using 2-ΔΔCt.
Cell transfection
The expression of HOTAIR was silenced utilizing specific small interfering RNA (si-HOTAIR) purchased from Ribobio (Guangzhou, China). Spermatocytes were cultured in six-well plate or 10-mm plates in DMEM with 10% FBS and without antibiotics for 4-6 h. When the cells reached approximately 60% confluence, si-mrhl or si-control were transfected by using Lipofectamine RNAiMAX reagent (Life Technologies) in accordance to manufacturer’s instructions.
Superoxide dismutase activity assay
Superoxide dismutase (SOD) activity was determined using the SOD assay kit according to the manufacturer’s instructions.
Statistical analysis
Statistical analyses were performed using SPSS 13.0 statistical analysis software and were performed using either an analysis of variance (ANOVA) or Student’s t-test. Data are expressed as mean ± standard deviation. All experiments were repeated at least three times. Association analysis was performed using the Spearman correlation test. P < 0.05 was considered to indicate a statistically significant difference.
Results
Low expression of HOTAIR and Nrf2 in the ejaculated spermatozoa from asthenozoospermic and oligoasthenozoospermic patients
This study included 45 asthenozoospermic patients, 45 oligoasthenozoospermic patients and 45 healthy fertile controls (Table 1). There were no significant differences in mean age, ejaculate volume and pH among three groups. However, the motility, vitality and morphology were significantly lower in asthenozoospermic and oligoasthenozoospermic patients. Subsequently, the expression levels of HOTAIR and Nrf2 were measured by Real-Time PCR in the ejaculated spermatozoa samples from three groups. Notably, both of HOTAIR and Nrf2 expression were obviously reduced in the samples from asthenozoospermic patients and oligoasthenozoospermic patients compared to those from healthy control (Figure 1A and 1B). There were no significant differences of HOTAIR and Nrf2 expression between asthenozoospermic patients and oligoasthenozoospermic patients.
Table 1.
Demographic and semen characteristics in controls and patients
| Parameter | Controls (n = 45) | Asthenozoospermic (n = 45) | Oligoasthenozoospermic (n = 45) |
|---|---|---|---|
| Age (years) | 33.4 ± 3.1 | 33.8 ± 3.6 | 34.3 ± 4.2 |
| Volume (ml) | 3.62 ± 1.52 | 3.34 ± 1.31 | 3.51 ± 1.3 |
| Concentration (106/mL) | 62.9 ± 36.7 | 53.6 ± 33.3 | 9.1 ± 5.5*,# |
| pH | 7.54 ± 0.18 | 7.51 ± 0.12 | 7.53 ± 0.14 |
| Progressive Motility (%) | 65 ± 10 | 21 ± 9* | 17 ± 7* |
| Viability (%) | 85 ± 8 | 71 ± 9* | 68 ± 7* |
| Normal sperm morphology (%) | 65 ± 6.13 | 41.83 ± 4.25* | 38.01 ± 3.64* |
There were significant differences of sperm concentration, progressive motility viability and normal sperm morphology between control group and patients group.
There was significant difference of sperm concentration between asthenozoospermic group and oligoasthenozoospermic group.
Figure 1.
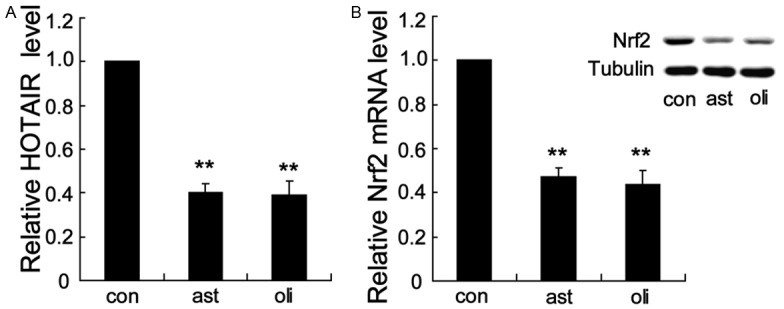
Low expression of HOTAIR and Nrf2 in the ejaculated spermatozoa from asthenozoospermic and oligoasthenozoospermic patients. HOTAIR (A) and Nrf2 (B) expression levels were obviously reduced in the samples from asthenozoospermic patients and oligoasthenozoospermic patients compared to those from healthy control. **P < 0.01, indicate significant differences from the healthy control groups. con: healthy control, ast: asthenozoospermic patients, oli: oligoasthenozoospermic patients.
HOTAIR expression level were positively correlated with Nrf2 expression level and SOD activity
We performed Spearman’s correlation analysis to analyze the correlation between HOTAIR expression level and Nrf2 expression level. Our results showed that there was a significant positive correlation between HOTAIR expression level and Nrf2 expression level in asthenozoospermic patients (r = 0.84, P < 0.001) and in oligoasthenozoospermic patients (Figure 2A and 2B) (r = 0.83, P < 0.001). Significant positive correlations were also observed between HOTAIR expression level and SOD activity in patients (r = 0.63, P < 0.01 in asthenozoospermic patients; r = 0.64, P < 0.01 in oligoasthenozoospermic patients) (Figure 2C and 2D).
Figure 2.
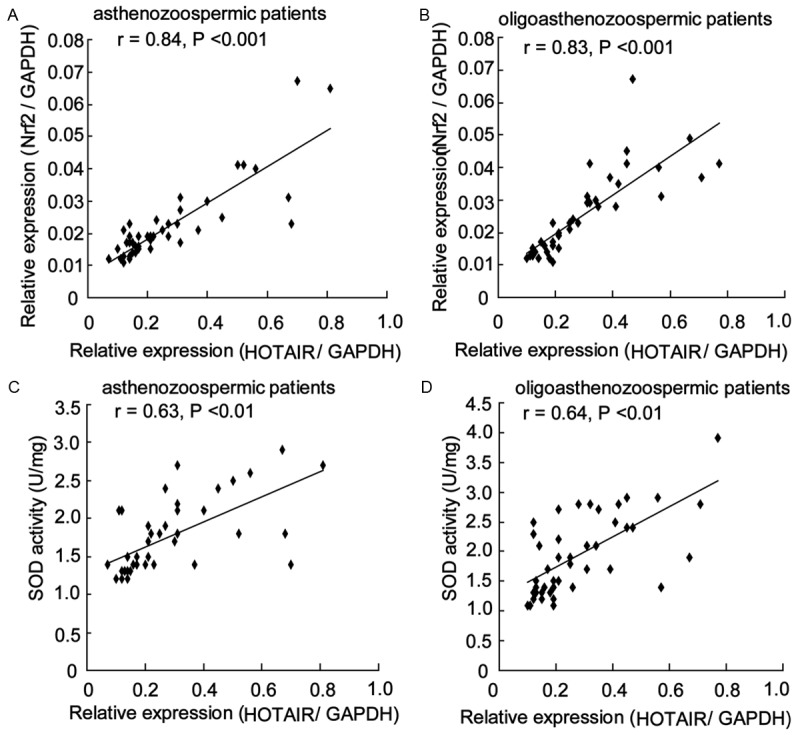
HOTAIR expression levels were positively correlated with Nrf2 expression level and SOD activity. Spearman’s correlation analysis revealed that there was a significant positive correlation between HOTAIR expression level and Nrf2 expression level in asthenozoospermic patients (A) and in oligoasthenozoospermic patients (B). Significant positive correlations were also observed between HOTAIR expression level and SOD activity in asthenozoospermic patients (C) and in oligoasthenozoospermic patients (D).
HOTAIR expression levels were positively correlated with sperm quality in patients
To explore the clinical relevance of HOTAIR expression to asthenozoospermia and oligoasthenozoospermia, we further conducted a correlation analysis of HOTAIR expression with sperm parameters including sperm concentration, motility and vitality in patients. The expression level of HOTAIR was positively correlated with sperm progressive motility and vitality in 90 semen samples (from 45 asthenozoospermic patients and 45 oligoasthenozoospermic patients), but not associated with sperm concentration (Table 2). These data suggested that HOTAIR expression were clinically involved in asthenozoospermia and oligoasthenozoospermia probably via influencing sperm motility and vitality.
Table 2.
Correlations between HOTAIR RNA level and sperm concentration, motility and vitality in patients
| Parameter | r | P |
|---|---|---|
| Sperm concentration | 0.24 | N.S |
| Progressive Motility | 0.71 | < 0.01 |
| Viability | 0.65 | < 0.01 |
| Normal sperm morphology | 0.53 | < 0.01 |
Low level of histone H4 acetylation of the Nrf2 gene promoter in the ejaculated spermatozoa from asthenozoospermic and oligoasthenozoospermic patients
It has been reported that the level of histone H4 acetylation was significantly lower in ejaculated spermatozoa of infertile men [18]. To explore whether the low Nrf2 expression level was associated with aberrant H4 acetylation in Nrf2 gene promoter, we performed ChIP-qPCR assay using spermatozoa with antiacetyl histone H4 antibodies. Compared to control, ejaculated spermatozoa from asthenozoospermic and oligoasthenozoospermic patients displayed a significant decrease of histone H4 acetylation (Figure 3). These results indicated that Nrf2 expression was low in spermatozoa from asthenozoospermic and oligoasthenozoospermic patients by mediating deacetylation of histone H4 at the Nrf2 gene promoter.
Figure 3.
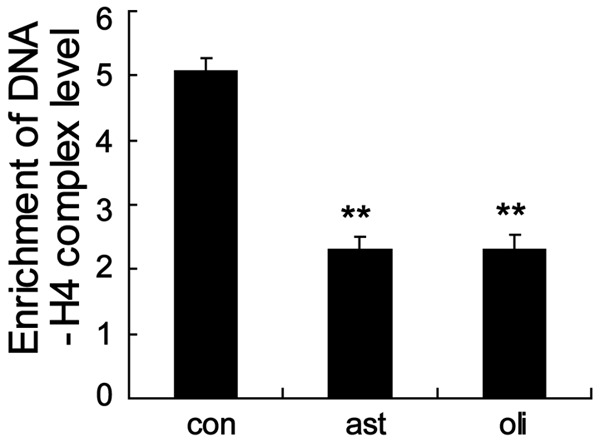
Low level of histone H4 acetylation of the Nrf2 gene promoter in the ejaculated spermatozoa from asthenozoospermic and oligoasthenozoospermic patients. ChIP-qPCR assay revealed that histone H4 acetylation in Nrf2 promoter was significantly decreased in ejaculated spermatozoa from asthenozoospermic and oligoasthenozoospermic patients. **P < 0.01, indicate significant differences from the healthy control groups. con: healthy control, ast: asthenozoospermic patients, oli: oligoasthenozoospermic patients.
Silence HOTAIR inhibited Nrf2 expression and SOD activity in spermatocytes
To determine whether HOTAIR could regulate Nrf2 expression, we decided to silence HOTAIR and analyze its effect on Nrf2 gene expression in GC1-spg cells. As shown in Figure 4B, HOTAIR expression was obviously decreased in GC1-spg cells after transfection with HOTAIR siRNA (si-HOTAIR). Indeed, Nrf2 mRNA and protein expression was significantly decreased after treatment with si-HOTAIR. Moreover, SOD activity was reduced upon the knockout of HOTAIR in GC1-spg cells (Figure 4C).
Figure 4.
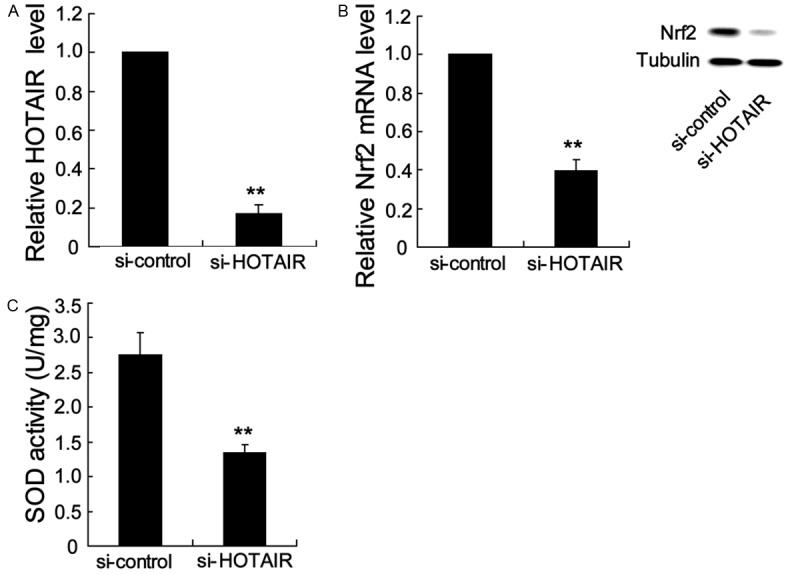
Silence HOTAIR inhibited Nrf2 expression and SOD activity in spermatocytes. GC1-spg cells were transfected with si-HOTAIR (100 nM) for 48 h, the HOTAIR expression (A), Nrf2 mRNA and protein expression (B) and SOD activity (C) were obviously decreased. **P < 0.01, indicate significant differences from the respective control groups.
Silence HOTAIR inhibited level of histone H4 acetylation in the Nrf2 gene promoter
To determine whether HOTAIR regulated Nrf2 expression via mediating histone H4 acetylation in the Nrf2 gene promoter, we decided to silence HOTAIR and analyze its effect on Nrf2 gene expression in Gc1-Spg cells. Notably, our results revealed that silence HOTAIR significantly suppressed the level of histone H4 acetylation in the Nrf2 gene promoter (Figure 5), indicating that HOTAIR regulated Nrf2 expression via mediating histone H4 acetylation in the Nrf2 gene promoter.
Figure 5.
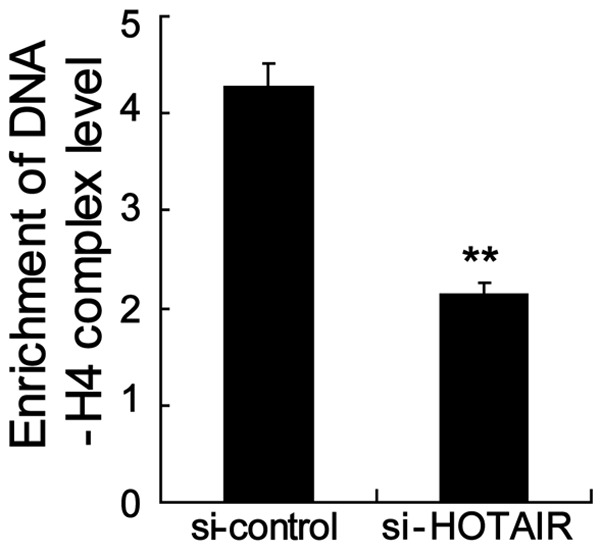
Silence HOTAIR inhibited level of histone H4 acetylation in the Nrf2 gene promoter. ChIP-qPCR assay revealed that silence HOTAIR significantly suppressed the level of histone H4 acetylation in the Nrf2 gene promoter. **P < 0.01, indicate significant differences from the respective control groups.
Discussion
lncRNAs have been shown to play multiple regulatory roles in many cellular processes including spermatogenesis [11,19]. Recently, some lncRNAs, such as Mrhl, HongrES2 and Tsx have been discovered in regulation of spermatogenesis [14]. However, the role and the molecular mechanism of lncRNAs in the spermatozoa of asthenozoospermic and oligoasthenozoospermic patients are still unclear. In the present study, we have identified that sperm HOTAIR levels in asthenozoospermic and oligoasthenozoospermic patients were significantly lower than those in control subjects and sperm HOTAIR levels are positively correlated with sperm Nrf2 expression level.
Previous studies demonstrated that the level of Nrf2 expression in spermatozoa from asthenozoospermic and oligoasthenozoospermic pa-tients was low [10], which were also confirmed by our data. Furthermore, we found that low level of histone H4 acetylation of the Nrf2 gene promoter in spermatozoa from these infertile patients. It is well known that the decrease of histones acetylation leads to transcription inactivation [20]. Therefore, we drew a conclusion that the decrease of Nrf2 expression in infertile men was caused by the reduction of histone acetylation in Nrf2 promoter.
It is interesting to note that Nrf2 expression is downregulated under HOTAIR gene silencing, indicating that there was a linkage between Nrf2 and HOTAIR expression level in spermatocytes. It is well known that activation of histone modifications to specific target gene is one of the molecular mechanisms of lncRNAs regulation [13,21]. Our data also revealed that silence HOTAIR inhibited level of histone H4 acetylation in the Nrf2 gene promoter. Thus, HOTAIR regulated Nrf2 expression by hyperacetylation of histone H4 in Nrf2 promoter. Chen et al. demonstrated that Nrf2 expression level were associated with sperm quality and the antioxidant gene expression [10]. Combined with our results, it is suggested that HOTAIR may be involved in protecting spermatozoa against oxidative damage.
SOD is a key enzyme for scavenging ROS in spermatozoa and can prevent the decrease of sperm motility [22,23]. As predicted, SOD activity was positively correlated with sperm HOTAIR expression level. Thus, the reduction of HOTAIR expression levels in spermatozoa may lead to enhanced oxidative stress, which would further impair sperm motility and vitality, as seen in asthenozoospermic and oligoasthenozoospermic patients.
In conclusion, our observations demonstrated that sperm HOTAIR expression levels were significantly inhibited in asthenozoospermic and oligoasthenozoospermic patients, which correlated with damage of sperm motility and vitality. Decreased HOTAIR expression led to the decrease of Nrf2 expression and SOD activity. This study shed light on the mechanisms of ROS related defects in sperm function.
Disclosure of conflict of interest
None.
References
- 1.Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22:1506–1512. doi: 10.1093/humrep/dem046. [DOI] [PubMed] [Google Scholar]
- 2.De Kretser DM. Male infertility. Lancet. 1997;349:787–790. doi: 10.1016/s0140-6736(96)08341-9. [DOI] [PubMed] [Google Scholar]
- 3.Curi SM, Ariagno JI, Chenlo PH, Mendeluk GR, Pugliese MN, Sardi Segovia LM, Repetto HE, Blanco AM. Asthenozoospermia: analysis of a large population. Arch Androl. 2003;49:343–349. doi: 10.1080/01485010390219656. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal A, Gupta S, Sharma R. Oxidative stress and its implications in female infertilitya clinician’s perspective. Reprod Biomed Online. 2005;11:641–650. doi: 10.1016/s1472-6483(10)61174-1. [DOI] [PubMed] [Google Scholar]
- 5.Lamond S, Watkinson M, Rutherford T, Laing K, Whiting A, Smallwood A, Nargund G, Campbell S, Banerjee S. Gene-specific chromatin damage in human spermatozoa can be blocked by antioxidants that target mitochondria. Reprod Biomed Online. 2003;7:407–418. doi: 10.1016/s1472-6483(10)61884-6. [DOI] [PubMed] [Google Scholar]
- 6.Macanovic B, Vucetic M, Jankovic A, Stancic A, Buzadzic B, Garalejic E, Korac A, Korac B, Otasevic V. Correlation between sperm parameters and protein expression of antioxidative defense enzymes in seminal plasma: a pilot study. Dis Markers. 2015;2015:436236. doi: 10.1155/2015/436236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng Q, Kalabus JL, Zhang J, Blanco JG. A conserved antioxidant response element (ARE) in the promoter of human carbonyl reductase 3 (CBR3) mediates induction by the master redox switch Nrf2. Biochem Pharmacol. 2012;83:139–148. doi: 10.1016/j.bcp.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esakky P, Hansen DA, Drury AM, Moley KH. Modulation of cell cycle progression in the spermatocyte cell line [GC-2spd(ts) Cell-Line] by cigarette smoke condensate (CSC) via arylhydrocarbon receptor-nuclear factor erythroid 2-related factor 2 (Ahr-Nrf2) pathway. Biol Reprod. 2014;90:9. doi: 10.1095/biolreprod.113.113225. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura BN, Lawson G, Chan JY, Banuelos J, Cortés MM, Hoang YD, Ortiz L, Rau BA, Luderer U. Knockout of the transcription factor NRF2 disrupts spermatogenesis in an age-dependent manner. Free Radic Biol Med. 2010;49:1368–1379. doi: 10.1016/j.freeradbiomed.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen K, Mai Z, Zhou Y, Gao X, Yu B. Low NRF2 mRNA expression in spermatozoa from men with low sperm motility. Tohoku J Exp Med. 2012;228:259–266. doi: 10.1620/tjem.228.259. [DOI] [PubMed] [Google Scholar]
- 11.Caley DP, Pink RC, Trujillano D, Carter DR. Long noncoding RNAs, chromatin, and development. ScientificWorldJournal. 2010;10:90–102. doi: 10.1100/tsw.2010.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berghoff EG, Clark MF, Chen S, Cajigas I, Leib DE, Kohtz JD. Evf2 (Dlx6as) lncRNA regulates ultraconserved enhancer methylation and the differential transcriptional control of adjacent genes. Development. 2013;140:4407–16. doi: 10.1242/dev.099390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klattenhoff CA, Scheuermann JC, Surface LE, Bradley RK, Fields PA, Steinhauser ML, Ding H, Butty VL, Torrey L, Haas S, Abo R, Tabebordbar M, Lee RT, Burge CB, Boyer LA. Braveheart, a long noncoding RNA required for cardiovascular lineage commitment. Cell. 2013;152:570–583. doi: 10.1016/j.cell.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luk AC, Chan WY, Rennert OM, Lee TL. Long noncoding RNAs in spermatogenesis: insights from recent high-through puttranscriptome studies. Reproduction. 2014;147:R131–141. doi: 10.1530/REP-13-0594. [DOI] [PubMed] [Google Scholar]
- 15.Woo CJ, Kingston RE. HOTAIR lifts noncoding RNAs to new levels. Cell. 2007;129:1257–1259. doi: 10.1016/j.cell.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Ye P, Wang T, Liu WH, Li XC, Tang LJ, Tian FZ. Enhancing HOTAIR/MiR-10b drive liver normal stem cells to arise malignant transformation tendency through inducing epithelial-mesenchymal transitions. Rejuvenation Res. 2015;18:332–40. doi: 10.1089/rej.2014.1642. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. Laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 5th edition. Geneva: World Health Organization; 2010. pp. 10–56. [Google Scholar]
- 18.Kim JH, Jee BC, Lee JM, Suh CS, Kim SH. Histone acetylation level and histone acetyltransferase/deacetylase activity in ejaculatedsperm from normozoospermic men. Yonsei Med J. 2014;55:1333–1340. doi: 10.3349/ymj.2014.55.5.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang M, Li W, Tian H, Hu T, Wang L, Lin Y, Li Y, Huang H, Sun F. Sequential expression of long noncoding RNA as mRNA gene expression in specific stages of mouse spermatogenesis. Sci Rep. 2014;4:5966. doi: 10.1038/srep05966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turner BM. Histone acetylation and control of gene expression. J Cell Sci. 1999;99:13–20. doi: 10.1242/jcs.99.1.13. [DOI] [PubMed] [Google Scholar]
- 21.Yap KL, Li S, Muñoz-Cabello AM, Raguz S, Zeng L, Mujtaba S, Gil J, Walsh MJ, Zhou MM. Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4a. Mol Cell. 2010;38:662–674. doi: 10.1016/j.molcel.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dandekar SP, Nadkarni GD, Kulkarni VS, Punekar S. Lipid peroxidation and antioxidant enzymes in male infertility. J Postgrad Med. 2002;48:186–189. [PubMed] [Google Scholar]
- 23.Cocchia N, Pasolini MP, Mancini R, Petrazzuolo O, Cristofaro I, Rosapane I, Sica A, Tortora G, Lorizio R, Paraggio G, Mancini A. Effect of sod (superoxide dismutase) protein supplementation in semen extenders on motility, viability, acrosome status and ERK (extracellular signal-regulated kinase) protein phosphorylation of chilled stallion spermatozoa. Theriogenology. 2011;75:1201–1210. doi: 10.1016/j.theriogenology.2010.11.031. [DOI] [PubMed] [Google Scholar]


