Abstract
Objective: The purpose of this study was to investigate the expression of A-kinase anchor protein 95 (AKAP95), cell cycle protein E1 (cyclinE1) and D1 (cyclinD1), and gap junction protein connexin 43 (Cx43) in ovarian cancer tissues, the relationship between four proteins and clinicopathologic parameters, and the correlation between these proteins. Methods: The expression of proteins in 54 cases of ovarian cancer tissues was detected by immunohistochemical method. Results: The positive expression rates of AKAP95, cyclinD1 and cyclinE1 in ovarian cancer tissues were 72.22%, 66.67% and 79.63%, respectively, which were higher than that of ovarian pericarcinoma tissues expressing as 33.33%, 25% and 8.30% (P<0.05). The positive expression rate of Cx43 in ovarian cancer tissues was 40.74%, which was lower than that of ovarian pericarcinoma tissues expressing as 75%; respectively, and the difference was statistically significant between groups (P<0.05). The expression of cyclinD1 in ovarian cancer tissues was related to the histologic type (P<0.05) while it showed no correlation with the degree of differentiation (P>0.05). Additionally, the expression of AKAP95, Cx43 and cyclinE1 in ovarian cancer tissues showed no correlation with the degree of differentiation or the histologic type (P>0.05). Protein expressions of AKAP95, Cx43 and cyclinE1 were correlated with each other (P<0.05), and the expressions of cyclinD1, cyclinE1 and Cx43 were also correlated with each other (P<0.05). However, AKAP95 and cyclinD1 showed no correlation (P>0.05). Conclusion: AKAP95, cyclinD1 and cyclinE1 play an important role in promoting the process of ovarian cancer formation. The tumor inhibitory effects of Cx43 protein on the pathogenesis of ovarian cancer were weakened. The expression of cyclinD1 in ovarian cancer tissues is related to the histologic type while it shows no correlation with the degree of differentiation. Additionally, the expression of AKAP95, Cx43 and cyclinE1 in ovarian cancer tissues shows no correlation with the degree of differentiation or the histologic type. AKAP95 expression is correlated with Cx43 and cyclinE1 expression; Cx43 expression is correlated with AKAP95, cyclinD1 and cyclinE1 expression; cyclinE1 expression is correlated with AKAP95, Cx43, cyclinD1 expression; cyclinD1 expression is correlated with Cx43 and cyclinE1 expression, while AKAP95 and cyclinD1 show no correlation.
Keywords: AKAP95, cyclinE1, cyclinD1, Cx43, correlation, ovarian cancer
Introduction
The transition disorder of G1 stage into S stage is one of the important reasons for tumor formation, and cyclinE and cyclinD are important members of cyclin family which are responsible for G1/S stage transition. cyclinD combining with CDK4/6 and activating CDK4/6 is essential for the entry of G0 stage into G1 stage, which is prepared for cell G1/S stage transition [1]. The combination of cyclinE with CDK2 promots cell G1/S stage transition [2]. AKAP95 can combine with cyclinD/E through the subunits of PKA R II [3,4]. Cx43 inhibits tumor growth by increasing the degradation of skp2 [5]. Skp2, a member of the F-BOX family, can adjust the activity of cyclinE-CDK2 by the ubiquitin proteasome system, and further affect the cell cycle progression [6]. However, no findings in AKAP95 protein expression of ovarian cancer tissues have been reported. Therefore, in the present study, the expressions of AKAP95, Cx43, cyclinD1 and cyclinE1 protein in ovarian cancer tissues were analyzed, and the correction between these proteins was further explored.
Materials and methods
Source of tumor
Ovarian cancer tissue samples of 54 cases with definite pathologic diagnosis were collected from ovarian carcinoma surgical specimens in the First Affiliated Hospital of Liaoning Medical University between 2010 and 2011. The mean age of patients was 50.13 ± 11.92. A total of 7 cases were well differentiated, 31 cases were moderately differentiated and 16 cases were poorly differentiated. 20 cases were serous adenocarcinoma, 11 cases were mucinous adenocarcinoma, 15 cases were endometrioid adenocarcinoma, and 8 cases were clear cell carcinoma. Samples of some patients (n = 12) in the control group were collected from the tissues near to ovarian cancer tissues above 2 cm, which were all through pathological identification and found no cancer cells. All patients had signed informed consent.
Reagents and methods
Anti-AKAP8 monoclonal antibody (ab72196) and anti-cyclinE1 monoclonal antibody (ab33911) were purchased from Abcam (Burlingame, California, USA), anti-connexin43 (C-20) polyclonal antibody and anti-cyclinD1 (DSC-6) monoclonal antibody were purchased from Santa Cruz, Inc. (Dallas, Texas, USA), and UltraSensitiveTM SP (Mouse/Rabbit) IHC Kit was purchased from MAIXINBiotech. Co., Ltd. (FuZhou, FuJian, China). All samples were fixed in formalin, embedded in paraffin, and serially sectioned for about 4 μm. The sections were then undertaken immunohistochemical detection using S-P method, colorationed with DAB and counterstained with hematoxylin.
Criteria for judgment of positive expression
Brown yellow indicated positive expression of protein and no brown yellow indicated negative expression of protein. Each section was randomly selected from 10 different points of view, and 200 tumor cells in each view were counted. The percentage of the number of positive cells to the total cells was used as criteria for judgment which was shown as follows: no brown staining to lower than 10% brown staining was recorded as “-”, more than or equal to 10% and lower than 25% brown staining was recorded as “+-”, more than or equal to 25% and lower than 50% brown staining was recorded as “+”, more than or equal to 50% and lower than 75% brown staining was recorded as “++”, and brown staining more than or equal to 75% brown staining was recorded as “+++”. When the data were statistically processed, “+-” and “-” were regarded as negative expression, and “+”, “++”, and “+++” were regarded as positive expression.
Statistical analysis
SPSS13.0 software package (SPSS Inc., Chicago, IL, USA) was used to analyze the data for X2 test, Fishers exact test and Spearman rank correlation analysis. Test level was α = 0.05.
The experiment was approved by the Medical Ethics Committee of School of Public Health in Xiamen University.
Results
Expression of AKAP95, Cx43, cyclinD1 and cyclinE1 in ovarian cancer tissues
As shown in Table 1, positive expression rates of AKAP95 (Figure 1), cyclin D1 (Figure 2) and cyclinE1 (Figure 3) protein in ovarian cancer tissues were 72.22%, 66.67% and 79.63%, respectively, which were statistically significant higher than that of ovarian pericarcinoma tissues expressing as 33.33%, 25.00% and 8.3% (P<0.05). The positive rate of Cx43 protein (Figure 4) in ovarian cancer was 75%, which was statistically significant lower than that of ovarian pericarcinoma tissues (P<0.05).Statistical analysis was performed by X2 test or Fishers exact test.
Table 1.
Expression of AKAP95, cyclinD1, cyclinE1 and Cx43 in ovarian cancer tissues
| Protein | Characteristics | Ovarian cancer tissues | Ovarian pericarcinoma tissues | X2 | P |
|---|---|---|---|---|---|
| AKAP95 | Positive | 39 | 4 | 6.540 | 0.011 |
| Negative | 15 | 8 | |||
| cyclinE1 | Positive | 43 | 1 | 22.458 | <0.001 |
| Negative | 11 | 11 | |||
| cyclinD1 | Positive | 36 | 3 | 7.051 | 0.008 |
| Negative | 18 | 9 | |||
| Cx43 | Positive | 22 | 9 | 4.626 | 0.031 |
| Negative | 32 | 3 |
Figure 1.

Expression of AKAP95 in ovarian cancer tissues, ×400. A: AKAP95 was expressed in the nuclei of ovarian pericarcinoma tissues; B: AKAP95 was expressed weakly in the cytoplasm, and highly in some nuclei of ovarian serous adenocarcinoma tissues; C: AKAP95 was highly expressed in the nuclei of ovarian clear cell adenocarcinoma tissues; D: AKAP95 was weakly expressed in the nuclei of ovarian clear cell adenocarcinoma tissues; E: AKAP95 was highly expressed in the nuclei of ovarian endometrioid adenocarcinoma tissues; F: AKAP95 was negatively expressed in the nuclei of ovarian endometrioid adenocarcinoma tissues.
Figure 2.

Expression of cyclinD1 in ovarian cancer tissues, ×400. A and B: CyclinD1 protein was weakly expressed in the cytoplasm and a few nuclei of ovarian serous adenocarcinomatissues; C and D: CyclinD1 protein was moderately expressed in the nucleus and cytoplasm of ovarian clear cell carcinoma tissues; E: CyclinD1 protein was highly expressed in the cytoplasm of ovarian mucinous adenocarcinoma tissues; F: CyclinD1 protein was highly expressed in the nuclei of ovarian mucinous adenocarcinoma tissues; G: CyclinD1 protein was moderately expressed in the nuclei of ovarian mucinous adenocarcinoma tissues; H: CyclinD1 protein was moderately expressed in the nuclei of ovarian endometrioid carcinoma tissues; I: CyclinD1 protein was moderately expressed in the cytoplasm of ovarian endometrial adenoid cancer tissues; J: CyclinD1 protein was negatively expressed in ovarian endometrioid adenocarcinoma tissues.
Figure 3.

Expression of cyclinE1 in ovarian cancer tissues, ×400. A and B: CyclinE1 was highly expressed in the nuclei of ovarian serous adenocarcinoma tissues; C: CyclinE1 was negatively expressed in the nuclei of ovarian serous adenocarcinoma tissues; D: CyclinE1 was highly expressed in the nuclei of in ovarian clear cell carcinoma tissues; E: CyclinE1 was weakly expressed in the cytoplasm of ovarian clear cell carcinoma tissues; F: CyclinE1 was weakly expressed in the cytoplasm of ovarian mucinous adenocarcinoma tissues; G and H: CyclinE1 was highly expressed in the nuclei of ovarian endometrioid adenocarcinoma tissues; I: cyclinE1 was weakly expressed in the cytoplasm of ovarian endometrial adenocarcinoma tissues.
Figure 4.
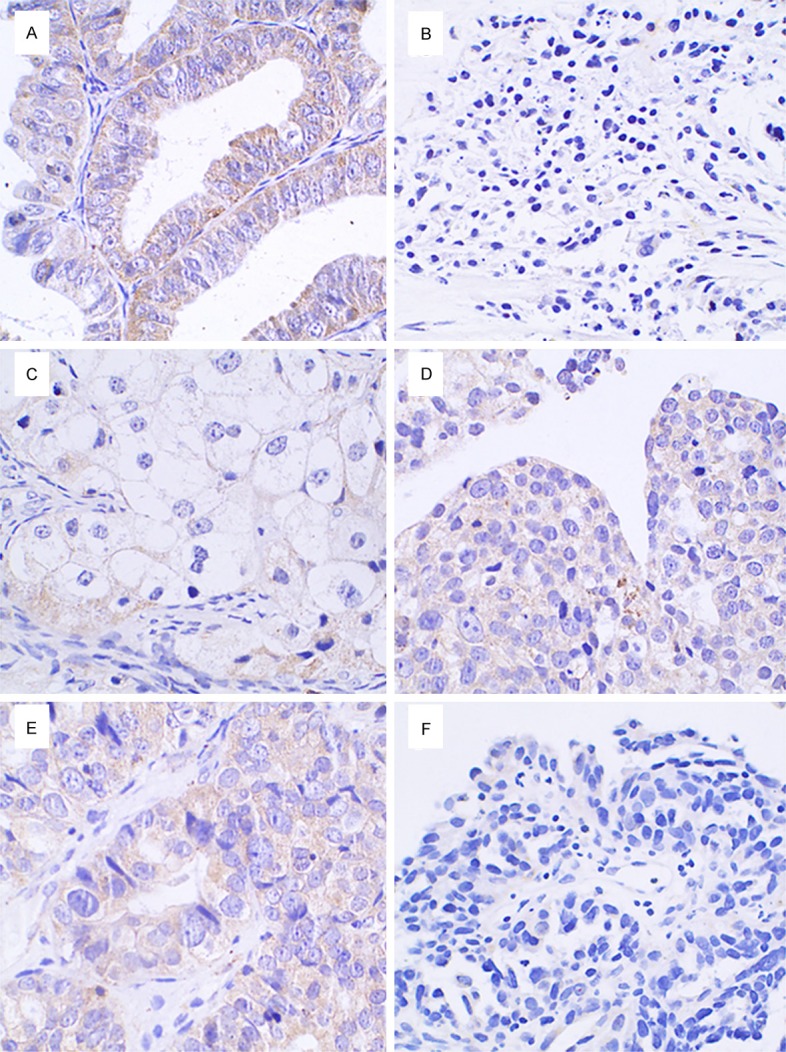
Expression of Cx43 in ovarian cancer tissues, ×400. A: Cx43 was highly expressed in the cytoplasm of ovarian serous adenocarcinoma tissues; B: Cx43 was negatively expressed in the cytoplasm of ovarian serous adenocarcinoma tissues; C: Cx43 was weakly expressed in the cytoplasm of ovarian clear cell carcinoma tissues; D and E: Cx43 was highly expressed in the cytoplasm of ovarian endometrial adenocarcinoma cancer tissues; F: Cx43 was negatively expressed in the cytoplasm of ovarian endometrial adenocarcinoma cancer tissues.
Relationship between protein expression and clinicopathological parameters
The results in Table 2 showed that cyclinD1 protein expression was related to the types of ovarian cancer tissues (P<0.05) while had no correlation with the degree of tissue differentiation (P>0.05). AKAP95, Cx43 and cyclinE1 protein expression had no correlation with the degree of tissue differentiation and the Histologic types of ovarian cancer (P>0.05).Statistical analysis was performed by X2 test or Fishers exact test.
Table 2.
Relationship between the expression of four proteins and clinicopathological parameters
| Pathological parameters | Number of cases | AKAP95 | |||
|
| |||||
| Positive | Negative | X2 | P | ||
|
| |||||
| Degree of differentiation | |||||
| High | 7 | 4 | 3 | 2.618 | 0.340 |
| Moderate | 31 | 25 | 6 | ||
| Low | 16 | 10 | 6 | ||
| Histologic type | |||||
| Endom-etrioid | 15 | 14 | 1 | 6.536 | 0.122 |
| Serous property | 20 | 12 | 9 | ||
| Mucinous | 11 | 8 | 3 | ||
| Clear cell | 8 | 6 | 2 | ||
|
| |||||
| Pathological parameters | Number of cases | cyclinD1 | |||
|
| |||||
| Positive | Negative | X2 | P | ||
|
| |||||
| Degree of differentiation | |||||
| High | 7 | 3 | 4 | 4.135 | 0.156 |
| Moderate | 31 | 24 | 7 | ||
| Low | 16 | 9 | 7 | ||
| Histologic type | |||||
| Endom-etrioid | 15 | 14 | 1 | 8.732 | 0.047 |
| Serous property | 20 | 12 | 8 | ||
| Mucinous | 11 | 5 | 6 | ||
| Clear cell | 8 | 5 | 3 | ||
|
| |||||
| Pathological parameters | Number of cases | cyclinE1 | |||
|
| |||||
| Positive | Negative | X2 | P | ||
|
| |||||
| Degree of differentiation | |||||
| High | 7 | 4 | 3 | 5.446 | 0.077 |
| Moderate | 31 | 28 | 3 | ||
| Low | 16 | 11 | 5 | ||
| Histologic type | |||||
| Endom-etrioid | 15 | 14 | 1 | 2.864 | 0.475 |
| Serous property | 20 | 15 | 5 | ||
| Mucinous | 11 | 8 | 3 | ||
| Clear cell | 8 | 6 | 2 | ||
|
| |||||
| Pathological parameters | Number of cases | Cx43 | |||
|
| |||||
| Positive | Negative | X2 | P | ||
|
| |||||
| Degree of differentiation | |||||
| High | 7 | 1 | 6 | 3.143 | 0.224 |
| Moderate | 31 | 15 | 16 | ||
| Low | 16 | 6 | 10 | ||
| Histologic type | |||||
| Endom-etrioid | 15 | 7 | 8 | 3.091 | 0.395 |
| Serous property | 20 | 8 | 12 | ||
| Mucinous | 11 | 4 | 7 | ||
| Clear cell | 8 | 1 | 7 | ||
Correlation analysis of the expression of four proteins
The relationships between the expressions of four proteins in ovarian cancer tissues are shown in Tables 3, 4, 5, 6, 7 and 8. Results suggested that AKAP95 was correlated with Cx43 and cyclinE1 (P<0.05); Cx43 was correlated with AKAP95, CyclinD1, and cyclinE1 (P<0.05); cyclinE1 was correlated with AKAP95, Cx43 and cyclinD1 (P<0.05); CyclinD1 was correlated with cyclinE1 and Cx43 (P<0.05). Moreover, there was no association between AKAP95 and cyclinD1 protein (P>0.05). The correlation between four proteins was performed by Spearman rank correlation analysis.
Table 3.
Relationship between AKAP95 protein expression and cyclinE1 protein expression in ovarian cancer tissues
| AKAP95 | cyclinE1 | r s | P | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| - | +- | + | ++ | +++ | |||
| - | 2 | 1 | 1 | 2 | 7 | 0.284 | 0.013 |
| +- | 3 | 1 | 1 | 1 | 0 | ||
| + | 2 | 2 | 4 | 1 | 3 | ||
| ++ | 0 | 0 | 5 | 2 | 4 | ||
| +++ | 0 | 0 | 1 | 5 | 10 | ||
Note: rs is Spearman rank correlation coefficient.
Table 4.
Relationship between AKAP95 protein expression and cyclinD1 protein expression in ovarian cancer tissues
| AKAP95 | cyclinD1 | r s | P | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| - | +- | + | ++ | +++ | |||
| - | 1 | 2 | 2 | 1 | 1 | 0.21 | 0.128 |
| +- | 2 | 1 | 1 | 4 | 0 | ||
| + | 3 | 2 | 3 | 3 | 1 | ||
| ++ | 2 | 1 | 3 | 1 | 4 | ||
| +++ | 2 | 2 | 3 | 4 | 5 | ||
Note: rs is Spearman rank correlation coefficient.
Table 5.
Relationship between AKAP95 protein expression and Cx43 protein expression in ovarian cancer tissues
| AKAP95 | Cx43 | rs | P | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| - | +- | + | ++ | +++ | |||
| - | 3 | 2 | 0 | 2 | 0 | 0.381 | 0.004 |
| +- | 5 | 2 | 1 | 0 | 0 | ||
| + | 4 | 5 | 1 | 1 | 1 | ||
| ++ | 1 | 4 | 6 | 0 | 0 | ||
| +++ | 2 | 4 | 4 | 6 | 0 | ||
Note: rs is Spearman rank correlation coefficient.
Table 6.
Relationship between cyclinE1 protein expression and cyclinD1 protein expression in ovarian cancer tissues
| cyclinE1 | cyclinD1 | rs | P | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| - | +- | + | ++ | +++ | |||
| - | 4 | 1 | 1 | 0 | 1 | 0.288 | 0.035 |
| +- | 2 | 2 | 0 | 0 | 0 | ||
| + | 0 | 1 | 5 | 4 | 4 | ||
| ++ | 2 | 3 | 2 | 1 | 3 | ||
| +++ | 2 | 1 | 4 | 8 | 3 | ||
Note: rs is Spearman rank correlation coefficient.
Table 7.
Relationship between cyclinE1 protein expression and Cx43 protein expression in ovarian cancer tissues
| cyclinE1 | Cx43 | r s | P | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| - | +- | + | ++ | +++ | |||
| - | 4 | 3 | 0 | 0 | 0 | 0.484 | <0.001 |
| +- | 3 | 1 | 0 | 0 | 0 | ||
| + | 3 | 5 | 6 | 0 | 0 | ||
| ++ | 3 | 3 | 2 | 3 | 0 | ||
| +++ | 2 | 5 | 4 | 6 | 1 | ||
Note: rs is Spearman rank correlation coefficient.
Table 8.
Relationship between cyclinD1 protein expression and Cx43 protein expression in ovarian cancer tissues
| cyclinD1 | Cx43 | r s | P | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| - | +- | + | ++ | +++ | |||
| - | 6 | 3 | 1 | 0 | 0 | 0.550 | <0.001 |
| +- | 1 | 6 | 1 | 0 | 0 | ||
| + | 6 | 2 | 3 | 1 | 0 | ||
| ++ | 2 | 4 | 2 | 4 | 1 | ||
| +++ | 0 | 2 | 5 | 4 | 0 | ||
Note: rs is Spearman rank correlation coefficient.
Discussion
Ovarian cancer (OC) is one of the most common malignant tumors in the female genital system. Ovarian cancer comes on secretly and has no typical symptom at the early stage of the development, resulting in the highest mortality rate in all gynecologic cancers [7]. Losing control of G1/S stage transition is the key step of the cancer. In this study, the expression of AKAP95, Cx43, and G1/S phase transition related proteins including cyclinD1 and cyclinE1 in 54 cases of ovarian cancer, and the correlation between these proteins were investigated.
AKAP95 has the function of anchoring the protein kinase A (PKA) in the nuclear [16]. The expression of AKAP95 protein in rectal cancer tissues was higher than that of nearby tissues [22], indicating that the expression of AKAP95 might be associated with the occurrence of rectal cancer. This study showed that the expression of AKAP95 protein in ovarian cancer tissues was also higher than that of ovarian pericarcinoma tissues, and associated with the expression of cyclinE1 protein. cyclin D/E combined with RII subunit of PKA throng AKAP95 and produced two kinds of complexes cyclin-CDKs and cyclinD/E-AKAP95-PKA [3,4]. CyclinD/E-CDK2/4/6 played a key role in promoting G1 phase process and G1/S stage transition. AKAP95 protein was highly expressed, and associated with cyclinE1 protein expression, demonstrating that AKAP95 protein might play a role in the process of G1 phase and G1/S phase transition. In the present study, it was not detected the correlation between AKAP95 and CyclinD1 due to two reasons. One reason is that CyclinD mainly exerts its functions in the early and middle G1 stage [1], cyclinE mainly plays a role in the mid and late G1 stage [2]. Therefore, it is inferred that AKAP95 protein might be more likely functional in the G1/S transition stage but not in the early and mid G1 stage. Another reason is that cyclinD1 protein has tissue specificity.
Three subtypes, cyclinD1, D2 and D3 have tissue specificity, but at least one subtype is expressed in each tissue, which primarily plays a role in the early and mid G1 stage [8]. CyclinE has two subtypes E1 and E2 which mainly play a role in G1/S transition stage [12]. The expression of cyclinD1 [9-11] and cyclinE1 [10,13] was increased in various tumor tissues, and high expression of cyclinD1 and cyclinE1 was found in this study, which was consistent with relevant reported literature [9-11,13]. CyclinD combined with CDK4/6 and formed the complex which could phosphorylate pRb to make the pRb-E2F complex detached and the binding site of E2F1-3 exposed for activating gene transcription required for G1/S stage transition [14]. Furthermore, E2F1-3 could induce the expression of cyclinE which could form cyclinE/CDK2 complexes with CDK2 and further phosphorylated pRb, leading to the formation of a positive feedback loop signal [15]. Therefore, the correlation between cyclinD1 expression and cyclinE1 expression in ovarian carcinoma tissues further provided the evidence for above studies, indicating that cyclinD1 and cyclinE1 might play a synergistic effect in tumor formation.
Cx43 protein exerted anti-cancer effects mainly through the formation of gap junction (GJ) [17]. Expression of Cx43 protein in a variety of tumor tissues including gastrointestinal stromal tumor tissues and human brain glioma tumor tissues was decreased [18,19], which was consistent with this study. Previous studies showed that Cx43 increased Skp2 degradation through up-regulation of Skp2 self ubiquitination, thereby reducing the degradation of p27 (CDKI) [5,20] which had inhibitory effects on cyclin/CDK [21,23,24] and further promoted Cx43 to inhibit G1/S stage transition. This study showed that Cx43 was related to AKAP95, cyclin D1 and E1, which was consistent with the reported literatures. It was suggested that Cx43 protein affected cyclin/CDK activity through different pathways, thus affecting the G1/S stage transition.
In addition, the previous findings expressed that cyclinD1 expression had no correlation with pathological types in bladder carcinoma [9] while was not consistent with pathological types in gastric cancer [10]. This study also showed that cyclinD1 expression in ovarian cancer tissue had relevance to the histologic type. The difference might be associated with tissue-specificity of cyclin D1.
Acknowledgements
This work is supported by National Natural Science Foundation (No. 81071927), Xiamen Science and Technology Bureau funded project (3502Z20144006), Xiamen University Training Programs of Innovation and Entrepreneurship for Undergraduates (No. 2015Y0823 and No. 2015X0453).
Disclosure of conflict of interest
None.
References
- 1.Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL. CyclinD as a therapeutic target in cancer. Nat Rev Cancer. 2011;11:558–572. doi: 10.1038/nrc3090. [DOI] [PubMed] [Google Scholar]
- 2.Koff A, Giordano A, Desai D, Yamashita K, Harper JW, Elledge S, Nishimoto T, Morgan DO, Franza BR, Roberts JM. Formation and activation of a cyclin E-cdk2 complex during the G1 phase of the human cell cycle. Science. 1992;257:1689–1694. doi: 10.1126/science.1388288. [DOI] [PubMed] [Google Scholar]
- 3.Arsenijevic T, Degraef C, Dumont JE, Roger PP, PirsonI I. G1/S Cyclins interact with regulatory subunit of PKA via A-kinase anchoring protein, AKAP95. Cell Cycle. 2006;5:1217–1222. doi: 10.4161/cc.5.11.2802. [DOI] [PubMed] [Google Scholar]
- 4.Arsenijevic T, Degraef C, Dumont JE, Roger PP, Pirson I. A novel Partner for D-tyPecyclins: Protein kinase A-anchoring Protein AKAP95. Biochem J. 2004;378:673–679. doi: 10.1042/BJ20031765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang YW, Nakayama K, Nakayama K, Morita I. A novel route for Connexin 43 to inhibit cell proliferation: negative regulation of S-phase kinase-associated protein (skp2) Cancer Res. 2003;63:1623–1630. [PubMed] [Google Scholar]
- 6.Lim MS, Adamson A, Lin Z, Perez-Ordonez B, Jordan RC, Tripp S, Perkins SL, Elenitoba-Johnson KS. Expression of Skp2, a p27(Kip1) ubiquitinligase, in malignant lymPhoma: correlation with p27(Kip1) and proliferation index. Blood. 2002;100:2950–2956. doi: 10.1182/blood.V100.8.2950. [DOI] [PubMed] [Google Scholar]
- 7.Hall M, Rustin G. Recurrent ovarian cancer: when and how to treat. Curr Oncol Rep. 2011;13:459–471. doi: 10.1007/s11912-011-0199-3. [DOI] [PubMed] [Google Scholar]
- 8.Sicinska E, Aifantis L, Le Cam L, Swat W, Borowski C, Yu Q, Ferrando AA, Levin SD, Geng Y, von Boehmer H, Sicinski P. Requirement for cyclin D3 in lymphocyte development and T cell leukemias. Cancer Cell. 2003;4:451–461. doi: 10.1016/s1535-6108(03)00301-5. [DOI] [PubMed] [Google Scholar]
- 9.Kopparapu PK, Boorjian SA, Robinson BD, Downes M, Gudas LJ, Mongan NP, Persson JL. Expression of Cyclin D1 and Its Association with Disease Characteristics in Bladder Cancer. Anticancer Res. 2013;33:5235–5242. [PMC free article] [PubMed] [Google Scholar]
- 10.Aoyagi K, Koufuji K, Yano S, Murakami N, Terasaki Y, Yamasaki Y, Takeda J, Tanaka M, Shirouzu K. Immunohistochemical Study on the Expression of Cyclin D1 and E in Gastric Cancer. Kurume Med J. 2000;47:199–203. doi: 10.2739/kurumemedj.47.199. [DOI] [PubMed] [Google Scholar]
- 11.Reis-Filho JS, Savage K, Lambros MB, James M, Steele D, Jones RL, Dowsett M. Cyclin D1 protein overexpression and CCND1 amplification in breast carcinomas: an immunohistochemical and chromogenic in situhybridisation analysis. Mod Pathol. 2006;19:999–1009. doi: 10.1038/modpathol.3800621. [DOI] [PubMed] [Google Scholar]
- 12.Koff A, Cross F, Fisher A, Schumacher J, Leguellec K, Philippe M, Roberts JM. Human cyclin E, a new cyclin that interacts with two members of the CDC2 gene family. Cell. 1991;66:1217–1228. doi: 10.1016/0092-8674(91)90044-y. [DOI] [PubMed] [Google Scholar]
- 13.Etemadmoghadam D, Au-Yeung G, Wall M, Mitchell C, Kansara M, Loehrer E, Batzios C, George J, Ftouni S, Weir BA, Carter S, Gresshoff I, Mileshkin L, Rischin D, Hahn WC, Waring PM, Getz G, Cullinane C, Campbell LJ, Bowtell DD. Resistance to CDK2 inhibitors is associated with selection of polyploid cells in CCNE1-amplified ovarian cancer. Clin Cancer Res. 2013;19:5960–5971. doi: 10.1158/1078-0432.CCR-13-1337. [DOI] [PubMed] [Google Scholar]
- 14.Knudsen ES, Knudsen KE. Tailoring to RB: tumour suppressor status and therapeutic response. Nat Rev Cancer. 2008;8:714–724. doi: 10.1038/nrc2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malumbres M, Barbacid M. To cycle or not to cycle: a critical decision in cancer. Nat Rev Cancer. 2001;1:222–231. doi: 10.1038/35106065. [DOI] [PubMed] [Google Scholar]
- 16.Coghlan VM, Langeberg LK, Fernandez A, Lamb NJ, Scott JD. Cloning and characterization of AKAP 95, a nuclear protein that associates with the regulatory subunit of typeII cAMP-dependent protein kinase. J Biol Chem. 1994;269:7658–7665. [PubMed] [Google Scholar]
- 17.Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Güldenagel M, Deutsch U, Söhl G. Structural and Functional Diversity of Connexin Genes in the Mouse and Human Genome. Biol Chem. 2002;383:725–737. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- 18.Nishitani A, Hirota S, Nishida T, Isozaki K, Hashimoto K, Nakagomi N, Matsuda H. Differential expression of connexin43 in gastrointestinal stromal tumours of gastric andsmall intestinal origin. J Pathol. 2005;206:377–382. doi: 10.1002/path.1799. [DOI] [PubMed] [Google Scholar]
- 19.Soroceanu L, Manning TJ Jr, Sontheimer H. Reduced exression of connexin-43 and functional gap junctioncoupling in humangliomas. Glia. 2001;33:107–117. doi: 10.1002/1098-1136(200102)33:2<107::aid-glia1010>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 20.Zhang YW, Morita I, Ikeda M, Ma KW, Murota S. Connexin43 suppresses proliferation of osteosarcoma U2OS cells through post-Transcriptional regulation of p27. Oncogene. 2001;20:4138–4149. doi: 10.1038/sj.onc.1204563. [DOI] [PubMed] [Google Scholar]
- 21.Nourse J, Firo E, Flanagan WM, Coats S, Polyak K, Lee MH, Massague J, Crabtree GR, Roberts JM. Interleukin-2-mediat elimination of the p27kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature. 1994;372:570–573. doi: 10.1038/372570a0. [DOI] [PubMed] [Google Scholar]
- 22.Qi F, Yuan Y, Zhi X, Huang Q, Chen Y, Zhuang W, Zhang D, Teng B, Kong X, Zhang Y. Synergistic effects of AKAP95, Cyclin D1, Cyclin E1, and Cx43 in the development of rectal cancer. Int J Clin Exp Pathol. 2015;8:1666–1673. [PMC free article] [PubMed] [Google Scholar]
- 23.Guo H, Lu Y, Wang J, Liu X, Keller ET, Liu Q, et al. Targeting the Notch signaling pathway in cancer therapeutics. Thoracic Cancer. 2014;5:473–486. doi: 10.1111/1759-7714.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu YJ, Tang Y, Li ZF, Li Z, Zhao Y, Wu ZJ, Su Q. Expression and significance of Rac1, Pak1 and Rock1 in gastric carcinoma. Asia Pac J Clin Oncol. 2014;10:e33–e39. doi: 10.1111/ajco.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]


