Abstract
Characteristic features of asthma include airway inflammation and hyperactivity, mucus hypersecretion, mucosal edema, and airway remodeling. These features could be due to pathological water transport across pulmonary epithelia and aquaporins (AQPs) have recently been isolated as key proteins in fluid transportation in the human respiratory tract. We aimed to evaluate the role of aquaporins in the pathogenesis of asthma and their possible use a diagnostic marker of the disease. A total of 110 hospitalized and outpatients with mild to moderate adult-onset asthma were invited to participate in this study and 34 submitted an induced sputum sample adequate for analysis. The amount of AQP1, AQP5 and MUC5AC were measured with ELISA assay. The amount of IL-2, IL-4, IL-6, IL-10, TNF-α, IFN-γ and IL-17 in both serum and sputum were measured with Cytometry Bead Array (CBA kit). Our results suggest that sputum AQP5, AQP1 and MUC5AC are all in a good correlation (r=0.498 between AQP5 and AQP1, r=0.529 and r=0.661 between MUC5AC and AQP5 or AQP1, respectively, all P<0.05). The AUC value for AQP1 and AQP5 to diagnose asthma were 0.729 and 0.745, respectively. In conclusion, water homeostasis plays an important role in maintaining adequate fluid transportation within the lung and is involved in the pathogenesis of asthma. Our results suggest that AQP may influence pulmonary physiology that their dysfunction can contribute to pulmonary pathogenesis, such as asthma. Moreover, their quantification could serve as biomarkers for the diagnosis of asthma.
Keywords: Asthma, aquaporin 1, aquaporin 5, disease models, humans
Introduction
Asthma is the most common chronic respiratory disease and is associated with increased morbidity and mortality. Airway inflammation and hyperactivity, mucus hypersecretion, mucosal edema, and airway remodeling are characteristic features of the disease [1,2]. Pulmonary epithelial water transport is a vital component of lung physiology; fluid movement between the airspace and cellular and/or interstitial and vascular compartments contribute to an adequate airway fluid balance [3,4]. Under pathological conditions, dysregulation of transepithelial water transport appears to play an important role in the development of pulmonary edema [3,4]. However, the detailed mechanisms and specific contribution of water transportation in the development of pulmonary edema is still unclear.
Aquaporins (AQPs) constitute a family of proteins highly expressed in the membrane of tissue cells involved in water transportation (e.g., blood vessels, urinary, respiratory and digestive tract) [4-9]. They are water-permeable channels located within the cell membrane, or pore proteins, that play an important role in selective water transfer through the cell wall. To date, 13 different AQPs have been identified and cloned in mammals. Among those, AQP1 and AQP5 constitute the principal routes for osmotically driven water transport in the human respiratory tract. While AQP1 is expressed in microvascular endothelial cells (MECs) and surrounding connective tissue cells, AQP5 is present in the apical membrane of airway epithelial cells [6,8]. AQP1 may help regulate the permeability of plasma to the vascular walls [10], while AQP5 may be involved in the formation of mucus within the respiratory tract [6,11,12]. Loss of function experiments have documented that AQPs are important for fluid transportation in various tissues [10], their involvement in the pathogenesis of asthma, however, has yet to be demonstrated.
In this study, we measured AQPs, cytokines and cell counts in blood and induced sputum samples from mild to moderate adult-onset asthma patients. The correlation between AQPs expression level and its possible use as a diagnostic marker for asthma was also explored.
Materials and methods
Subjects
Both hospitalized and outpatients with mild to moderate adult-onset asthma, according to the Chinese Medical Association of Respiratory Disease Branch Asthma Study Group diagnostic criteria, were recruited in the department of respiratory medicine of the Chinese Traditional Medicine Hospital of Xinjiang Medical University between March, 2012 and July, 2013. Patients with severe asthma or acute exacerbation of asthma, those with a history of cardiac or other respiratory diseases, those who reported a respiratory tract infection less than two weeks before sputum collection or received glucocorticosteroids less than a month before collection, current or past smokers (0.5 package/day) were excluded from the study, as well as pregnant women and those who refused to participate in research. Furthermore, eleven healthy subjects without any respiratory diseases were recruited from the surrounding community of hospital as control between March and December 2012. This study was approved by the Chinese Traditional Medicine Hospital of Xinjiang Medical University’s ethics committee. All patients were informed of the study’s experimental nature and gave written informed consent.
Sample collection and handling
A 3 ml fasting venous blood sample was collected in the morning, centrifuged under 4°C for 20 minutes at 3,000 rpm for 20 min and subsequently stored at -80°C until analysis of serum IL-2 (sIL-2), sIL-4 sIL-6, sIL-10, sTNF-α, sIFN-γ, and sIL-17 using a Cytometry Bead Array (CBA kit).
Induction and handling of sputum was conducted as previously reported [13-16]. Prior to sputum induction, patients with a FEV1 <80% were administered inhaled beta2-agonist (salbutamol, 200~400 µg, GSK, catalogue number: KR0160) to prevent secondary bronchospasm during the procedure, a FEV1 testing was reapplied. If the FEV1 is still <80%, the sputum induction was canceled. Sputum induction was performed using an inhalation of atomized hypertonic sterile saline (3%) at 4 ml per minute, during a total 20 minutes. Procedures were immediately interrupted if patients felt uncomfortable. Under the guidance of physicians, patients were encouraged to actively cough up sputum at 5, 10, 15 and 20 minutes following inhalation of hypertonic saline. Sputum plugs weighing over 0.3 gram were collected with sterile forceps and deposited in a dry and sterile vial, to which fresh sputum-lysis buffer was added (4 times larger in volume). Specimens were then incubated for 15 minutes at 37°C and vortexted to homogeneity. Cells and supernatant were subsequently separated by centrifugation under 4°C, at 3000 g for 10 minutes; supernatant was removed and stored at -80°C until further measures of AQP1, AQP3, AQP4, AQP5 and MUC5AC using an ELISA assay, while IL-2, IL-4, IL-6, IL-10, TNF-α, IFN-γ, IL-172 were subsequently measured using a Cytometry Bead Array (CBA kit).
Cell pellets were resuspended with PBS and viability measured with the Trypan Blue exclusion method. Sputum samples with cell viability >50% were processed for further analysis and underwent cell count. Samples were then further centrifuged for 5 minutes at 500 g and processed for cytological examination. Cell smears were made and stained with hematoxylin and eosin. At least 400 non cubic epithelial cells were counted and classified under light microscope. Epithelial cells, alveolar macrophages, neutrophils, squamous cells, eosinophils and basophils were classified and counted under microscope. Samples with >20% squamous cells were discarded as large number of squamous cells indicates pollution.
Pulmonary function test
Spirometry was performed using pulmonary function impulse oscillometry (Impulse Oscillation Detector (Master Screen IOS) for Pulmonary Function, Jaegere, Germany). Pulmonary capacity and flow-volume loop were determined and FEV1 value and FEV1% of predicted normal value were recorded, as well as the PEF% of predicted normal value.
Statistical analysis
Statistics were analyzed with MP Pro 10.0. Mean values are presented with their corresponding standard errors, and mean values between patients and controls were tested with t-test. For multiple comparisons, F-test was firstly applied and, if SD was not uniform, Welch test was applied to test for significance. In all instances, tests with P-values under α=0.05 were considered statistically significant.
We analyzed the correlation between eosinophils, AQP1, AQP5, MUC5AC and IFN-γ. MUC5AC was included as it is an important readout for mucus hypersecretion in the pulmonary tracts and associated with chronic obstructive pulmonary disease [17-19]; IFN-γ was included because it showed significant difference between asthma patient and healthy controls in our primary analysis (Table 1).
Table 1.
Physiological parameters
| Asthma group | Control group | P | |
|---|---|---|---|
| N=34 | N=11 | ||
| Gender (F/M) | 28/6 | 8/3 | 0.795 |
| Age | 42.35±8.67 | 38.82±9.84 | 0.262 |
| BMI | 23.07±3.98 | 23.19±2.53 | 0.933 |
| Course of disease | 2.5 (0.3, 25) | - | |
| ACT | 19.03±2.74 | - | |
| Eosinophils (%) | 6.56±14.98 | 0.50±0.28 | 0.024 |
| Neutrophils (%) | 48.79±24.35 | 27.45±11.39 | 0.000 |
| Macrophages (%) | 42.03±21.91 | 67.64±9.27 | 0.000 |
| sputum AQP5 | 469.39±153.65 | 565.12±101.68 | 0.086 |
| sputum AQP1 | 0.89±0.21 | 0.79±0.39 | 0.372 |
| sputum MUC5AC | 65.33±17.84 | 57.84±12.48 | 0.223 |
| Baseline predrug FVC (% predicted) | 97.68±15.99 | ||
| Baseline predrug FEV 1 (% predicted) | 81.61±10.84 | ||
| IL-2 | 1.83±0.48 | 1.80±0.51 | 0.881 |
| IL-4 | 0.67±0.41 | 0.46±0.30 | 0.221 |
| IL-6 | 2.47±1.35 | 2.02±0.87 | 0.340 |
| IL-10 | 0.68±0.50 | 0.64±0.60 | 0.845 |
| TNF-α | 6.64±22.50 | 1.73±1.13 | 0.523 |
| IFN-γ | 2.22±0.69 | 1.64±0.60 | 0.029 |
| IL-17 | 19.75±3.91 | 17.01±6.09 | 0.213 |
| sIL-2 | 1.35±0.69 | 1.03±1.13 | 0.573 |
| sIL-4 | 0.15±0.16 | 0.18±0.31 | 0.830 |
| sIL-6 | 105.27±113.54 | 59.65±46.82 | 0.528 |
| sIL-10 | 0.31±0.31 | 0.04±0.08 | 0.183 |
| sTNF-α | 1.95±1.84 | 2.23±0.91 | 0.807 |
| sIFN-γ | 1.82±1.31 | 1.70±0.40 | 0.881 |
| sIL-17 | 23.95±15.46 | 17.94±5.19 | 0.538 |
*Values are represented as mean ± S.E., P<0.05 was considered significant.
Results
Subjects and sample selection
During the study period, 110 subjects were invited to participate in the study. Among those, 76 subjects met the exclusion criteria and 34 patients with adequate samples were accepted for final analysis (Figure 1).
Figure 1.
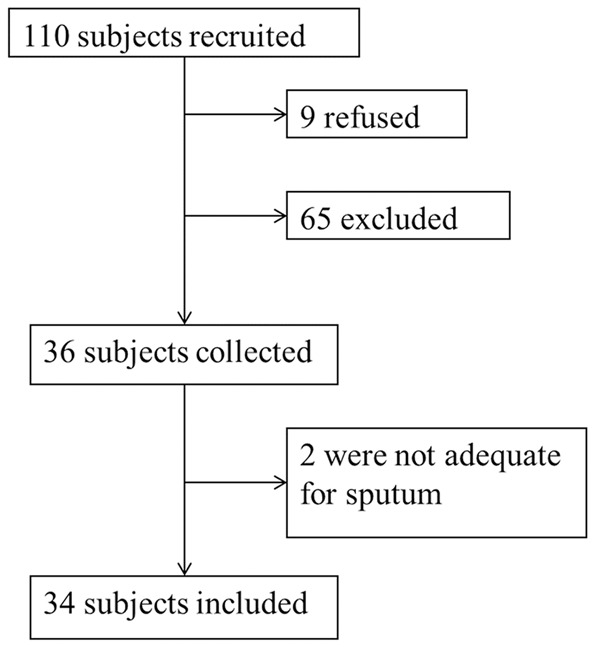
Workflow for subjects and sample selection.
Physiological parameters
We measured several physiological parameters (Table 1). There was no statistically significant difference in gender, age and BMI between asthma patients and healthy controls. Among those multiple parameters we measured, asthma patients had significantly higher counts of both eosinophils (6.56 vs. 0.50, P=0.024), neutrophils (48.79 vs. 27.45, P<0.001), and IFN-γ (2.22 vs. 1.64, P=0.029) while and macrophages were significantly lower in the asthma group (42.03 vs. 67.64, P<0.001).
Correlation among eosinophils, AQP1, AQP5, MUC5AC and IFN-γ
Results of the correlation analysis between eosinophil counts, AQP1, AQP5, MUC5AC and IFN-γ are presented in Table 2. Sputum AQP5 was inversely correlated with eosinophil counts. Sputum AQP1 showed positive correlation with sputum AQP5, sputum MUC5AC was positively correlated with both sputum AQP5 and sputum AQP1. No correlation was found between IFN eosinophil counts, sputum AQP5, sputum AQP1 or MUC5AC.
Table 2.
Summary of correlation among eosinophils, AQP1, AQP5, MUC5AC and IFN-γ
| Eosinophils | sputum AQP5 | sputum AQP1 | MUC5AC | |
|---|---|---|---|---|
| sputum AQP5 | -0.386* | |||
| sputum AQP1 | -0.201 | 0.498* | ||
| sputum MUC5AC | -0.279 | 0.529* | 0.661* | |
| IFN | 0.114 | -0.145 | 0.075 | 0.155 |
P<0.05.
For a more comprehensive analysis, we fitted linear regression models to evaluate the association between measured levels of MUC5AC, sputum AQP1 and sputum AQP5. Results were also consistent with the summary analyses showing that sputum AQP5 was positively correlated with sputum AQP1 (Figure 2) and sputum MUC5AC was positively correlated with sputum AQP1 (Figure 3) and sputum AQP5 (Figure 4).
Figure 2.
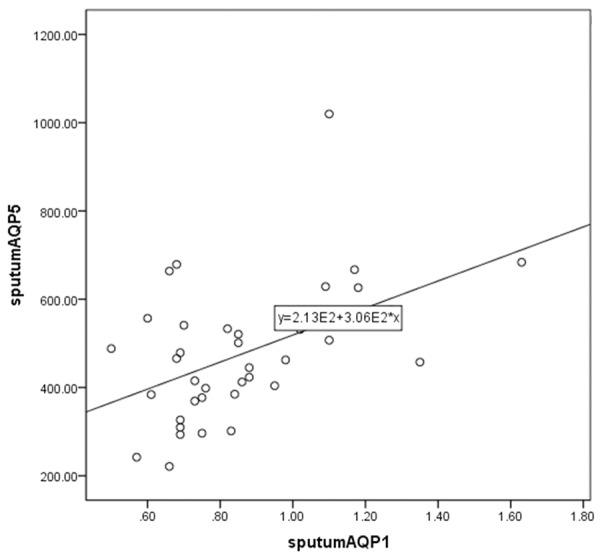
Scatter plot and linear-regression analysis of correlation between sputum AQP1 and sputum AQP5 (R2=0.248).
Figure 3.
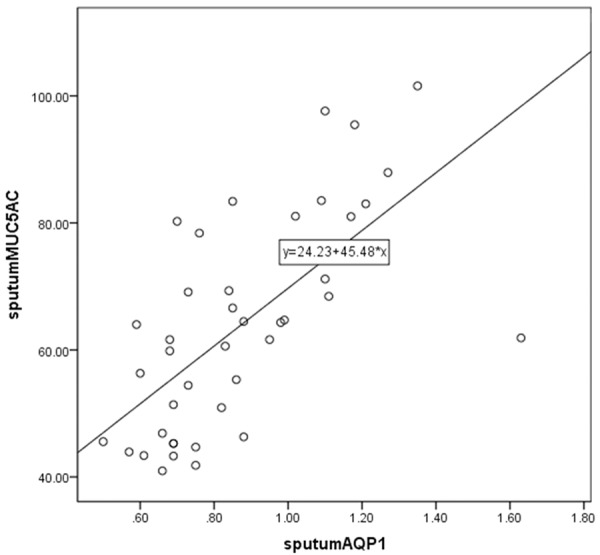
Scatter plot and linear-regression analysis of correlation between sputum MUC5AC and sputum AQP1 (R2=0.437).
Figure 4.
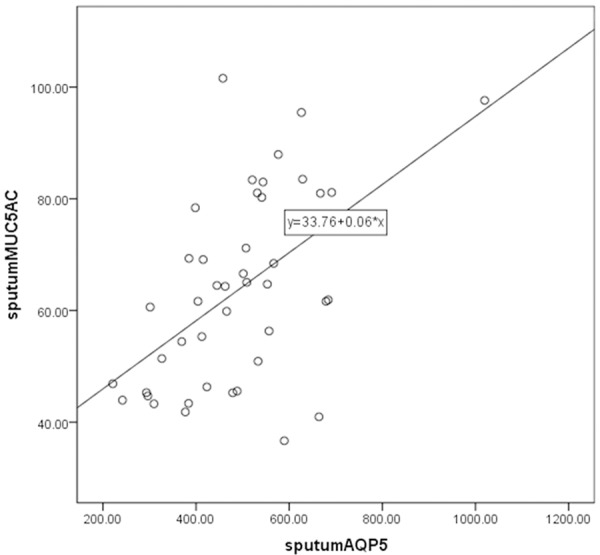
Scatter plot and linear-regression analysis of correlation between sputum MUC5AC and sputum AQP5 (R2=0.280).
Assessment of AQP1 and AQP5 in asthma diagnosis
We further assessed whether AQP1 and AQP5 level in induced sputum could be used as a diagnostic marker for asthma by analyzing their receiver operating characteristic curve (ROC, Figure 5A and 5B). The AUC value for AQP1 and AQP5 was 0.729 and 0.745 (Table 3), respectively, indicating either of them could serve as moderate markers in the diagnosis of asthma (AUC>0.7, [20]). The best cutoff value for diagnostic purposes was .0.69 for AQP1 and 419.23 for AQP5 (Table 3).
Figure 5.
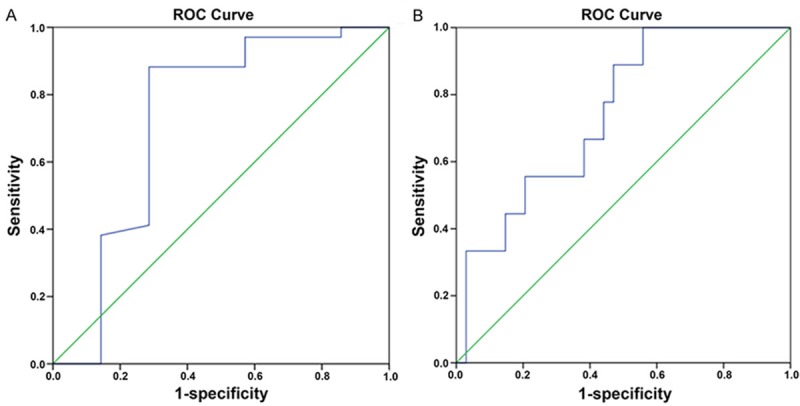
Receiver operating characteristic (ROC) of AQP1 (A) and AQP5 (B).
Table 3.
Summary for AUC, cutoff value, sensitivity and specificity of AQP5 and AQP1
| AUC | Cutoff | Sensitivity | Specificity | |
|---|---|---|---|---|
| AQP5 | 0.745 | 419.23 | 1 | 0.441 |
| AQP1 | 0.729 | 0.69 | 0.882 | 0.714 |
Discussion
By analyzing blood and induced sputum samples from adult-onset asthma patients, we carried out the first study to monitor a variety of contents, including different cell types, related proteins and cytokines, and compared their differences between patient and healthy people. We found that, compared to healthy controls, asthma patients had significantly higher counts of both eosinophils and neutrophils while macrophages were significantly lower. Meanwhile, IFN-γ concentrations increased significantly in asthma patients. Furthermore, sputum AQP5 were negatively correlated with eosinophil counts, sputum AQP1 were positively correlated with sputum AQP5, and sputum MUC5AC showed positive correlation with both sputum AQP5 and sputum AQP1. However, IFN did not show correlation with either eosinophil counts, sputum AQP5, sputum AQP1 or MUC5AC. Moreover, we assessed whether AQP1 and AQP5 could be used as diagnostic marker for asthma in clinics using receiver operating characteristic (ROC) assay. We found that AQP1 and AQP5 could be used as moderate markers of asthma, suggesting that their level can assist in the diagnosis of asthma in the clinical setting; the optimal cutoff values were estimated at 419.23 for AQP1 and 0.69 for AQP5. Our results not only provide direct evidence of correlation between asthma and both AQP1 and AQP5, but also indicate that these two proteins could be used as clinical markers of disease.
Water homeostasis plays an important role in maintaining adequate fluid transportation within the lung and is involved in the pathogenesis of asthma [3-5,7-9,11]. AQP1, AQP3, AQP4 and AQP5 have been found to be expressed in mammalian pulmonary tissue [5,21]. In 2001, Kreda et al. documented the distribution of AQPs in human airways using in situ hybridization and immunohistochemistry staining [22]. Moreover, King et al. further showed that vascular permeability was impaired in AQP1 knockout mice and that congenital AQP1 deficiency leads to less thickening of blood vessel walls following intravenous saline perfusion in human [23] indicating that AQP1 is required for maintaining permeability of pulmonary vasculature. Our findings are consistent with this hypothesis, as asthma also led to decreased expression of AQP1 in our patients compared to healthy controls. However, how AQPs contributes to the pathogenesis asthma is not clear, but there are indications that AQPs may influence pulmonary physiology in a variety of ways and that their dysfunction can contribute to pulmonary pathogenesis. Firstly, AQP1 is the predominant type if AQPs found in pulmonary tissue; it mediates water transportation between airway and pulmonary microvasculature. Upregulation of AQP1 in asthma may increase the water level in pulmonary tissue [24], promote the allergic response and secretions in airway, and promote leakage of inflammatory corpuscle [25]. It can also lead to pathological angiogenesis and promote airway remodeling, a characteristic feature of asthma [26,27]. Those effects are consistent with our findings that AQP1 shows significant positive correlation with MUC5AC in induced sputum supernatant. Secondly, AQP5 is involved in glandular secretion, fluid clearance in airway and pulmonary tissue and in maintaining a normal fluid surface in airways. AQP5 decrements can lead to decreased fluid secretion and elevated mucoprotein concentrations in airway. Furthermore, elevated inflammatory factors and cytokines may also lead to airway injury and asthma progression via the downregulation of AQP5 [25,28,29]. Those studies all suggest that AQP dysfunctions likely contribute to the pathogenesis and progression of asthma.
Although we have found that the sputum concentration level of several cell types were altered in asthma patients, AQP expression was also higher but we did not observe a statistically significant change in AQPs. It is possible that because we included patients suffering from both mild and moderate asthma in the primary analysis, could have underestimated, or diluted any difference possibly seen in patients with moderate asthma. However, we were unable to evaluate AQP expression in patients suffering from severe asthma, as sputum induction is contraindicated in patients. We believe, however, that differences in AQP expression should be more obvious in patients suffering from severe asthma and that any changes in AQP expression would be significant among this group. Also, our sample size consisting of 34 patients was likely too small and our study was underpowered to detect differences and a larger sample size is needed for future studies to draw convincing conclusions.
In summary, we have characterized cell types and counts, cytokine and aquaporin expression in induced sputum from adult-onset mild to moderate asthma patients. We found that aquaporin expression showed a tendency towards correlation with asthma. Furthermore, we found that the level of aquaporins could be used as moderate diagnostic marker for asthma.
Acknowledgements
The special funds of innovation and development of Xinjiang Uygur Autonomous Region Research Institute (grant NO. 2015008).
Disclosure of conflict of interest
None.
References
- 1.Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, Gibson P, Ohta K, O’Byrne P, Pedersen SE, Pizzichini E, Sullivan SD, Wenzel SE, Zar HJ. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–178. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan AF, Schatz M, Wenzel SE, Vanderweil SG, Camargo CA Jr. A profile of U. S. asthma centers, 2006. Ann Allergy Asthma Immunol. 2007;99:419–423. doi: 10.1016/S1081-1206(10)60566-2. [DOI] [PubMed] [Google Scholar]
- 3.Borok Z, Verkman AS. Lung edema clearance: 20 years of progress: invited review: role of aquaporin water channels in fluid transport in lung and airways. J Appl Physiol (1985) 2002;93:2199–2206. doi: 10.1152/japplphysiol.01171.2001. [DOI] [PubMed] [Google Scholar]
- 4.Song Y, Verkman AS. Aquaporin-5 dependent fluid secretion in airway submucosal glands. J Biol Chem. 2001;276:41288–41292. doi: 10.1074/jbc.M107257200. [DOI] [PubMed] [Google Scholar]
- 5.Ishibashi K, Hara S, Kondo S. Aquaporin water channels in mammals. Clin Exp Nephrol. 2009;13:107–117. doi: 10.1007/s10157-008-0118-6. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen S, King LS, Christensen BM, Agre P. Aquaporins in complex tissues. II. Subcellular distribution in respiratory and glandular tissues of rat. Am J Physiol. 1997;273:C1549–1561. doi: 10.1152/ajpcell.1997.273.5.C1549. [DOI] [PubMed] [Google Scholar]
- 7.Song Y, Jayaraman S, Yang B, Matthay MA, Verkman AS. Role of aquaporin water channels in airway fluid transport, humidification, and surface liquid hydration. J Gen Physiol. 2001;117:573–582. doi: 10.1085/jgp.117.6.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verkman AS. Role of aquaporins in lung liquid physiology. Respir Physiol Neurobiol. 2007;159:324–330. doi: 10.1016/j.resp.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verkman AS, Matthay MA, Song Y. Aquaporin water channels and lung physiology. Am J Physiol Lung Cell Mol Physiol. 2000;278:L867–879. doi: 10.1152/ajplung.2000.278.5.L867. [DOI] [PubMed] [Google Scholar]
- 10.Bai C, Fukuda N, Song Y, Ma T, Matthay MA, Verkman AS. Lung fluid transport in aquaporin-1 and aquaporin-4 knockout mice. J Clin Invest. 1999;103:555–561. doi: 10.1172/JCI4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King LS, Agre P. Pathophysiology of the aquaporin water channels. Annu Rev Physiol. 1996;58:619–648. doi: 10.1146/annurev.ph.58.030196.003155. [DOI] [PubMed] [Google Scholar]
- 12.Matsuzaki T, Hata H, Ozawa H, Takata K. Immunohistochemical localization of the aquaporins AQP1, AQP3, AQP4, and AQP5 in the mouse respiratory system. Acta Histochem Cytochem. 2009;42:159–169. doi: 10.1267/ahc.09023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pin I, Gibson PG, Kolendowicz R, Girgis-Gabardo A, Denburg JA, Hargreave FE, Dolovich J. Use of induced sputum cell counts to investigate airway inflammation in asthma. Thorax. 1992;47:25–29. doi: 10.1136/thx.47.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korevaar DA, Westerhof GA, Wang J, Cohen JF, Spijker R, Sterk PJ, Bel EH, Bossuyt PM. Diagnostic accuracy of minimally invasive markers for detection of airway eosinophilia in asthma: a systematic review and meta-analysis. Lancet Respir Med. 2015;3:290–300. doi: 10.1016/S2213-2600(15)00050-8. [DOI] [PubMed] [Google Scholar]
- 15.Lee WY, Southworth T, Booth S, Singh D. High- and low-dose allergen challenges in asthmatic patients using inhaled corticosteroids. Br J Clin Pharmacol. 2015;79:523–532. doi: 10.1111/bcp.12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turgut T, Ilhan N, Deveci F, Akpolat N, Erden ES, Muz MH. Glutathione and nitrite levels in induced sputum at COPD patients and healthy smokers. J Thorac Dis. 2014;6:765–771. doi: 10.3978/j.issn.2072-1439.2014.04.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morcillo EJ, Cortijo J. Mucus and MUC in asthma. Curr Opin Pulm Med. 2006;12:1–6. doi: 10.1097/01.mcp.0000198064.27586.37. [DOI] [PubMed] [Google Scholar]
- 18.Rogers DF, Barnes PJ. Treatment of airway mucus hypersecretion. Ann Med. 2006;38:116–125. doi: 10.1080/07853890600585795. [DOI] [PubMed] [Google Scholar]
- 19.Evans CM, Kim K, Tuvim MJ, Dickey BF. Mucus hypersecretion in asthma: causes and effects. Curr Opin Pulm Med. 2009;15:4–11. doi: 10.1097/MCP.0b013e32831da8d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang DD, Zhou XH, Freeman DH Jr, Freeman JL. A non-parametric method for the comparison of partial areas under ROC curves and its application to large health care data sets. Stat Med. 2002;21:701–715. doi: 10.1002/sim.1011. [DOI] [PubMed] [Google Scholar]
- 21.Ablimit A, Hasan B, Lu W, Qin W, Wushouer Q, Zhong N, Upur H. Changes in water channel aquaporin 1 and aquaporin 5 in the small airways and the alveoli in a rat asthma model. Micron. 2013;45:68–73. doi: 10.1016/j.micron.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Kreda SM, Gynn MC, Fenstermacher DA, Boucher RC, Gabriel SE. Expression and localization of epithelial aquaporins in the adult human lung. Am J Respir Cell Mol Biol. 2001;24:224–234. doi: 10.1165/ajrcmb.24.3.4367. [DOI] [PubMed] [Google Scholar]
- 23.King LS, Nielsen S, Agre P, Brown RH. Decreased pulmonary vascular permeability in aquaporin-1-null humans. Proc Natl Acad Sci U S A. 2002;99:1059–1063. doi: 10.1073/pnas.022626499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kraft M. The distal airways: are they important in asthma? Eur Respir J. 1999;14:1403–1417. doi: 10.1183/09031936.99.14614039. [DOI] [PubMed] [Google Scholar]
- 25.Wang K, Feng YL, Wen FQ, Chen XR, Ou XM, Xu D, Yang J, Deng ZP. Decreased expression of human aquaporin-5 correlated with mucus overproduction in airways of chronic obstructive pulmonary disease. Acta Pharmacol Sin. 2007;28:1166–1174. doi: 10.1111/j.1745-7254.2007.00608.x. [DOI] [PubMed] [Google Scholar]
- 26.Belge H, Devuyst O. Aquaporin-1-a water channel on the move. Nephrol Dial Transplant. 2006;21:2069–2071. doi: 10.1093/ndt/gfl162. [DOI] [PubMed] [Google Scholar]
- 27.Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005;434:786–792. doi: 10.1038/nature03460. [DOI] [PubMed] [Google Scholar]
- 28.Krane CM, Fortner CN, Hand AR, McGraw DW, Lorenz JN, Wert SE, Towne JE, Paul RJ, Whitsett JA, Menon AG. Aquaporin 5-deficient mouse lungs are hyperresponsive to cholinergic stimulation. Proc Natl Acad Sci U S A. 2001;98:14114–14119. doi: 10.1073/pnas.231273398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.James AL, Pare PD, Hogg JC. The mechanics of airway narrowing in asthma. Am Rev Respir Dis. 1989;139:242–246. doi: 10.1164/ajrccm/139.1.242. [DOI] [PubMed] [Google Scholar]


