Abstract
MicroRNAs (miRNAs) are a class of small non-coding RNAs that have been suggested to play critical roles in tumorigenesis. Recently, miR-152 was reported to be dysregulated in some human cancers. However, the function and mechanism of miR-152 in non-small cell lung cancer (NSCLC) is still unclear. In the present study, our findings showed that the expression of miR-152 was significantly down-regulated and neuropilin-1 was up-regulated in the NSCLC specimens. Moreover, the levels of miR-152 and neuropilin-1 were inversely correlated. Bioinformatics analyses and luciferase reporter assay showed that miR-152 targeted the 3’-UTR of neuropilin-1 mRNA to inhibit its translation. Furthermore, overexpression of miR-152 inhibited neuropilin-1 mediated cell invasiveness, while down-regulated expression of miR-152 increased neuropilin-1 mediated cell invasiveness in NSCLC cells. Together, these findings indicated that miR-152 suppression in NSCLC cells might promote neuropilin-1 mediated cancer metastasis and suggested a new therapeutic application of miR-152 in the treatment of NSCLC.
Keywords: Non-small cell lung cancer, miR-152, neuropilin-1, migration, invasion
Introduction
Lung cancer is one of the most malignant cancers and the leading cause of cancer-related deaths worldwide, and non-small cell lung cancer (NSCLC) accounts for 80% of primary lung cancer [1,2]. Despite recent advances in clinical and experimental oncology, the 5-year survival rate of NSCLC patients is still around 15% [3]. However, the molecular mechanisms underlying the development and progression of NSCLC are currently still poorly understood [4]. Thus, exploring the potential mechanisms will be useful to find new therapeutic targets and strategies for the treatment of NSCLC.
MicroRNAs (miRNAs), which are a class of non-coding small RNA comprised of about 18 to 23 nucleotides, bind to the complimentary recognition sequences in the 3’-untranslated region (3’-UTR) of target mRNA, resulting in translational inhibition or target mRNA degradation [5,6]. MiRNAs are implicated in the regulation of various cellular processes, including cell growth, differentiation, apoptosis, and organ development [7,8]. Emerging evidence shows that numerous miRNAs participate in the regulation of NSCLC initiation and progression [9]. For example, Mei et al. showed that miR-141 promotes the proliferation of NSCLC cells by regulating expression of PHLPP1 and PHLPP2 [10]. Liu et al. reported that miR-196a promotes NSCLC cell proliferation and invasion through targeting HOXA5 [11]. Wang et al. found that miR-203 suppresses the proliferation and migration and promotes the apoptosis of lung cancer cells by targeting SRC [12]. However, the role of miR-152 in NSCLC cells remains unclear.
In this study, we showed that the expression of miR-152 was significantly decreased and the levels of neuropilin-1 were increased in NSCLC tissues compared to paired non-tumor tissues. The expression level of miR-152 and neuropilin-1 were inversely correlated in NSCLC tissues. Bioinformatics analyses showed that miR-152 targeted the 3’-UTR of neuropilin-1 mRNA to inhibit its translation, which was confirmed by luciferase reporter assay. Furthermore, overexpression of miR-152 inhibited neuropilin-1 mediated cell invasiveness, while down-regulated expression of miR-152 increased neuropilin-1 mediated cell invasiveness in NSCLC cells. Together, our findings suggested that miR-152 suppression may be the cause of the increased levels of neuropilin-1, as well as the augmented cancer metastases in NSCLC.
Materials and methods
Cell lines
A human NSCLC cell line A549 was obtained from the American Type Culture Collection (ATCC, USA). The A549 cell line was cultured in DMEM medium (Invitrogen) supplemented with 10% fetal bovine serum (FBS) (Invitrogen). Cells were maintained at 37°C in a humidified atmosphere with 5% CO2.
Patient sample collection
Paired NSCLC and adjacent non-tumor tissues (located more than 5 cm away from the tumors) were obtained from 36 patients who underwent primary surgical resection of NSCLC between 2011 and 2013 at Huaihe Hospital of HeNan University. None of the patients had received preoperative adjuvant therapy. These samples were snap-frozen in liquid nitrogen after resection. Prior patient consent and approval from the ethics committees of HeNan University were obtained for the use of these clinical materials for research purposes.
Plasmids transfection
MiR-153-expressing and antisense plasmids (anti) were prepared with general method. Transfection was performed with Lipofectamine 2000 reagent (Invitrogen), according to the instructions of the manufacturer. The cells that were transfected with plasmid expressing a scrambled sequence were used as control (NC). Transfected cells expressing miR-152, or anti-miR-152, or control (NC) was purified by flow cytometry based on GFP.
Western blot
Cultured cells were lysed in RIPA buffer with 1% PMSF. Protein was loaded and separated by SDS-PAGE gel and transferred onto PVDF membrane (Bio-Rad). The blots were probed with primary antibodies at 4°C overnight and subsequently incubated with HRP-conjugated secondary antibodies. Signals were visualized using ECL Substrates (Pierce). GAPDH was used as an endogenous control.
RNA isolation and quantitative real-time PCR (qRT-PCR)
Total RNA was isolated with Trizol (Invitrogen) according to the manufacturer’s protocol. MicroRNA was isolated with the mirVana miRNA Isolation Kit (Ambion). Expression of miR-152 was detected using TaqMan MicroRNA Assay primers (ABI). U6 or GAPDH was used as an internal control. Experiments were performed on the ABI Prism 7500 HT. The relative expression level was calculated by normalization with the signal for U6 expression using 2-ΔΔCt method.
Luciferase-reporter activity assay
Luciferase reporters were successfully constructed using molecular cloning technology. Target sequence for neuropilin-1 miRNA 3’-UTR clone was purchased from Creative Biogene (Shirley). miR-152 modified A549 cells were seeded in 24-well plates for 24 h, after which they were transfected with 1 μg of Luciferase-reporter plasmids per well. Luciferase activities were measured using the dual-luciferase-reporter gene assay kit (Promega) according to the manufacturer’s instructions.
Migration and invasion assays
Migration and invasion were examined using a transwell chamber (Millipore). For the migration assay, 1×105 transfected cells were plated into the upper chamber, and cultured in DMEM, while DMEM with 10% FBS was added to the lower chamber. After 24 h incubation at 37°C, cells remaining on the upper surface of membrane were removed, and membrane was stained with 20% methanol and 0.5% crystal violet. Cell images were obtained using an inverted microscope (Olympus). For invasion assays, the upper chamber was precoated with Matrigel (BD).
Statistical analysis
All statistical analyses were performed using SPSS version 18.0 software. Bivariate correlations were calculated by Spearman’s Rank Correlation Coefficients. All data were statistically analyzed using one-way ANOVA with a Bonferroni correction, followed by Fisher’s exact test for comparison of two groups. P<0.05 was considered significant.
Results
Decreased miR-152 and increased neuropilin-1 are detected and inversely correlate in NSCLC
In the NSCLC samples, we detected significantly higher levels of neuropilin-1 (Figure 1A) and significantly lower levels of miR-152 in NSCLC (Figure 1B) compared to the adjacent non-tumor tissues from the same NSCLC patient. To figure out the relationship between miR-152 and neuropilin-1, we examined the correlation using the 36 NSCLC specimens. A strong inverse correlation between miR-152 and neuropilin-1 was detected (Figure 1C, P<0.05). These data suggest the presence of a causal link between miR-152 and neuropilin-1 in NSCLC.
Figure 1.
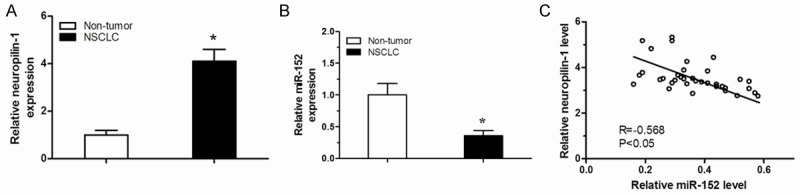
Decreased miR-152 and correlated increased neuropilin-1 are detected in NSCLC tissues. qRT-PCR on miR-152 and Western blot for neuropilin-1 were performed on paired NSCLC and the adjacent non-tumor tissues. NSCLC contained increased neuropilin-1 levels (A) and decreased miR-152 levels (B) compared to adjacent non-tumor tissues. (C) Correlation tests were performed between neuropilin-1 and miR-152. *P<0.05.
MiR-152 targets 3’-UTR of neuropilin-1 mRNA to inhibit its translation
Since our data showed a relationship between miR-152 and neuropilin-1 in NSCLC cells, we checked whether miR-152 may target neuropilin-1 mRNA. Our finds revealed that the miR-152 binding sites in the 3’-UTR of neuropilin-1 mRNA ranged from 2135th base site to 2142th base site (Figure 2A). In order to figure out whether miR-152 may regulate the translation of neuropilin-1 in NSCLC, we used a human NSCLC cell line, A549, to either overexpress miR-152 or to inhibit miR-152 through transfection of the cells with a miR-152-expressing plasmid (A549-miR-152), or with a plasmid carrying miR-152 antisense (A549-anti-miR-152), respectively, the A549 cells were also transfected with a plasmid carrying a scrambled sequence as a control (A549-NC). Co-expression of a GFP reporter in these plasmids allowed purification of transfected cells by flow cytometry. The overexpression or inhibition of miR-152 in A549 cells was confirmed by qRT-PCR (Figure 2B). miR-152-modified A549 cells were then transfected with 1 μg of neuropilin-1 3’-UTR luciferase reporter plasmid. The luciferase activities were quantified in these cells, indicating that miR-152 targets 3’-UTR of neuropilin-1 mRNA to inhibit its translation (Figure 2C).
Figure 2.
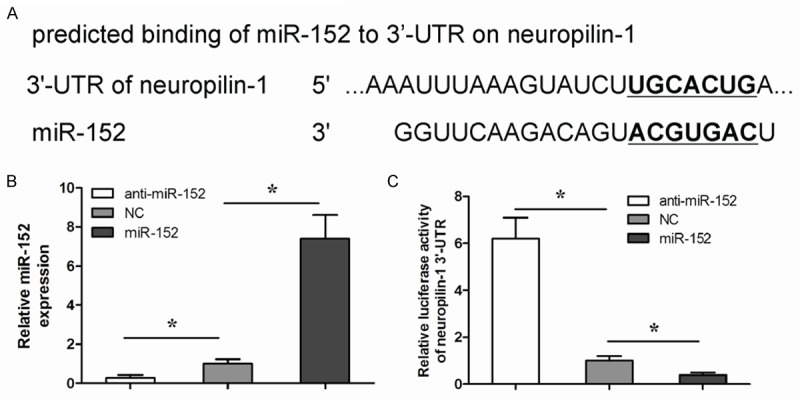
MiR-152 targets 3’-UTR of neuropilin-1 mRNA to inhibit its translation. A. Bioinformatic analyses of binding of miR-152 to the 3’-UTR of neuropilin-1 mRNA. B. We either overexpressed miR-152 or inhibited miR-152 in NSCLC cell line A549, by transfection of the cells with a miR-152-expressing plasmid (A549-miR-152), or with a plasmid carrying miR-152 antisense (A549-anti-miR-152). The A549 cells were also transfected with a plasmid carrying a scrambled sequence as a control (A549-NC). The overexpression or inhibition of miR-152 in A549 cells was confirmed by qRT-qPCR. C. miR-152-modified A549 cells were then transfected with 1 μg of neuropilin-1 3’-UTR luciferase reporter plasmid. The luciferase activities were quantified in these cells. *P<0.05.
miR-152 inhibits neuropilin-1 in NSCLC cells
Our data showed that alteration of miR-152 in A549 cells did not change mRNA of neuropilin-1 (Figure 3A). However, overexpression of miR-152 significantly decreased neuropilin-1 protein levels in A549 cells, while inhibition of miR-152 significantly increased neuropilin-1 levels in A549 cells, by Western blot (Figure 3B). These finding demonstrated that miR-152 could suppress neuropilin-1 protein translation in NSCLC cells.
Figure 3.
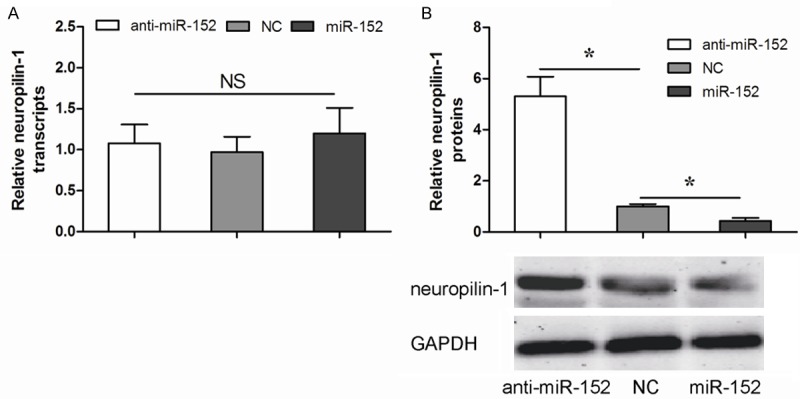
miR-152 decreases neuropilin-1 protein in NSCLC cells. A. The mRNA levels of neuropilin-1 in miR-152-modified A549 cells by qRT-PCR. B. The protein levels of neuropilin-1 in miR-152 modified A549 cells by Western blot. *P<0.05.
miR-152 suppresses cell migration and invasion in NSCLC cells
Transwell migration assay showed that the increased expression of miR-152 resulted in decreases in cell migration ability of A549 cells (Figure 4A). Similarly, depletion of miR-152 resulted in increases in cell migration ability of A549 cells (Figure 4A). Furthermore, transwell invasion assay reported that overexpression of miR-152 resulted in decreases in cell invasion ability of A549 cells (Figure 4B). Similarly, the depletion of miR-152 resulted in increases in cell invasion ability of A549 cells (Figure 4B). Together, our results indicated that miR-152 inhibited NSCLC cell metastases through neuropilin-1 suppression (Figure 5).
Figure 4.
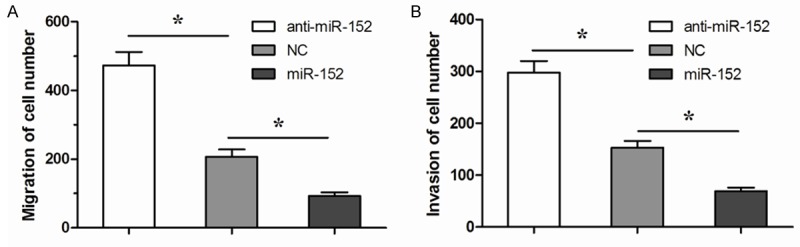
miR-152 inhibits NSCLC cell migration and invasion. A. Overexpression of miR-152 resulted in decreases in cell migration ability of A549 cells, while depletion of miR-152 resulted in increases in cell migration ability of A549 cells in a transwell cell migration assay. B. Overexpression of miR-152 resulted in decreases in cell invasion ability of A549 cells, while depletion of miR-152 resulted in increases in cell invasion ability of A549 cells in a transwell cell invasion assay. *P<0.05.
Figure 5.
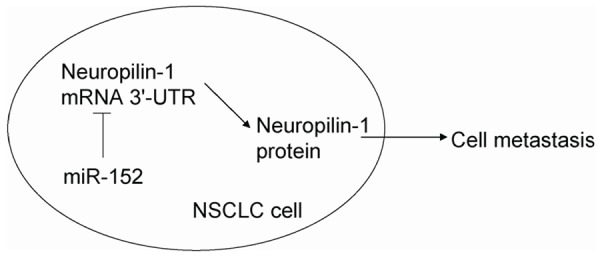
Schematic of the model. MiR-152 inhibits NSCLC cell metastasis possibly through suppression of neuropilin-1.
Discussion
miRNAs have been reported to play essential roles in carcinogenesis and tumor progression as either tumor suppressors or oncogenes [13]. For example, Yang et al. showed that miR-506 is down-regulated in clear cell renal cell carcinoma and exerts its anti-cancer function by directly targeting FLOT1 [14]. Xu et al. reported that miR-32 is down-regulated in osteosarcoma and inhibits osteosarcoma cell proliferation and invasion by targeting Sox9 [15]. Wang et al. found that miR-106a is up-regulated in gastric cancer and inhibits the extrinsic apoptotic pathway by targeting FAS [16].
miR-152 is a member of the miR-148/152 family which includes miR-148a/b and miR-152 [17]. Aberrant expression of miR-152 was thought to contribute to the malignant phenotype in various types of cancer. For example, Zhai et al. showed that miR-152 might act as a tumor suppressor to inhibit gastric cancer cell proliferation and motility by targeting CD151 [18]. Ma et al. miR-152 could function as a tumor suppressor in glioblastoma stem cells by targeting Krüppel-like factor 4 [19]. Zhu et al. found that miR-152 could act as a tumor suppressor that targets TGFα in prostate cancer [20]. However, the role of miR-152 in the NSCLC cell metastasis remains unknown.
Neuropilins, including neuropilin-1 and neuropilin-2, are multifunctional non tyrosine kinase receptors that were first identified based on their critical roles in the developing nervous system [21]. Subsequent study showed neuropilin-1 functions as a receptor for the VEGF-A isoform VEGF-165 in both endothelial cells and some tumor cells [22]. Many studies showed that neuropilin-1 is up-regulated in various tumor types and is expressed in different tumor vasculatures [23], indicating that neuropilin-1 plays important roles in tumor progression.
In this study, our data revealed that the levels of miR-152 in NSCLC tissues were inversely correlated with the levels of neuropilin-1 in NSCLC patients. Overexpression of miR-152 suppressed NSCLC cell migration and invasion, while depletion of miR-152 increased NSCLC cell migration and invasion. Then, we modified miR-152 levels in NSCLC cells and found that it did not affect neuropilin-1 mRNA but altered the protein. These data were further strengthened by promoter luciferase assay. Moreover, miR-152 mediated changes in neuropilin-1 seemed to result in changes in cell metastasis.
In conclusion, our study proposes a model that regulates NSCLC metastases. neuropilin-1 promotes NSCLC cell migration and invasion. MiR-152 inhibits neuropilin-1 protein translation and subsequently suppresses cell metastasis. Thus, the present study suggests that miR-152 might be a potential therapeutic target for treating NSCLC.
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–594. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstraw P, Ball D, Jett JR, Le Chevalier T, Lim E, Nicholson AG, Shepherd FA. Non-smallcell lung cancer. Lancet. 2011;378:1727–1740. doi: 10.1016/S0140-6736(10)62101-0. [DOI] [PubMed] [Google Scholar]
- 4.Engelman JA, Janne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2008;14:2895–2899. doi: 10.1158/1078-0432.CCR-07-2248. [DOI] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez-Garcia I, Miska EA. MicroRNA functions in animal development and human disease. Development. 2005;132:4653–4662. doi: 10.1242/dev.02073. [DOI] [PubMed] [Google Scholar]
- 9.Lin PY, Yu SL, Yang PC. MicroRNA in lung cancer. Br J Cancer. 2010;103:1144–1148. doi: 10.1038/sj.bjc.6605901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mei Z, He Y, Feng J, Shi J, Du Y, Qian L, Huang Q, Jie Z. MicroRNA-141 promotes the proliferation of non-small cell lung cancer cells by regulating expression of PHLPP1 and PHLPP2. FEBS Lett. 2014;588:3055–3061. doi: 10.1016/j.febslet.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 11.Liu XH, Lu KH, Wang KM, Sun M, Zhang EB, Yang JS, Yin DD, Liu ZL, Zhou J, Liu ZJ. MicroRNA-196a promotes non-small cell lung cancer cell proliferation and invasion through targeting HOXA5. BMC Cancer. 2012;12:348. doi: 10.1186/1471-2407-12-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang N, Liang H, Zhou Y, Wang C, Zhang S, Pan Y, Wang Y, Yan X, Zhang J, Zhang CY, Zen K, Li D, Chen X. miR-203 suppresses the proliferation and migration and promotes the apoptosis of lung cancer cells by targeting SRC. PLoS One. 2014;9:e105570. doi: 10.1371/journal.pone.0105570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kent O, Mendell J. A small piece in the cancer puzzle: microRNAs as tumor suppressors and oncogenes. Oncogene. 2006;25:6188–6196. doi: 10.1038/sj.onc.1209913. [DOI] [PubMed] [Google Scholar]
- 14.Yang FQ, Zhang HM, Chen SJ, Yan Y, Zheng JH. MiR-506 Is Down-Regulated in Clear Cell Renal Cell Carcinoma and Inhibits Cell Growth and Metastasis via Targeting FLOT1. PLoS One. 2015;10:e0120258. doi: 10.1371/journal.pone.0120258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu JQ, Zhang WB, Wan R, Yang YQ. MicroRNA-32 inhibits osteosarcoma cell proliferation and invasion by targeting Sox9. Tumour Biol. 2014;35:9847–53. doi: 10.1007/s13277-014-2229-x. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Liu M, Zhu H, Zhang W, He S, Hu C, Quan L, Bai J, Xu N. miR106a is frequently upregulated in gastric cancer and inhibits theextrinsic apoptotic pathway by targeting FAS. Mol Carcinog. 2013;52:634–646. doi: 10.1002/mc.21899. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Zhan Z, Xu L, Ma F, Li D, Guo Z, Li N, Cao X. MicroRNA-148/152 impair innate response and antigen presentation of TLRtriggered dendritic cells by targeting CaMKIIα. J Immunol. 2010;185:7244–7251. doi: 10.4049/jimmunol.1001573. [DOI] [PubMed] [Google Scholar]
- 18.Zhai R, Kan X, Wang B, Du H, Long Y, Wu H, Tao K, Wang G, Bao L, Li F, Zhang W. miR-152 suppresses gastric cancer cell proliferation and motility by targeting CD151. Tumour Biol. 2014;35:11367–11373. doi: 10.1007/s13277-014-2471-2. [DOI] [PubMed] [Google Scholar]
- 19.Ma J, Yao Y, Wang P, Liu Y, Zhao L, Li Z, Li Z, Xue Y. MiR-152 functions as a tumor suppressor in glioblastoma stem cells by targeting Krüppel-like factor 4. Cancer Lett. 2014;355:85–95. doi: 10.1016/j.canlet.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Zhu C, Li J, Ding Q, Cheng G, Zhou H, Tao L, Cai H, Li P, Cao Q, Ju X. miR152 controls migration and invasive potential by targeting TGFα in‐prostate cancer cell lines. Prostate. 2013;73:1082–1089. doi: 10.1002/pros.22656. [DOI] [PubMed] [Google Scholar]
- 21.Bielenberg DR, Pettaway CA, Takashima S, Klagsbrun M. Neuropilins in neoplasms: expression, regulation, and function. Exp Cell Res. 2006;312:584–593. doi: 10.1016/j.yexcr.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 22.Pan Q, Chanthery Y, Liang WC, Stawicki S, Mak J, Rathore N, Tong RK, Kowalski J, Yee SF, Pacheco G. Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell. 2007;11:53–67. doi: 10.1016/j.ccr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 23.Miao HQ, Lee P, Lin H, Soker S, Klagsbrun M. Neuropilin-1 expression by tumor cells promotes tumor angiogenesis and progression. FASEB J. 2000;14:2532–2539. doi: 10.1096/fj.00-0250com. [DOI] [PubMed] [Google Scholar]


