Abstract
Objective: The present study was designed to explore the clinical values of microRNA-129 (miR-129) expression in peripheral blood mononuclear cells for prostate cancer patients and the role of miR-129 in the proliferation of prostate cancer. Methods: The peripheral blood mononuclear cells were isolated form blood simple from 98 patients confirmed with prostate cancer and 56 matched healthy volunteers. Reverse transcription quantitative real-time polymerase chain reaction (qRT-PCR) was employed to determine the expression level of miR-129 in peripheral blood mononuclear cells. Cox proportional hazards regression models and Kaplan-Meier analysis were used to evaluate the association of miR-129 expression with clinical and pathological characteristics of prostate cancer patients. The effect of miR-129 on the proliferation of prostate cancer cells in vitro was also determined. Results: Reverse transcription quantitative real-time polymerase chain reaction (qRT-PCR) results showed that the expression of miR-129 was dramatically down-regulated in peripheral blood mononuclear cells for prostate cancer patients in comparison with healthy controls (P<0.05). The decrease in miR-129 expression in peripheral blood mononuclear cells was significantly associated with aggressive clinical pathological features such as histological grade (P=0.010), high preoperative PSA level (P=0.002), pathological stage (P=0.011), high Gleason score (P=0.005), lymph node metastasis (P=0.002), angiolymphatic invasion (P=0.004), biochemical recurrence (P=0.001). The prostate cancer patients with a low miR-129 expression in peripheral blood mononuclear cells had an obviously shorter BCR-free survival compared with high miR-129 expression (P<0.001). The Cox multivariate analysis established that the miR-129 expression may be an independent prognostic factor for biochemical recurrence (BCR)-free survival prostate cancer patients (P=0.000). The results of in vitro CCK-8 assays, as well as proliferating cell nuclear antigen (PCNA) and phosphorylated histone-3 (P-H3) (markers of proliferation) indicated that miR-129 overexpression markedly retarded the proliferation of PC-3 and DU-145 cells. Conclusions: Our results provide the first evidence that the miR-129is significantly downregulated in prostate cancer patients and multivariate analysis confirmed that miR-129 is a novel independent prognostic factor for prostate cancer. Overexpression of miR-129 exerts tumor suppressive functions and abrogates prostate cancer growth.
Keywords: miR-129, prostate cancer, peripheral blood mononuclear cells, prognosis, proliferation
Introduction
MicroRNAs (miRNAs) are a class of short non-coding RNAs averaging 20 to 24 nucleotides in length that post-transcriptionally regulate gene expressions by binding to the 3’ end of tun translated regions (3’UTRs) of target mRNAs, resulting in the degradation or translational inhibition of targeted genes [1]. miRNAs play both dual roles as oncogenic miRNAs and tumor-suppressive miRNAs, and are closely linked with the development and progression in various cancers [2]. The deregulated miRNAs expression has been extensively found in tumor tissue and play a pivotal role in cellular biological pathways including cell proliferation, metastasis, cell cycle and invasion during the process of carcinogenesis [3]. The clinical relevance of miRNAs in cancer has been widely explored in recent years [4].
Prostate cancer is one of the most prevalent malignancies in males worldwide and is the second most reasons for cancer-related deaths among men [5]. Prostate cancer is clinically presented with heterogeneous multifocal and highly aggressive features [6]. The prognosis of prostate cancer patients with localized or regional disease is relatively favorable, but the total 5-year survival rate is only 29% [7]. The mechanisms in which the carcinogenesis and progression of prostate cancer remain to be further elucidated. Conventional prognostic factor of serum prostate-specific antigen (PSA) is already available for earlier diagnosis of prostate cancer, but the PSA measurement is insufficient to identify insignificant prostate cancer. Accumulating studies have demonstrated that the subjects with prostate cancer of equivalent PSA level may have different clinical outcomes due to the molecularly heterogeneous subtypes [8]. Current techniques are limited to distinguish the prostate cancer patients that are most at risk of metastasis and death. Therefore, the novel, specific and efficient diagnostic and prognostic biomarkers for prostate cancer are needed to be explored for prediction of aggressive prostate cancer and improvement of clinical outcome of prostate cancer patients. In recent years, miRNAs have been to function as molecular prognostic biomarkers for many cancers. For example, it is reported thatmiR-188-5p level is significantly down-regulated in metastatic prostate cancer tissues and is predicted to be an independent prognostic factor for biochemical recurrence-free survival and overall survival in prostate cancer patients [9]. In addition, the miR-221 is also progressively down-regulated in prostate cancer patients with lymph node-metastasis and is employed as a biomarker for clinical prognosis of prostate cancer patients at high-risk [10]. However, the miR-96 is overexpressed in prostate cancer tissues and is closely related with the poor median survival of 3 years [11]. These results suggested that miRNAs are emerging as potential prognostic biomarkers or useful therapeutic target in prostate cancer. Several miRNAs such as miR-143 [12] miR-29a [13] and miR-124 [14]have been proven to be critical mediators in the growth and metastasis of prostate cancer. The miR-129 was recently found to be down-regulated in gastric cancer [15], colorectal cancer [16], liver cancer [17] and lymphocytic leukaemia [18]. MiR-129 is verified to a diagnostic and prognostic biomarker in gastrointestinal cancer associated with potential of tumor suppressor activity including the inhibition of tumorigenesis, proliferation, invasion and disease progression [19]. However, the role of miR-129 in peripheral blood mononuclear cells of prostate cancer patients remains largely elusive. In the present study, we aimed to explore the association of miR-129 level in peripheral blood mononuclear cells with clinicopathological factors and prognosis the prostate cancer.
Material and methods
Ethics statement
The approval of this study protocol was obtained from the Ethics Committee of Jinling Hospital and the written informed consent was provided from all subjects. All experiments were performed in accordance with relevant guidelines and regulations. This study conformed to the principles outlined in the Declaration of Helsinki.
Subjects
A total of 98 prostate cancer patients who underwent radical prostatectomy were included from Department of Urology, Jinling Hospital, School of Medicine, Nanjing University ranged from 2000 to 2007. The ages of 98 prostate cancer patients varied from 42 to 71 years (median, 60 years). The patients enrolled in this study did not receive any treatment including chemotherapy, radiation therapy, androgen deprivation treatment before radical prostatectomy. All paraffin-embedded tissues in each sample were pathologically diagnosed with prostate cancer on the HE-stained tissue section. The histopathological grading for all cases was confirmed by experienced pathologists. The clinicopathological and demographic data pre- and post-operation were preserved in medical records. The biochemical and clinicopathological parameters in each patient such as clinical stage, Gleason score, margin status, angiolymphatic invasion status, seminal vesicle invasion status, and biochemical relapse were all recorded. The summary of patient’s clinicopathological characteristics is shown in (Table 1). The biochemical recurrence time was set as the period from surgical treatment to the assay of two successive values of serum PSA level ≥0.2 ng/ml. The date of prostatectomy was recognized as the beginning of the follow-up period. The patients died from unexpected events or other diseases rather than prostate cancer were excluded from this study. In addition, 56 matched healthy volunteers (age from 43 to 74 years (median, 61 years) were served as the control group. Twenty mL of peripheral blood cells in all cases were collected and peripheral blood mononuclear cells were separated gradient density (Tianjin Hao Yang Biological Manufacture Co., Ltd, Tianjing, China) according to the instructions of the manufacturer. The cell pellet was frozen at -80°C until further use [20].
Table 1.
Correlation of miR-129 expression in peripheral blood mononuclear cells with 98 prostate cancer patients’ clinicopathologic features
| Variable | All Cases (%) | miR-129 expression | χ2 | P | |
|---|---|---|---|---|---|
|
| |||||
| Low (%) | High (%) | ||||
| Age | |||||
| ≤60 | 65 (66.3%) | 35 (53.8%) | 30 (46.2%) | 1.478 | 0.224 |
| >60 | 33 (33.7%) | 22 (66.7%) | 11 (33.3%) | ||
| Histological grade | |||||
| G1+G2 | 52 (53.1%) | 24 (46.2%) | 28 (53.8%) | 6.566 | 0.010 |
| G3 | 46 (46.9%) | 33 (71.7%) | 13 (28.3%) | ||
| Preoperative PSA | |||||
| <4 ng/mL | 3 (3.1%) | 0 (0%) | 3 (100%) | 12.181 | 0.002 |
| 4-10 ng/mL | 21 (21.4%) | 7 (33.3%) | 14 (66.7%) | ||
| >10 ng/mL | 74 (75.5%) | 50 (67.6%) | 24 (32.4%) | ||
| Pathological stage | |||||
| I+II | 57 (58.2%) | 27 (47.4%) | 30 (52.6%) | 6.524 | 0.011 |
| III+IV | 41 (41.8%) | 30 (73.2%) | 11 (26.8%) | ||
| Gleason score | |||||
| <7 | 35 (35.7%) | 14 (40.0%) | 21 (60.0%) | 10.543 | 0.005 |
| 7 | 24 (24.5%) | 13 (54.2%) | 11 (45.8%) | ||
| >7 | 39(39.8%) | 30 (76.9%) | 9 (23.1%) | ||
| Lymph node metastasis | |||||
| Negative | 82 (83.7%) | 42 (51.2%) | 40 (48.8%) | 9.952 | 0.002 |
| Positive | 16 (16.3%) | 15 (93.8%) | 1 (6.2%) | ||
| Surgical margin status | |||||
| Negative | 80 (81.6%) | 46 (57.5%) | 34 (42.5%) | 0.079 | 0.779 |
| Positive | 18 (18.4%) | 11 (61.1%) | 7 (38.9%) | ||
| Angiolymphatic invasion | |||||
| Negative | 74 (75.6%) | 37 (50.0%) | 37 (50.0%) | 8.275 | 0.004 |
| Positive | 24 (24.4%) | 20 (83.3%) | 4 (16.7%) | ||
| Biochemical recurrence | |||||
| Negative | 70 (71.4%) | 32 (45.7%) | 38 (54.3%) | 10.815 | 0.001 |
| Positive | 28 (28.6%) | 25 (89.3%) | 3 (10.7%) | ||
RNA isolation and quantitative real-time PCR
The Trizol reagent (Life Technologies, Gaithersburg, MD, U.S.A.) was used to extract the total RNA in peripheral blood mononuclear cells of 98 subjects with prostate cancer and 56 matched healthy volunteers according to the manufacturer’s instructions. The concentrations and purity of RNA were measured at the optical density at 260 and 280 nm. The reverse transcription of RNA was then performed using PrimeScript RT-PCR kit (Takara, Japan). The reversed cDNA was served as template and was then suffered to quantitative real-time polymerase chain reaction (qRT-PCR) detection using the SYBR Premix Ex Taq TM (Takara, Otsu, Shiga, Japan) on a StepOnePlus system (Applied Biosystems). The U6 for miR-129 and GAPDH for mRNAs were served as internal controls as described before. Three independent experiments in each sample were performed and the relative proportion of target gene expression was quantified by normalizing the targeted gene level to that of internal control by the ΔΔCt method. Primer sequences used are as follows: miR-129 forward: 5’-GATACTCACTTTTTGCGGTCT-3’; reverse: 5’-GTGCAGGGTCCGAGGT-3’; U6: forward: 5’-CGCTTCGGCAGCACATATAC-3’; reverse: 5’-CAGGGGCCATGCTAATCTT-3’.
Cell culture and transfection
The prostate cell lines PC-3 and DU145 were purchased from the Cell Bank of Chinese Academy of Sciences (Shanghai, China). The cells were cultured in RPMI 1640 medium supplemented with 10% FBS coupled with 1% penicillin/streptomycin and 1% nonessential amino acids and 1% (mg/ml) sodium pyruvate at 37°C in a humidified incubator with 5% CO2. The day before transfection, PC-3 and DU145 cells were seeded in six-well plates, and the transient transfection of miR-129 precursor or negative miRNA (Ambion, Carlsbad, CA, USA) were conducted with Lipofectamine 2000 Transfection Reagent (Invitrogen) following manufacturer’s instructions.
Cell proliferation assay
The cell proliferation was evaluated by Cell Counting Kit-8 (CCK-8, Dojindo, Japan) in accordance with the manufacturer’s suggestions. The OD450 absorbance was determined at day 1, 2 and 4 post transfection. After 96 h transfection, the cells were harvested to determine the markers of proliferation PCNA and phosphorylated histone H3 (P-H3).
Western blot
The PC-3 and DU-145 prostate cell lines were transfected with miR-129 precursor or negative miRNA for 96 h and were harvested in lysis buffer (Beyotime Biotechnology, China) supplemented with HaltTM protease inhibitor cocktail EDTA-free (Pierce). The total protein concentration in the supernatant was quantified with the Bradford assay (BCA; Pierce, Santa Cruz, CA, USA). Equal amounts of protein in each sample were loaded onto an SDS gel and transferred to immobilon polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA). The membrane was incubated with designed primary antibodies including PCNA (Santa Cruz, CA, USA), P-H3 (Santa Cruz, CA, USA), GAPDH (Santa Cruz, CA, USA) overnight at 4°C. The positive signals from HRP-coupled secondary antibodies (Santa Cruz, CA, USA) were visualized by enhanced chemiluminescence detection kit (Thermo Scientific, Rockford, IL, USA). The densitometric analysis of the band intensities was measured and normalized to the band intensities of GAPD Husing the Image J software (NIH, USA).
Statistical analysis
All data about continuous variables were expressed as mean ± SD. SPSS 19.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The Kolmogorov-Smirnov test was used to check the data distribution normality. Comparisons between two groups were made by Student’s t test. One-way or two-way ANOVA followed by post hoc Bonferroni test was used when multiple comparisons were made. The test for categorical variables was made by chi-square test, and the small cell variables were compared by Fisher’s exact test. Survival analysis was conducted with the Kaplan-Meier method. Multivariate analysis was carried out with the Cox proportional hazards model. There receiver operating characteristic (ROC) curve was applied to evaluate the diagnostic efficacy. P values less than 0.05 was considered statistically significant.
Results
Down-regulation of miR-129 expression in peripheral blood mononuclear cells of prostate cancer patients
We measured the miR-129 expression in peripheral blood mononuclear cells of 98 subjects with prostate cancer and 56 matched healthy volunteers by qRT-PCR. The results showed that the expression of miR-129 at the mRNA level in peripheral blood mononuclear cells was significantly down-regulated in the prostate cancer patients, compared with the healthy controls (P<0.05, Figure 1).
Figure 1.
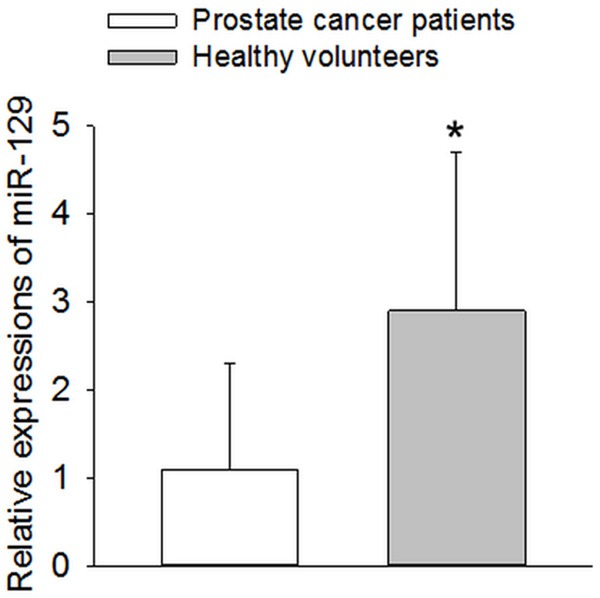
Expression of miR-129 in peripheral blood mononuclear cells in healthy volunteers and patients with prostate cancer. The expression levels of miR-129 were detected and analyzed in peripheral blood mononuclear cells of 98 subjects with prostate cancer and 56 matched healthy volunteers by qRT-PCR analysis. The results showed that miR-129 expression level was significantly decreased in prostate cancer patients compared to controls. *P<0.05 vs. Prostate cancer patients.
Diagnostic efficacy of miR-129 in prostate cancer patients
The evaluation of diagnostic efficacy of miR-129 in peripheral blood mononuclear cells of prostate cancer patients was performed by calculating the area under the receiver operating characteristic curve. The ROC curve analysis revealed that AUC was 0.846 (95 % CI=0.559-0.998, P=0.013). When the cutoff value=1.03, the diagnostic sensitivity (88.9 %) and specificity (66.7%) reached their peak values (P<0.05, Figure 2). Thus the miR-129 expression was further classified into the low expression group (n=miR-129 expression <1.03, n=57) and high expression group (miR-129 expression ≥1.03, n=41) as the threshold ROC curve value of 1.03.
Figure 2.
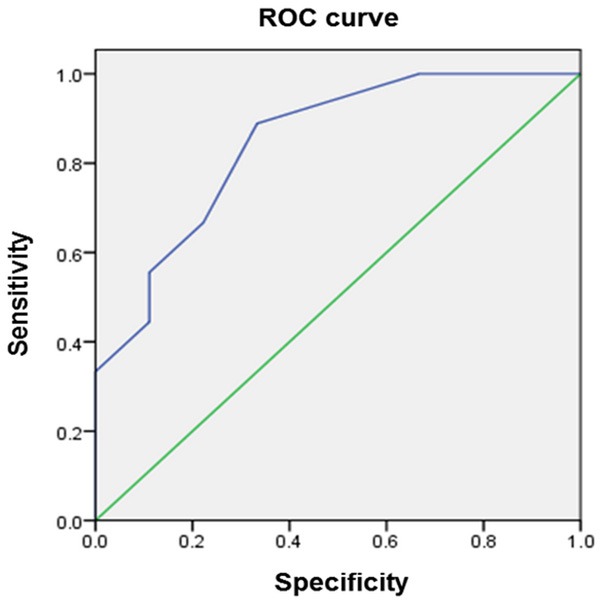
Assessment of the diagnostic efficacy of miR-129 in peripheral blood mononuclear cells of prostate cancer patients by calculating the area under the receiver operating characteristic curve. (AUC=0.846, P=0.013).
Correlation of miR-129 expression with clinical parameters of prostate cancer patients
As seen in Table 1, the low expression of miR-129 in peripheral blood mononuclear cells of prostate cancer patients is closely correlated with aggressive clinical pathological parameters such as histological grade (P=0.010), high preoperative PSA level (P=0.002), pathological stage (P=0.011), high Gleason score (P=0.005), lymph node metastasis (P=0.002), angiolymphatic invasion (P=0.004), biochemical recurrence (P=0.001). No association was found between the expression level of miR-129 and other clinical factors including age and surgical margin status (all P>0.05).
Relationship between miR-129 expression and biochemical recurrence free survival
To assess the possible prognostic value of miR-129 in peripheral blood mononuclear cells, the biochemical recurrence (BCR)-free survival in 98 prostate cancer patients undergoing radical prostatectomy was carried out via calculating the cumulative survival curves with the Kaplan-Meier method. The assessment in total prostate cancer patients revealed that the low expression level of miR-129 in peripheral blood mononuclear cells was strongly correlated with adverse biochemical recurrence free survival of prostate cancer patients. The Kaplan-Meier curves plotted between high or low miR-129 expression and BCR-free survival disclosed that prostate cancer patients with a low miR-129 expression in peripheral blood mononuclear cells had an obviously shorter BCR-free survival compared with high miR-129 expression (P<0.001, Figure 3). As summarized in Table 2, the univariate survival analysis with Cox proportional hazards model revealed that a significant impact of well-known clinicopathological prognostic features including miR-129 expression (P=0.000), histological grade (P=0.017), pathological stage (P=0.015), lymph node metastasis (P=0.011) and angiolymphatic invasion (P=0.028) were significantly associated with BCR-free survival in prostate cancer patients. Since variables observed to have a prognostic influence by univariate analysis may covariate, we conducted a multivariate analysis of relationship of miR-129 expression with the BCR-free survival of patients with prostate cancer. The Cox multivariate analysis established the significance of miR-126 expression (P=0.000), and other clinicopathologic parameters including histological grade (P=0.000), pathological stage (P=0.031), lymph node metastasis (P=0.000) and angiolymphatic invasion (P=0.000) for independent prognostic predictors of BCR-free survival of prostate cancer patients (Table 3).
Figure 3.
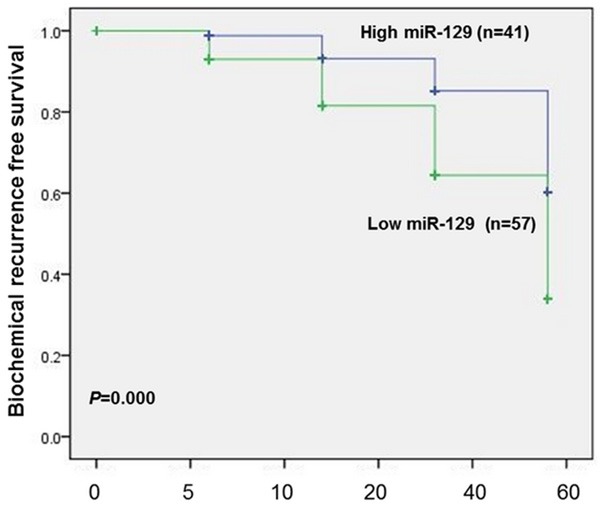
Biochemical recurrence (BCR)-free survival curves for two groups defined by low and high expression of miR-129 in subjects with prostate cancer. The patients with higher miR-129 expression had significantly longer BCR-free survival after radical prostatectomy than patients with lower miR-129 expression did (P<0.001).
Table 2.
Univariate survival analysis of biochemical recurrence (BCR)-free survival in 98 prostate cancer patients
| Variables | BCR-free survival | ||
|---|---|---|---|
|
|
|||
| Exp (B) | 95% CI | P value | |
| Age (≤60 vs. >60) | 1.601 | 0.325-1.266 | 0.247 |
| Histological grade (G1+G2 vs. G3) | 2.216 | 0.145-1.446 | 0.017 |
| Preoperative PSA (<10 ng/mL vs. ≥10 ng/mL) | 1.095 | 0.594-0.775 | 0.796 |
| Pathological stage (T1-2 vs. T3-4) | 2.578 | 0.186-1.708 | 0.015 |
| Gleason score (4-6 vs. 7-10) | |||
| Lymph node metastasis (Negative vs. Positive) | 2.693 | 0. 229-1.752 | 0.011 |
| Surgical margin status (Negative vs. Positive) | 1.283 | 0.447-0.945 | 0.483 |
| Angiolymphatic invasion (Negative vs. Positive) | 1.472 | 0.041-0.732 | 0.028 |
| miR-129 expression (High vs. Low) | 3.968 | 0.622-2.135 | 0.000 |
Table 3.
Multivariate survival analysis of biochemical recurrence (BCR)-free survival in 98 prostate cancer patients
| Variables | BCR-free survival | ||
|---|---|---|---|
|
|
|||
| Exp (B) | 95% CI | P value | |
| Histological grade (G1+G2 vs. G3) | 3.737 | 0.674-1.962 | 0.000 |
| Pathological stage (T1-2 vs. T3-4) | 1.802 | 0.053-1.125 | 0.031 |
| Lymph node metastasis (Negative vs. Positive) | 3.222 | 0.676-1.664 | 0.000 |
| Angiolymphatic invasion (Negative vs. Positive) | 3.213 | 0.516-1.818 | 0.000 |
| miR-129 expression (High vs. Low) | 5.113 | 0.846-2.418 | 0.000 |
Effect of miR-129 on prostate cell growth
The miR-129 precursor or negative control was transfected to PC-3 and DU-145 prostate cell lines to determine its effect on the proliferation of prostate cell in vitro. As reflected in Table 4, the enforced overexpression of miR-129 effectively attenuated the proliferation rate of PC-3 and DU-145 prostate cell lines at day 2 and 4 post transfection determined with CCK8 assay (Table 4). Furthermore, the proliferating markers of PC-3 and DU-145 cell lines including PCNA and phosphorylated histone H3 (P-H3) was also obviously inhibited by miR-129 precursor transfection at 4 day (Figure 4).
Table 4.
CCK8 assay for the growth of indicated prostate cancer cell lines that were transfected with miR-129 precursor or negative miRNA and measured at day 1, 2, and 4 posttransfection (Absorbance, OD)
| Cell lines | Indicated transfection | 0 d | 1 d | 2 d | 4 d |
|---|---|---|---|---|---|
| PC-3 | negative miRNA | 0.196±0.018 | 0.346±0.029 | 0.689±0.074 | 0.879±0.128 |
| miR-129 | 0.205±0.021 | 0.341±0.038 | 0.422±0.048* | 0.601±0.088* | |
| DU-145 | negative miRNA | 0.202±0.019 | 0.427±0.052 | 0.822±0.099 | 1.128±0.115 |
| miR-129 | 0.219±0.023 | 0.422±0.043 | 0.621±0.049* | 0.814±0.098* |
Data, mean values ± SD of six independent experiments.
P<0.05 vs. negative miRNA.
Figure 4.
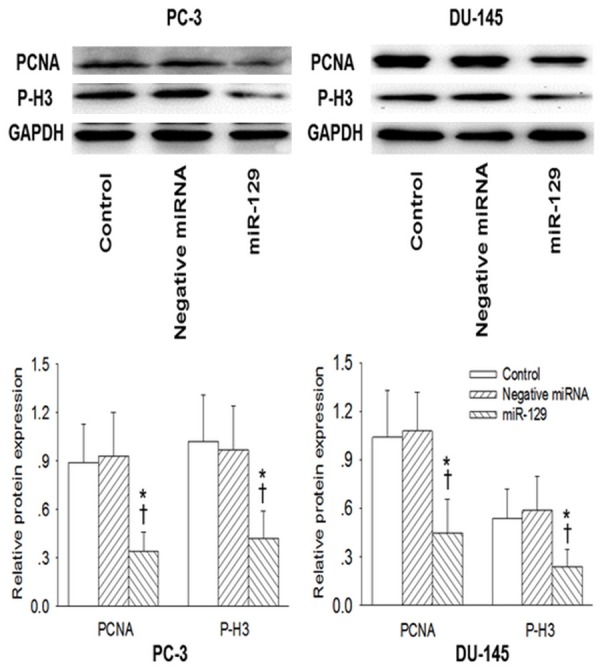
Effect of miR-129 on the proliferating cell nuclear antigen (PCNA) and phosphorylated histone H3 (P-H3) levels (markers of proliferation) in prostate cell lines. After 4 days transfection of miR-129 precursor or negative miRNA in PC-3 and DU-145 prostate cell lines, the PCNA and P-H3 were determined with Western Blot. Values are mean ± SD. *P<0.05 vs. Control; †P b 0.05 vs. Negative miRNA. n=3 for each group.
Discussion
The present study provides the new insights that the dysregulated miR-129 expression in peripheral blood mononuclear cells had the prospective high sensitivity and specificity for predicting the cancer progression and the biochemical recurrence (BCR)-free survival in prostate cancer patients.
Prostate cancer is one of the most prevalent cancers occurring among men [21]. Genetic and environmental factors have been demonstrated to be involved in its pathogenesis [22]. Epidemiological studies have found that race/ethnicity, obesity, disease family history, diet and aging are major risk factors in development of the disease prostate cancer [23]. The molecular biology and pathogenesis of prostate cancer is rarely complicated and remain largely unknown. No adequate biomarkers for predicting the tumor behavior is closely linked with the poor clinical outcome of prostate cancer. Recently, miRNAs have drawn enormous attention as biomarkers for detection of prostate cancer [24]. miRNAs are identified as important post-transcriptional regulators of targeted gene expressions and play critical roles in prostate cancer pathogenesis via controlling various pathways relevant to cancer development [24]. The most cancer types displayed the altered miRNA profiles and the susceptibility and progression of cancer are influenced by mutations, abnormal expression, or altered miRNA expression [25]. More and more studies demonstrate that miRNAs can be potentially characterized as the biomarker in the diagnosis and classification of human malignancies functioning as oncogenes or tumor suppressors [26].
The miR-129 profile is distinctly altered in colorectal cancer [16] and liver cancer [17] and miR-129 is a potential predictor in the diagnosis and prognosis of gastrointestinal cancer [19]. It has been implied that the dysregulated epithelial cells in the peripheral blood are closely associated with the unfavorable prognosis in disease-free and overall survival in prostate cancer patients [27]. Aberrant expression of subgroup k human endogenous retroviruses (HERV-K) in peripheral blood mononuclear cells was recognized as a better disease biomarker in prostate cancer detection especially in older men and smokers [28]. MiRNAs are stable and detectable in peripheral blood mononuclear cells isolated from patients by a real-time qRT-PCR with high sensitivity and specificity [29]. A recent study identifies an upregulation of miRNA-10b expression in peripheral blood mononuclear cells as a risk factor for poor prognosis of non-small cell lung cancer (NSCLC) patients [30]. However, the miR-129 expression in peripheral blood mononuclear cells of prostate cancer patients remains poorly understood. To our knowledge, this is the first study to provide compelling evidence that the miR-129 expression in peripheral blood mononuclear cells isolated from prostate cancer patients was significantly lower than the healthy subjects, the lower miR-129 expression tightly associated with poorer outcomes in prostate cancer patients. These results establish that the decreased miR-129 expression in the peripheral blood mononuclear cells may be a useful biomarker for prognosis of prostate cancer patients. However, the action of miR-129 in peripheral blood mononuclear cells and the underlying mechanisms resulting in the miR-129 downregulation in peripheral blood mononuclear cells of prostate cancer is still elusive. A possible explanation is that genetic polymorphism of miR-129 induces its abnormal expression in peripheral blood mononuclear cells, thus functioning as a tumor suppressor gene. Furthermore, the evaluation of diagnostic efficacy of miR-129 in peripheral blood mononuclear cells of prostate cancer patients was performed by calculating the area under the receiver operating characteristic curve. The ROC curve analysis revealed that diagnostic sensitivity and specificity ofmiR-129 in peripheral blood mononuclear cells are relatively high. Our study also disclosed the important risk factors for prostate cancer patients. The decrease in miR-129 expression in peripheral blood mononuclear cells was significantly associated with aggressive clinical pathological features such as histological grade, high preoperative PSA level pathological stage, high Gleason score, lymph node metastasis angiolymphatic invasion, biochemical recurrence. These results implicate that expression of miR-129 in peripheral blood mononuclear cells may be served as a potential candidate for early detection of prostate cancer.
The excessive proliferation was positively related with tumor development. Overexpression of miR-129 elicited a decrease in T24 and SW780 cells of bladder carcinoma cell lines [31]. Transfection withmiR-129 mimics obviously inhibited the proliferation and invasion of hepatocellular carcinoma cells [32]. In the present study, we showed that the enforced overexpression of miR-129 effectively attenuated the proliferation rate of PC-3 and DU-145 prostate cell lines at day 2 and 4 post transfection determined with CCK8 assay. Furthermore, the proliferating markers of PC-3 and DU-145 cell lines including PCNA and phosphorylated histone H3 (P-H3) was also obviously inhibited by miR-129 precursor transfection at 4 days. These results hintthatmiR-129 functions as tumor suppressor gene in prostate cancer and miR-129 may be defined as a potential treatment target for prostate cancer.
In summary, our data demonstrates the reduced miR-129 expression in peripheral blood mononuclear cells was correlated with the presence, metastasis and recurrence of prostate cancer patients. Determination of miR-129 is useful for distinguishing prostate cancer from cancer-free controls with high sensitivity and specificity. The miR-129 may an available biomarker for screening prostate cancer. Further studies are necessary to explore the biological effects of miR-129 in the prostate cancer cells and peripheral blood mononuclear cells isolated from prostate cancer patient in vitro.
Disclosure of conflict of interest
None.
References
- 1.Lan H, Lu H, Wang X, Jin H. MicroRNAs as potential biomarkers in cancer: opportunities and challenges. Biomed Res Int. 2015;2015:125094. doi: 10.1155/2015/125094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kohlhapp FJ, Mitra AK, Lengyel E, Peter ME. MicroRNAs as mediators and communicators between cancer cells and the tumor microenvironment. Oncogene. 2015;34:5857–68. doi: 10.1038/onc.2015.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiselev FL. [MicroRNA and cancer] . Mol Biol (Mosk) 2014;48:232–242. [PubMed] [Google Scholar]
- 4.Ergun S, Oztuzcu S. Oncocers: ceRNA-mediated cross-talk by sponging miRNAs in oncogenic pathways. Tumour Biol. 2015;36:3129–3136. doi: 10.1007/s13277-015-3346-x. [DOI] [PubMed] [Google Scholar]
- 5.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 6.Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–781. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Carroll PR. Early stage prostate cancer--do we have a problem with over-detection, overtreatment or both? J Urol. 2005;173:1061–1062. doi: 10.1097/01.ju.0000156838.67623.10. [DOI] [PubMed] [Google Scholar]
- 8.Wilt TJ, Ahmed HU. Prostate cancer screening and the management of clinically localized disease. BMJ. 2013;346:f325. doi: 10.1136/bmj.f325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H, Qi S, Zhang T, Wang A, Liu R, Guo J, Wang Y, Xu Y. miR-188-5p inhibits tumour growth and metastasis in prostate cancer by repressing LAPTM4B expression. Oncotarget. 2015;6:6092–6104. doi: 10.18632/oncotarget.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spahn M, Kneitz S, Scholz CJ, Stenger N, Rudiger T, Strobel P, Riedmiller H, Kneitz B. Expression of microRNA-221 is progressively reduced in aggressive prostate cancer and metastasis and predicts clinical recurrence. Int J Cancer. 2010;127:394–403. doi: 10.1002/ijc.24715. [DOI] [PubMed] [Google Scholar]
- 11.Haflidadottir BS, Larne O, Martin M, Persson M, Edsjo A, Bjartell A, Ceder Y. Upregulation of miR-96 enhances cellular proliferation of prostate cancer cells through FOXO1. PLoS One. 2013;8:e72400. doi: 10.1371/journal.pone.0072400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou P, Chen WG, Li XW. MicroRNA-143 acts as a tumor suppressor by targeting hexokinase 2 in human prostate cancer. Am J Cancer Res. 2015;5:2056–2063. [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Wan X, Qiang W, Li T, Huang W, Huang S, Wu D, Li Y. MiR-29a suppresses prostate cell proliferation and induces apoptosis via KDM5B protein regulation. Int J Clin Exp Med. 2015;8:5329–5339. [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang W, Mao YQ, Wang H, Yin WJ, Zhu SX, Wang WC. MiR-124 suppresses cell motility and adhesion by targeting talin 1 in prostate cancer cells. Cancer Cell Int. 2015;15:49. doi: 10.1186/s12935-015-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsai KW, Wu CW, Hu LY, Li SC, Liao YL, Lai CH, Kao HW, Fang WL, Huang KH, Chan WC, Lin WC. Epigenetic regulation of miR-34b and miR-129 expression in gastric cancer. Int J Cancer. 2011;129:2600–2610. doi: 10.1002/ijc.25919. [DOI] [PubMed] [Google Scholar]
- 16.Bandres E, Agirre X, Bitarte N, Ramirez N, Zarate R, Roman-Gomez J, Prosper F, Garcia-Foncillas J. Epigenetic regulation of microRNA expression in colorectal cancer. Int J Cancer. 2009;125:2737–2743. doi: 10.1002/ijc.24638. [DOI] [PubMed] [Google Scholar]
- 17.Chen X, Zhang L, Zhang T, Hao M, Zhang X, Zhang J, Xie Q, Wang Y, Guo M, Zhuang H, Lu F. Methylation-mediated repression of microRNA 129-2 enhances oncogenic SOX4 expression in HCC. Liver Int. 2013;33:476–486. doi: 10.1111/liv.12097. [DOI] [PubMed] [Google Scholar]
- 18.Wong KY, Yim RL, Kwong YL, Leung CY, Hui PK, Cheung F, Liang R, Jin DY, Chim CS. Epigenetic inactivation of the MIR129-2 in hematological malignancies. J Hematol Oncol. 2013;6:16. doi: 10.1186/1756-8722-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fesler A, Zhai H, Ju J. miR-129 as a novel therapeutic target and biomarker in gastrointestinal cancer. Onco Targets Ther. 2014;7:1481–1485. doi: 10.2147/OTT.S65548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collares CV, Evangelista AF, Xavier DJ, Rassi DM, Arns T, Foss-Freitas MC, Foss MC, Puthier D, Sakamoto-Hojo ET, Passos GA, Donadi EA. Identifying common and specific microRNAs expressed in peripheral blood mononuclear cell of type 1, type 2, and gestational diabetes mellitus patients. BMC Res Notes. 2013;6:491. doi: 10.1186/1756-0500-6-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsing AW, Chokkalingam AP. Prostate cancer epidemiology. Front Biosci. 2006;11:1388–1413. doi: 10.2741/1891. [DOI] [PubMed] [Google Scholar]
- 22.Rubin MA, Maher CA, Chinnaiyan AM. Common gene rearrangements in prostate cancer. J. Clin. Oncol. 2011;29:3659–3668. doi: 10.1200/JCO.2011.35.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gronberg H. Prostate cancer epidemiology. Lancet. 2003;361:859–864. doi: 10.1016/S0140-6736(03)12713-4. [DOI] [PubMed] [Google Scholar]
- 24.Wang YL, Wu S, Jiang B, Yin FF, Zheng SS, Hou SC. Role of MicroRNAs in Prostate Cancer Pathogenesis. Clin Genitourin Cancer. 2015;13:261–270. doi: 10.1016/j.clgc.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Villanueva A, Hoshida Y, Toffanin S, Lachenmayer A, Alsinet C, Savic R, Cornella H, Llovet JM. New strategies in hepatocellular carcinoma: genomic prognostic markers. Clin Cancer Res. 2010;16:4688–4694. doi: 10.1158/1078-0432.CCR-09-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mittal RD, Gangwar R, George GP, Mittal T, Kapoor R. Investigative role of pre-microRNAs in bladder cancer patients: a case-control study in North India. DNA Cell Biol. 2011;30:401–406. doi: 10.1089/dna.2010.1159. [DOI] [PubMed] [Google Scholar]
- 27.Tobias-Machado M, Fonseca F, Fantinato AP, Bendit I, Wroclawski ML, Wroclawski E, del Giglio A. Cytokeratin 19 expression by reverse transcriptase-polymerase chain reaction in the peripheral blood of prostate cancer patients. Tumori. 2005;91:248–252. doi: 10.1177/030089160509100307. [DOI] [PubMed] [Google Scholar]
- 28.Wallace TA, Downey RF, Seufert CJ, Schetter A, Dorsey TH, Johnson CA, Goldman R, Loffredo CA, Yan P, Sullivan FJ, Giles FJ, Wang-Johanning F, Ambs S, Glynn SA. Elevated HERV-K mRNA expression in PBMC is associated with a prostate cancer diagnosis particularly in older men and smokers. Carcinogenesis. 2014;35:2074–2083. doi: 10.1093/carcin/bgu114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie Y, Todd NW, Liu Z, Zhan M, Fang H, Peng H, Alattar M, Deepak J, Stass SA, Jiang F. Altered miRNA expression in sputum for diagnosis of non-small cell lung cancer. Lung Cancer. 2010;67:170–176. doi: 10.1016/j.lungcan.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang YL, Xu LP, Zhuo FL, Wang TY. Prognostic value of microRNA-10b overexpression in peripheral blood mononuclear cells of nonsmall-cell lung cancer patients. Tumour Biol. 2015;36:7069–75. doi: 10.1007/s13277-015-3366-6. [DOI] [PubMed] [Google Scholar]
- 31.Dyrskjot L, Ostenfeld MS, Bramsen JB, Silahtaroglu AN, Lamy P, Ramanathan R, Fristrup N, Jensen JL, Andersen CL, Zieger K, Kauppinen S, Ulhoi BP, Kjems J, Borre M, Orntoft TF. Genomic profiling of microRNAs in bladder cancer: miR-129 is associated with poor outcome and promotes cell death in vitro. Cancer Res. 2009;69:4851–4860. doi: 10.1158/0008-5472.CAN-08-4043. [DOI] [PubMed] [Google Scholar]
- 32.Zhai J, Qu S, Li X, Zhong J, Chen X, Qu Z, Wu D. miR-129 suppresses tumor cell growth and invasion by targeting PAK5 in hepatocellular carcinoma. Biochem Biophys Res Commun. 2015;464:161–167. doi: 10.1016/j.bbrc.2015.06.108. [DOI] [PubMed] [Google Scholar]


