Abstract
This study aimed to illustrate the potential effects of miR-155 in neuropathic pain and its potential mechanism. Spragure-Dawley (SD) rats were used for neuropathic pain model of bilateral chronic constriction injury (bCCI) construction. Effects of miR-155 expression on pain threshold of mechanical stimuli (MWT), paw withdrawal threshold latency (PMTL) and cold threshold were analyzed. Target for miR-155 was analyzed using bioinformatics methods. Moreover, effects of miR-155 target gene expression on pain thresholds were also assessed. Compared with the controls and sham group, miR-155 was overexpressed in neuropathic pain rats (P<0.05), but miR-155 slicing could significantly decreased the pain thresholds (P<0.05). Serum and glucocorticoid regulated protein kinase 3 (SGK3) was predicted as the target gene for miR-155, and miR-155 expression was negatively correlated to SGK3 expression. Furthermore, SGK3 overexpression could significantly decreased the pain thresholds which was the same as miR-155 (P<0.05). Moreover, miR-155 slicing and SGK3 overexpression could significantly decrease the painthreshold. The data presented in this study suggested that miR-155 slicing could excellently alleviate neuropathic pain in rats through targeting SGK3 expression. miR-155 may be a potential therapeutic target for neuropathic pain treatment.
Keywords: Neuropathic pain, miR-155, SGK3, mechanical allodynia, thermal hyperalgesia
Introduction
Neuropathic pain is a kind of direct or indirect pain that caused by the primary injury or dysfunction of somatosensory nervous system, and has been considered as the public health problem [1,2]. Papers reported that activation of cytokines and inflammatory mediator release would change the sensitivity of nerve cells, and then affecting the nerve plasticity, which leading to the neuropathic pain [3,4]. However, the definite pathogen mechanism for neuropathic pain still remains unclear.
miRNAs are some short endogenous RNAs known to post-transcriptionally repress gene expressions in animals and plants [5]. Recent evidences perform that miRNAs may involve in the processes for neuropathic pain regulation through targeting the 3’UTR of mRNAs [6]. For example, miR-96 and miR-182 are overexpressed in rat dorsal root ganglia but can be inhibited by the ligation of spinal ganglion [7], and intrathecal injection of miR-96 could anesis neuropathic pain via inhibiting Nav1.3 in [8]. Situation of similarity, miR-195 may enhance neuropathic pain via through regulating autophagy [9], which suggests that miRNAs may be potential therapeutic targets for neuropathic pain treatment.
Previous evidences reported that miR-155 has been widely proved to be involved in diseases progression and development, including involving in inflammatory reactions [10,11]. Paper refers that the majority targets for miR-155 are inflammation related proteins such as serum and glucocorticoid regulated protein kinase 3 (SGK3) [12]. SGK3 is an important inflammation signal protein which plays pivotal roles in signaling pathway and cell phosphorylation cascade [13,14]. Despite numerous studies have investigated the roles of miR-155 and SGK3 in neuropathic pain associated diseases respectively, but the correlations between miR-155 and SGK3 in regulating neuropathic diseases still remain underlying.
In this study, we constructed the rat neuropathic pain model and assessed the pain threshold nerve cells. Comprehensive experimental methods were used to detect the expressions of miR-155 in rat neuropathic model and the effects of miR-155 expression on SGK3 expression. This study aimed to investigate the correlations between miR-155 expression in neuropathic pain and its potential mechanism. Our study may provide theoretical basis for illustrating the therapeutic target role of miR-155 in neuropathic pain.
Materials and methods
Neuropathic pain model construction
All experimental procedures were approved by the Institute’s animal care center in accordance with the guidelines of the international association for the study of pain. Spragure-Dawley (SD) rats weighting at 250-350 g and hosed at the temperature of 22°C under a 12/12 h light/dark cycle with free access to food and water were chosen for the model construction in this study.
Bilateral chronic constriction injury (CCI) model of rats was constructed for neuropathic pain [15]. Briefly, rats were anesthetized by intraperitoneal injection of sodium pentobarbital with dose of 40-50 mg/kg. Then the sciatic nerve was exposed by blunt dissection through biceps femoris at the level of the middle of the thigh. Proximal to the sciatic’s trifurcation (above 2 mm distal to sciatic nerve), sciatic nerves were tied loosely using 4 ligatures (4.0 chromic gut) with about 1 mm spacing and the length of nerve thus was 4-5 mm long. After that, the artery on the surface of the sciatic nerve was just barely constricted and the degree of its circulation through the superficial epineurial vasculature was best to be retarded but not arrested when was observed using dissecting microscope (40 X magnification). The desired degree of constriction sometimes produced a small, brief twitch in the muscle around the exposure. After washed with physiological saline, the incision at muscle fascia, subcutaneous tissue and skin were discontinuous closed. An identical dissection was performed on the opposite side in every rat. Sham procedures comprised equal treatment but without ligation of the sciatic nerve were performed to prepare some rats as sham group. Rats were housed postoperatively in clear plastic cages with solid floors instead of wire mesh floors to avoid exacerbate discomfort arising from the affected hind paw. All surgical operations were performed by the same person.
Cell culture
Rats microgliacytes which were purchased from Sciencell (Carlsbad, CA, USA) were cultured in Dulbecco’s modified eagle medium (DMEM) supplemented with 10% hyclone, 100 IU/mL penicillin and 100 μg/mL streptomycin (Invitrogen, USA) in an atomosphere of 5% CO2 at 37°C. Microgliacytes were digested with 0.02% EDTA containing 0.05% trypsin (Sigma, USA) every 48 h for subculture. Cells from rats tissue: SD rats were sacrificed for the lumbar pool of the spinal cord collection, then the collected spinal cord was treated with precool Hanks balanced salt solution (HBSS) [16], followed with centrifugation at 400 g for 10 min for cells collection. After that, cells were put into 75% Percoll for centrifugation at 1000 g for 20 min. Isolated cells at 50/75% were washed with precool PBS buffer (PH 7.4). Cells were re-suspended using PBS buffer containing 1% bovine serum (Invitrogen).
Pain threshold assay
Mechanical allodynia
Pain threshold of sharp withdrawal threshold after mechanical stimuli (MWT) for rat microgliacytes in response to mechanical stimulation was assessed using pain gauge measurement (von Frey, IITC, USA) as described in previous study [17]. Briefly, rats were acclimated in transparent plastic cages with wire mesh floor for 30 min. Plantar surface of each hind paw was applied pressure from below with the calibrated Electronic von Frey filament and held for about 5 s. Then force applied at the time of sharp withdrawal was recorded.
Thernal hyperalgesia
Heat sensitivity was measured using paw withdrawal threshold latency (PMTL) in response to radiant heat based on the Hargreaves method [18]. Procedures were as follows: rats were placed in prespex boxes, and then a radiant heat source BME-410A beneath a glass-floor was focused on the center of the plantar surface of the hind paw. Heat intensity was approximately set up to 10 s to produce PWTL in normal animals and the cutoff time was set at 20 s to avoid tissue damage. The hind paws were given heat stimuli three times with greater than 3 min intervals between consecutive tests. Measurements were conducted at the internal time of between 8 ante meridiem and 2 post meridiem.
Cold allodynia
After acclimated in cages for 15 min, rats were gently injected with 0.1 mL acetone at the hind paw with the 1 mL syringe with a hose connection [19]. Reactions performed with rapid withdrawal, licking, shaking or lifting of the hind paw after the spread of the acetone over the planter surface were considered as positive. Measurement was performed three times for each hind paw with an interval of approximately 2 min between consecutive tests. The positive reaction frequency was considered as total member of withdrawal and measurements were conducted at the internal time of between 8 ante meridiem and 2 post meridiem.
qRT-PCR
Quantitative real-time polymerase chain reaction (qRT-PCR) was conducted to detect the mRNA expression of miR-155 in rats tissues [20]. Total RNA from rats cells was isolated using TRIzol Reagent (Invitrogen) as previously described [21] and then was treated with RNse-free Dnase I (Promega Biotech, USA). Concentration and purity for isolated RNA were measured using SMA 400 UV0VIS (Merinton, Shanghai, China). The purified RNA of 5 μL (0.5 μg/μL) with nuclease-free water was used for cDNA synthesis with the PrimerScript 1st Strand cDNA Synthesis Kit (Invitrogen). Expression of miR-155 in rat microgliacytes was detected using the SYBR ExScript RT-qPCR Kit (Takara, China). The total reaction system of 20 μL volume was as follows: 1 μL cDNA from the above PCR, 10 μL SYBR Premix EX Taq, 1 μL each of the primers (10 μM), and 7 μL ddH2O. The PCR program was as follows: pre-degeneration at 94°C for 5 min, denaturation at 94°C for 1 min; annealing at 56°C for 1 min; followed by 45 cycles, and extension at 72°C for 1 min. miR-155 expression was determined by the comparative threshold (Ct) cycle (2-ΔΔCt) method [22]. Primers used for miR-155 amplification were 5’-GCAGCTAGCCCAGGGTTG-3’ (sense) and 5’-GCAAAGCTTCAGTTAACCCGGCGGTGA-3’ (antisense). Phosphoglyceraldehyde dehydrogenase (GAPDH) was considered as the internal control.
Western blotting
Rat tissues were lapped with radioimmunoprecipitation (RIPA, Sangon Biotech, China) lysate containing phenylmethanesufonyl fluoride (PMSF, Sigma), and then were centrifuged at 12,000 rpm for 10 min at 4°C. Supertanant was collected for the measurement of protein concentrations using BCA protein assay kit (Pierce, Rochford, IL). For Western blotting assay [23], 20 μg protein per cell lysate was subjected to a 12% sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by transferred onto a Polyvinylidencefluoride (PVDF) membrane (Mippore). Then the PVDF membrane was blocked in Tris buffered saline Tween (TBST) containing 5% non-fat milk for 1 h. Consequently, the membrane was incubated with rabbit anti-human antibodies (miR-155, 1:100 dilution, Invitrogen) and overnight at 4°C. Then membrane was incubated with hoseradish peroxidase labeled goat anti-rat secondary antibody (1:1000 dilution) at room temperature for 1 h. Finally, PVDF membrane was washed with 1×TBST buffer for 10 min with 3 times. Detection was conducted using the development of X-ray after chromogenic substrate with an enhanced chemiluminescence (CEL) method. Additionally, GAPDH served as the internal control.
Statistical analysis
All experiments were conducted independently for 3 times. The data were expressed as mean ± standard deviation (SD). Total data were calculated with SPSS 17.0 (SPSS, San Diego, CA, USA) and significant differences between groups were analyzed using Student’s test or one-way analysis of variance (ANOVA). The P<0.05 was defined as statistically significant.
Results
Relative miR-155 expression in bCCI model rats
Compared with the control and sham group, relative miR-155 mRNA in bCCI rats was significantly increased (P<0.05, Figure 1A), indicating that miR-155 overexpression may be correlated with neuropathic pain in rats. Besides, neuropathic pain threshold assay showed that MWT and PMTL were significantly increased when miR-155 was down-regulated with ASO-miR-155 lentiviral vector transfection in rats, and the threshold up to maximum at 15 day after surgery (P<0.05, Figure 1B and 1C), but positive frequency for nerve sensitivity to cold was declined maximum at 15 day (P<0.05, Figure 1D), indicating that miR-155 down-regulation resulted in surgery rats bear high MWT and PMTL, and low temperature.
Figure 1.
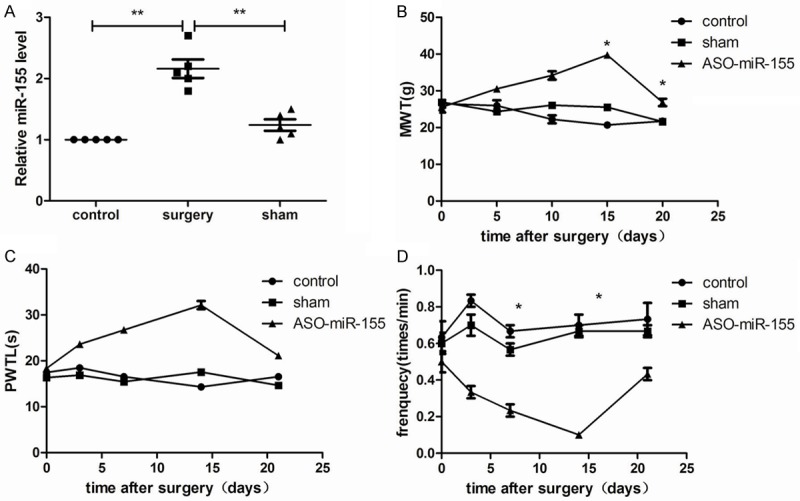
miR-155 expression in neuropathic pain model rats and effects of miR-155 expression on pain threshold. A: miR-155 was significantly overexpressed in neuropathic pain model rats compared with the control and sham control group; B: miR-155 down-regulation increased pain threshold of mechanical stimuli (NWT) at 15 day after surgery; C: miR-155 down-regulation increased pain threshold of paw withdrawal threshold latency (PMTL) at 15 day after surgery; D: miR-155 down-regulation decreased threshold of positive frequency. *P<0.05, compared with the control and the sham group.
SGK3 was a direct target for miR-155 in neuropathic pain model
Bioinformatics methods including TargetSvan, PicTar and miRanda databases were used to predict the target gene for miR-155 [24,25]. Data showed that SGK3 was a direct target for miR-155 (Figure 2A). Fluorescence vector for SGK3 was constructed to identify whether miR-155 could target the 3’-UTR of SGK3. The results showed that miR-155 expression could significantly decreased fluorescence density of SKG3-3’UTR compared with the control (Figure 2B). Western blotting analysis was used to investigate whether miR-155 could down-regulate SGK3 expression in neuropathic pain model, and the results showed that miR-155 expression could negatively regulate SGK3 expression (Figure 2C). Consequently, miR-155 was overexpressed in cells to verify SGK3 expression, and results showed that miR-155 could negatively regulate SGK3 expression endogenous (Figure 2D), which indicating that SGK3 was the direct target for miR-155.
Figure 2.
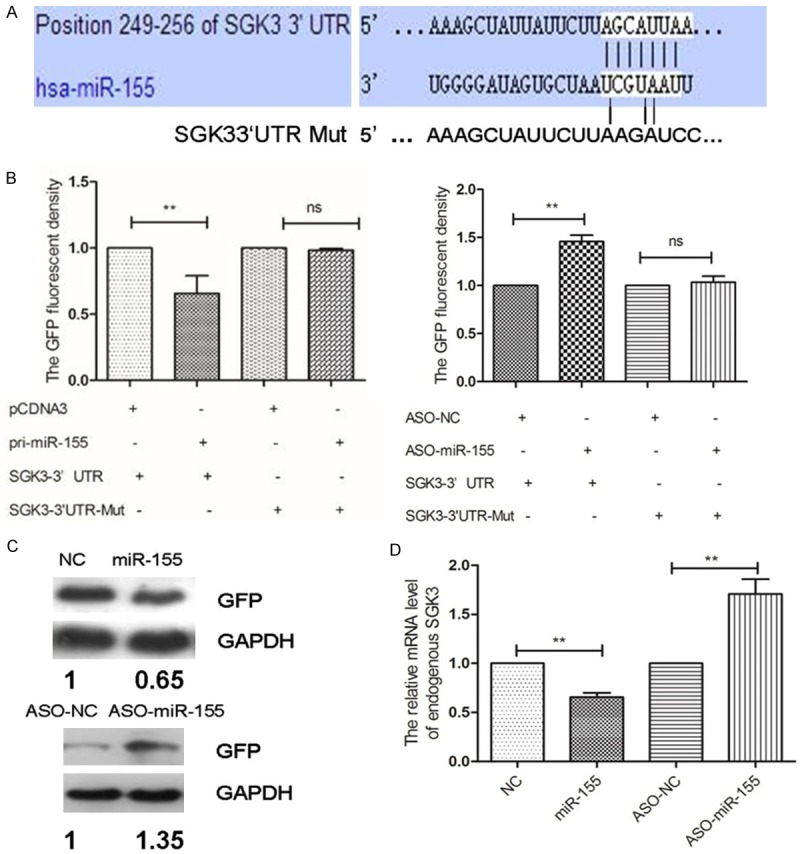
Target gene selections for miR-155 in neuropathic pain model rats. A: Bioinformatics analysis showed that SGK3 was the target for miR-155; B-D: Verification for SGK3 was the direct target gene for miR-155 both in vivo and in vitro. **P<0.01, compared with the controls.
Functional analysis of SGK3 in neuropathic pain model
In order to further analyze the potential mechanism of miR-155 regulating SGK3 in neuropathic pain model, SGK3 was overexpressed in N9 cells by transfecting with pcDNA-SGK3 plasma (Figure 3A). Further investigation showed that SGK3 overexpression could excellently improve the pain threshold for bCCI rats (P<0.01, Figure 3B-D), indicating that overexpression of SGK3 could alleviate the pain threshold for model rats.
Figure 3.
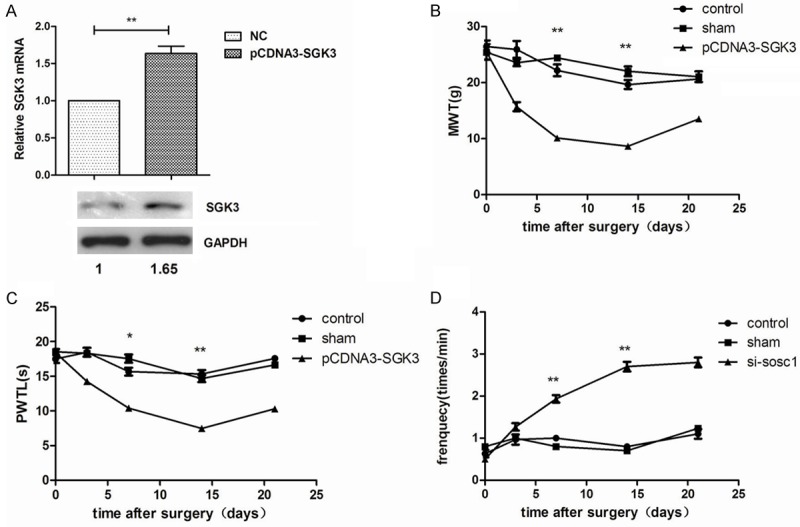
SGK3 expressions in neuropathic pain model rats and effects of SGK3 expression on pain threshold. A: relative SGK3 expression was increased when cells were transfected with pcDNA-SGK3 vector; B: SGK3 overexpression significantly decreased NWT at 15 day after surgery; C: SGK3 overexpression significantly decreased PMTL at 15 day after surgery; D: SGK3 overexpression significantly increased threshold of positive frequency. *P<0.05; **P<0.01, compared with the control and the sham group.
Remedial experiment
Remedial experiments were designed to further verify whether SGK3 was the direct target for miR-155 (Figure 4). Compared with the controls, SGK3 expression could rescue the effects of miR-155 expression on bCCI rats pain threshold, including MWT, PWTL and pain positive frequency with time increasing (Figure 4A-C), suggesting that SGK3 overexpression could rescue the effects of miR-155 overexpression on pain threshold in model rats.
Figure 4.
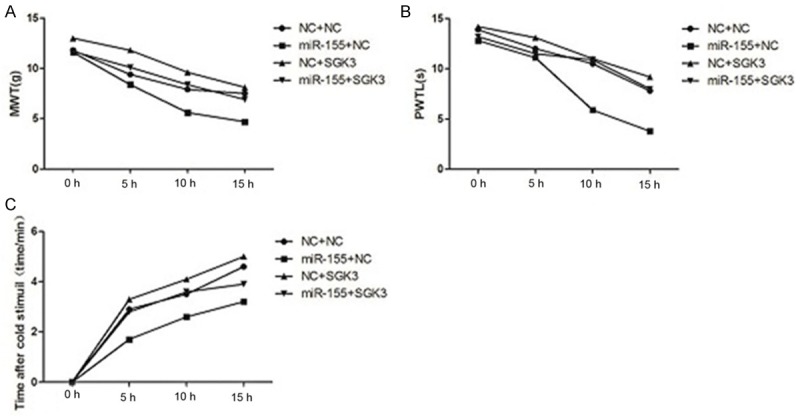
Remedial experiments for effects of SGK3 overexpression on pain threshold which were affected by miR-155 overexpression. A: SGK3 overexpression could rescue NWT which was decreased by miR-155 overexpression; B: SGK3 overexpression could rescue PMTL which was decreased by miR-155 overexpression; C: SGK3 overexpression could rescue cold threshold of positive frequency which was increased by miR-155 overexpression.
Discussion
Neuropathic pain has been considered as a common public health problem, which is caused by the primary injury or dysfunction of somatosensory nervous system [1,2]. The effects of miR-155 in neuropathic pain have not been fully reported. In this study, we used SD rats to construct the neuropathic pain model and then analyzed the influence of miR-155 in this kind of nervous system disease. The data showed that miR-155 slicing could significantly alleviate the pain thresholds, and SGK3 was the direct target for miR-155 and SGK3 overexpression could significantly alleviate the pain thresholds. Furthermore, experiments showed that miR-155 slicing and SGK3 up-regulation could well alleviate nerve pain, which suggesting the important roles of miR-155 and SGK3 in neuropathic pain.
It has been demonstrated that miR-155 played crucial roles in inflammatory reactions, such as miR-155 was overexpressed in lupus Treg cells [26]. miR-203 could regulate NPP through targeting Rap1a and its downstream signal MEK/ERK pathway [27], and miR-21 down-regulation could inhibit the pain threshold for rats in post-nerve injury [28]. Tan and his partners proved that miR-155 was overexpressed in neuropathic pain rats and miR-155 suppression could attenuate neuropathic pain via regulating suppressor of cytokine signaling 1 (SOCS1) pathway [29]. Coincidence with former evidences, our data showed that miR-155 was overexpressed in neuropathic pain rats and miR-155 slicing could alleviate the pain thresholds, suggesting the important roles of miR-155 in neuropathic pain.
Meanwhile, the association between SGK3 and neuropathic pain still remain unclear yet. Numerous evidences about SGK3 were mainly in malignancies, such as SGK3 mediated the INPP4B-dependent PI3K pathway in breast cancer cell migration [30], and SGK3 contributed the cell growth of BRAF-mutant melanoma [31]. In this study, SGK3 was predicted as the direct target for miR-155 in neuropathic pain, indicating that SGK3 may involve in neuropathic pain progression and development. On the other hand, miR-155 slicing could regulate the T/B cells production in autoimmune arthritis and miR-155 was overexpressed in gouty arthritis patients than that in healthy persons [32]. In this study, when miR-155 was suppressed and SGK3 expression was overexpressed, the pain thresholds were all improved compared with that in controls, suggesting that miR-155 down-regulation may function as a suppressor in contributing neuropathic pain via up-regulating SGK3 expression.
In conclusion, the data presented in this study suggested that miR-155 down-regulation played pivotal roles in inhibiting neuropathic pain through targeting SGK3 gene. SGK3 is the direct target gene for miR-155, and miR-155 slicing or SGK3 overexpression alleviates neuropathic pain in rats. Our study may provide theoretical basis for the exploration and application of miRNAs in neuropathic pain therapeutic treatment. Further studies are still needed to deep the protective mechanism for miR-155 in neuropathic pain.
Disclosure of conflict of interest
None.
References
- 1.Finnerup NB, Attal N, Haroutounian S, Mcnicol E, Baron R, Dworkin RH, Gilron I, Haanp M, Hansson P, Jensen TS. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162–173. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrett AM, Lucero MA, Le T, Robinson RL, Dworkin RH, Chappell AS. Epidemiology, Public Health Burden, and Treatment of Diabetic Peripheral Neuropathic Pain: A Review. Pain Med. 2007;8:S50–S62. doi: 10.1111/j.1526-4637.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 3.Evans LJ, Loescher AR, Boissonade FM, Whawell SA, Robinson PP, Andrew D. Temporal mismatch between pain behaviour, skin Nerve Growth Factor and intra-epidermal nerve fibre density in trigeminal neuropathic pain. BMC Neurosci. 2014;15:47–53. doi: 10.1186/1471-2202-15-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fox A, Patel H, Barnes P, Belvisi M. Release of nerve growth factor by human pulmonary epithelial cells: role in airway inflammatory diseases. Eur J Pharmacol. 2001;424:159–162. doi: 10.1016/s0014-2999(01)01138-4. [DOI] [PubMed] [Google Scholar]
- 5.Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakai A, Suzuki H. Emerging roles of microRNAs in chronic pain. Neurochem Int. 2014;77:58–67. doi: 10.1016/j.neuint.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Aldrich BT, Frakes EP, Kasuya J, Hammond DL, Kitamoto T. Changes in expression of sensory organ-specific microRNAs in rat dorsal root ganglia in association with mechanical hypersensitivity induced by spinal nerve ligation. Neuroscience. 2009;164:711–723. doi: 10.1016/j.neuroscience.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen HP, Zhou W, Kang LM, Yan H, Zhang L, Xu BH, Cai WH. Intrathecal miR-96 inhibits Nav1.3 expression and alleviates neuropathic pain in rat following chronic construction injury. Neurochem Res. 2014;39:76–83. doi: 10.1007/s11064-013-1192-z. [DOI] [PubMed] [Google Scholar]
- 9.Shi G, Shi J, Liu K, Liu N, Wang Y, Fu Z, Ding J, Jia L, Yuan W. Increased miR-195 aggravates neuropathic pain by inhibiting autophagy following peripheral nerve injury. Glia. 2013;61:504–512. doi: 10.1002/glia.22451. [DOI] [PubMed] [Google Scholar]
- 10.Lao G, Liu P, Wu Q, Zhang W, Liu Y, Yang L, Ma C. Mir-155 promotes cervical cancer cell proliferation through suppression of its target gene LKB1. Tumour Biol. 2014;35:11933–11938. doi: 10.1007/s13277-014-2479-7. [DOI] [PubMed] [Google Scholar]
- 11.Huang C, Li H, Wu W, Jiang T, Qiu Z. Regulation of miR-155 affects pancreatic cancer cell invasiveness and migration by modulating the STAT3 signaling pathway through SOCS1. Oncol Rep. 2013;30:1223–1230. doi: 10.3892/or.2013.2576. [DOI] [PubMed] [Google Scholar]
- 12.Tessier M, Woodgett JR. Serum and glucocorticoid-regulated protein kinases: Variations on a theme. J Cell Biochem. 2006;98:1391–1407. doi: 10.1002/jcb.20894. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Zhou D, Phung S, Masri S, Smith D, Chen S. SGK3 is an estrogen-inducible kinase promoting estrogen-mediated survival of breast cancer cells. Mol Endocrinol. 2011;25:72–82. doi: 10.1210/me.2010-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leong ML, Maiyar AC, Kim B, O’Keeffe BA, Firestone GL. Expression of the serum- and glucocorticoid-inducible protein kinase, Sgk, is a cell survival response to multiple types of environmental stress stimuli in mammary epithelial cells. J Biol Chem. 2003;278:5871–5882. doi: 10.1074/jbc.M211649200. [DOI] [PubMed] [Google Scholar]
- 15.Shen L, Li X, Wang H, Yu X, Huang Y. [Expressions of neuropathic pain-related proteins in the spinal cord dorsal horn in rats with bilateral chronic constriction injury] . Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2013;35:628–633. doi: 10.3881/j.issn.1000-503X.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Hanks JH. Hanks’ balanced salt solution and pH control. Methods in Cell Science. 1975;1:3–4. [Google Scholar]
- 17.Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115:71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Vanderlinden K, Giraldez J, Van Meirvenne M. Assessing reference evapotranspiration by the Hargreaves method in southern Spain. Journal of Irrigation and Drainage Engineering. 2004;130:184–191. [Google Scholar]
- 19.Jørum E, Warncke T, Stubhaug A. Cold allodynia and hyperalgesia in neuropathic pain: the effect of N-methyl-D-aspartate (NMDA) receptor antagonist ketamine-a double-blind, cross-over comparison with alfentanil and placebo. Pain. 2003;101:229–235. doi: 10.1016/S0304-3959(02)00122-7. [DOI] [PubMed] [Google Scholar]
- 20.Rhodes SD. Encyclopedia of Systems Biology. Springer; 2013. Quantitative Real-time Polymerase Chain Reaction; pp. 1807–1807. [Google Scholar]
- 21.Costa B, Comelli F, Bettoni I, Colleoni M, Giagnoni G. The endogenous fatty acid amide, palmitoylethanolamide, has anti-allodynic and anti-hyperalgesic effects in a murine model of neuropathic pain: involvement of CB(1), TRPV1 and PPARγ receptors and neurotrophic factors. Pain. 2008;139:541–550. doi: 10.1016/j.pain.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Schefe JH, Lehmann KE, Buschmann IR, Unger T, Funke-Kaiser H. Quantitative real-time RT-PCR data analysis: current concepts and the novel “gene expression’s CT difference” formula. J Mol Med (Berl) 2006;84:901–910. doi: 10.1007/s00109-006-0097-6. [DOI] [PubMed] [Google Scholar]
- 23.Kawasaki Y, Xu ZZ, Wang X, Park JY, Zhuang ZY, Tan PH, Gao YJ, Roy K, Corfas G, Lo EH. Distinct roles of matrix metalloproteases in the early-and late-phase development of neuropathic pain. Nat Med. 2008;14:331–336. doi: 10.1038/nm1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuse M, Nohata N, Kojima S, Sakamoto S, Chiyomaru T, Kawakami K, Enokida H, Nakagawa M, Naya Y, Ichikawa T. Restoration of miR-145 expression suppresses cell proliferation, migration and invasion in prostate cancer by targeting FSCN1. Int J Oncol. 2011;38:1093–1101. doi: 10.3892/ijo.2011.919. [DOI] [PubMed] [Google Scholar]
- 25.Hébert SS, Horré K, Nicolaï L, Papadopoulou AS, Mandemakers W, Silahtaroglu AN, Kauppinen S, Delacourte A, De Strooper B. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/β-secretase expression. Proc Natl Acad Sci U S A. 2008;105:6415–6420. doi: 10.1073/pnas.0710263105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Divekar AA, Dubey S, Gangalum PR, Singh RR. Dicer insufficiency and microRNA-155 overexpression in lupus regulatory T cells: an apparent paradox in the setting of an inflammatory milieu. J Immunol. 2011;186:924–930. doi: 10.4049/jimmunol.1002218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Huang Y, Ma C, Yu X, Zhang Z, Shen L. MiR-203 involves in neuropathic pain development and represses Rap1a expression in nerve growth factor differentiated neuronal PC12 cells. Clin J Pain. 2015;31:36–43. doi: 10.1097/AJP.0000000000000070. [DOI] [PubMed] [Google Scholar]
- 28.Sakai A, Suzuki H. Nerve injury-induced upregulation of miR-21 in the primary sensory neurons contributes to neuropathic pain in rats. Biochem Biophys Res Commun. 2013;435:176–181. doi: 10.1016/j.bbrc.2013.04.089. [DOI] [PubMed] [Google Scholar]
- 29.Tan Y, Yang J, Xiang K, Tan Q, Guo Q. Suppression of MicroRNA-155 Attenuates Neuropathic Pain by Regulating SOCS1 Signalling Pathway. Neurochem Res. 2014;40:550–560. doi: 10.1007/s11064-014-1500-2. [DOI] [PubMed] [Google Scholar]
- 30.Gasser JA, Inuzuka H, Lau AW, Wei W, Beroukhim R, Toker A. SGK3 Mediates INPP4BDependent PI3K Signaling in Breast Cancer. Mol Cell. 2014;56:595–607. doi: 10.1016/j.molcel.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scortegagna M, Lau E, Zhang T, Feng Y, Sereduk C, Yin H, De SK, Meeth K, Platt JT, Langdon CG. PDK1 and SGK3 Contribute to the Growth of BRAF-Mutant Melanomas and Are Potential Therapeutic Targets. Cancer Res. 2015;75:1399–412. doi: 10.1158/0008-5472.CAN-14-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bluml S, Bonelli M, Niederreiter B, Puchner A, Mayr G, Hayer S, Koenders MI, van den Berg WB, Smolen J, Redlich K. Essential role of microRNA-155 in the pathogenesis of autoimmune arthritis in mice. Arthritis Rheum. 2011;63:1281–1288. doi: 10.1002/art.30281. [DOI] [PubMed] [Google Scholar]


