Abstract
It is well known that ox-LDL plays key roles in the development of atherosclerosis, partly by inducing vascular smooth muscle cells (VSMCs) proliferation. Recent findings have revealed that microRNAs, a class of small noncoding RNAs, could regulate cell proliferation in many physiological and pathological conditions. However, the role and function of miRNAs on ox-LDL induced VSMC proliferation are not fully elucidated. In this study, we showed that ox-LDL could suppress miR-141 expression and inhibition of miR-141 could promote VSMCs proliferation. Moreover, we found that PAPPA was the direct target gene of miR-141. Overexpression of PAPPA impaired the miR-141-induced inhibition of proliferation in the VSMCs. Taken together; miR-141 may play important roles in ox-LDL-induced abnormal proliferation of the VSMC.
Keywords: Atherosclerosis, vascular smooth muscle cells, ox-LDL, miR-141, proliferation
Introduction
Cardiovascular disease is among the leading causes of death in developed countries [1]. Atherosclerosis is the most common cause of cardiovascular disease and is associated with significant morbidity and mortality [2]. However, its pathogenesis has not been fully understood. It is well known that oxidized low-density lipoprotein (ox-LDL) is one of the major risk factors of atherosclerosis [3]. Ox-LDL can promote the formation of foam cells derived from vascular smooth muscle cells (VSMCs) and regulate the proliferation, apoptosis, migration, and differentiation of VSMCs, which play critical roles in the pathogenesis of cardiovascular disease [4]. However, the detailed mechanism of how ox-LDL induces proliferation of VSMCs has not yet been completely understood.
MicroRNAs (miRNAs) are a class of noncoding small RNAs, usually 18-25 nucleotides in length. MiRNAs can repress translation and cleave mRNA by base-pairing to the 3’untranslated region of the target genes [5,6]. They have emerged as key regulators of diverse biological and pathological processes, including cell differentiation, apoptosis and proliferation [7]. Recently, increasing evidences have suggested that miRNAs play important roles in cardiovascular system [1,8]. For example, miR-21, miR-221, miR-222, and miR-145 were proved to play a regulative role in aberrant VSMC proliferation, which is a key pathological process of atherosclerosis [9-16]. MiR-143/145 is down-regulated in response to both vascular injury and PDGF treatment, and restoration of its expression attenuates the neointimal growth in the rat carotid artery after balloon injury [17]. MiRNAs also play a critical role in the pathogenesis of ox-LDL-mediated inflammation [18]. For instance, ox-LDL could regulate the expression of let-7g, which inhibited the expression of LOX-1 and OCT-1 in human coronary artery smooth muscle cells [19]. However, the roles of miRNAs in the proliferation of VSMCs induced by ox-LDL have not been yet been fully investigated.In this study, we found that miR-141 was down-regulated in VSMCs stimulated by ox-LDL. Restored expression of miR-141 in VSMCs was able to inhibit cell proliferation by targeting PAPP-A. Thus, our date suggested an important role of miR-141 in atherosclerosis pathogenesis, indicating its potential application in atherosclerosis therapy.
Materials and methods
Ethics statement and tissues samples
All experimental protocols were approved by the Clinical Research Ethics Committee of the Affiliated Hospital of Shandong Traditional Chinese Medicine University.
Vectors and cell culture
The siRNA of PAPPA was purchased from Sigma (Sigma-Aldrich, Oakville, ON, Canada). The binding site within 3’UTR sequence of PAPPA for miR-141 was cloned into the pMIR-REPORT luciferase construct (Ambion, Austin, TX). The mutant construct of PAPPA 3’UTR was generated using QuikChang Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA). Cell culture-related reagents were purchased from Gibco-Life Technologies. (Carlsbad, CA, USA) Human VSMCs is obtained from ATCC and cultured in the medium 231 supplemented with smooth muscle cell growth supplement (SMGC) at 37°C in a humidified atmosphere of 95% air and 5% CO2.
Cell transfection
The miR-141 mimics, inhibitors and scramble, which are non-homologous to the human genome, were synthesized by GenePharma (Shanghai, China) and transfected into the cells to a final oligonucleotide concentrtion of 10 nmol/L. All cell transfections were introduced using Dharma FECT1 Reagent (Dharmacon, TX, USA) according to the manufacturer’s instructions.
RNA isoltion and quantative realtime reverse transcription-PCR: Total RNA (2 μg) was reverse trascribed with 200 U of MMLV reverse transcriptase (Invitrogen) using Oligo (dT) primers or stem-loop primer in a 20 μL reaction mixture. Relative transcript levels of mRNA were determined by real-time PCR using the iQ5 Real-Time PCR Detection System (Bio-Rad, California, USA). The real-time PCR reaction included SYBR Green fluorescent dye (1x, Takara, Dalian, China), forward primers (1 μl, 10 μM), reverse primers (1 μl, 10 μM), qPCR mix (1×), and cDNA (1 μl). The sequences of the specific primers are shown in Table 1. To produce the melting curve, the reactions were subject to one step at 95°C for 30 s, followed by 45 cycles of 95°C for 5 s, 60°C for 10 s, and 72°C for 30 s. The relative gene expression was calculated by the ΔΔCt method. GAPDH or U6 was used as the internal control (Table 1).
Table 1.
Primer sequence
| Name | Sequence (5’-3’) |
|---|---|
| miRNA reverse transcription prime | |
| miRNA-141 | ATGTATGAGTGCAGGTCGGAGGTAT TCTCACTATAAGACTCAGCC |
| U6 snRNA | AAAATATGGAACGCTTCACGAATTTG |
| Real-time PCR primer sequence | |
| miRNA-141 | AAGACGTACTCAGGCCATGTCC |
| GACCCAAATGTCGCAGTCAG | |
| U6 snRNA | CTCGCTTCGGCAGCACATATACT |
| ACGCTTCACGAATTTGCGTGTC | |
| GAPDH | AATGGGCAGCCGTTAGGAAA |
| TGAAGGGGTCATTGATGGCA | |
| Ki-67 | TCCTTTGGTGGGCACCTAAGACCTG |
| PAPP-A | TGATGGTTGAGGTCGTTCCTTGATG |
| ATATCTCACGTGACCGAGGA | |
| AGCTGATGGTGCTGGAAGTC |
Cell proliferation assays
Cells were seeded in 96-well plates (1,000 cells per well) with complete culture medium (100 μl). Cells were cultured for another 24 h, 48 h, 72 h or 96 h. The supernatant was removed, and 100 μl of DMEM/F12 medium containing 10 μl of CCK8 was added to each well for incubating another 2 h at 37°C. The culture plates were then shaken for 10 min and the optical density (OD) values were read at 450 nm.
Dual luciferase assays
Cells were co-transfected with 0.4 μg of the reporter construct, 0.2 μg of pGL-3 control vector, and miR-141 or negative controls. Cells were harvested 24 h post-transfection and assayed with Dual Luciferase Assay (Promega, WI, USA) according to manufacturer’s instructions. Firefly luciferase values were normalized to Renilla, and the ratio of Firefly/Renilla values was reported.
Western blotting analysis
Western blot analysis was carried out using standard methods. Proteins were separated on 10% SDS-PAGE, and then transferred to PVDF membranes (Amersham, Buckinghamshire, UK). Membranes were blocked overnight with 5% non-fat dried milk and incubated for 2 h with anti- PAPPA antibody (Abcam, England) at 1:1000 dilution; anti-GAPDH antibody (Proteintech,Chicago, USA) at 1:50,000 dilution. After being washed with TBST (10 mM Tris, pH 8.0, 150 mM NaCl, and 0.1% Tween20), the membranes were incubated for 2 h with goat anti-rabbit antibody (zsgb-bio, Beijing, China) at 1:5000 dilution and 1:50000 dilution.
Statistical analysis
Each experiment was repeated at least three times. Statistical analysis was performed using SPSS 15.0. Data are presented as the mean ± standard deviation. Statistical analyses were done by analysis of variance (ANOVA) or Student’s t test. Statistical significance level was set at α = 0.05 (two-side).
Results
Expression of miR-141 was decreased in VSMCs induced by ox-LDL
A time-dependent induction of cell proliferation by ox-LDL (20 ng/ml) was observed with the maximal response at 72 h (Figure 1A). In addition, increasing concentrations of ox-LDL (0-60 ng/ml) significantly increased cell proliferation in a dose-dependent manner, with the maximal response at 40 ng/ml at 48 h (Figure 1B). Inhibition of miR-141 expression was observed in VSMCs after ox-LDL stimulation (Figure 1C). In addition, ox-LDL significantly inhibited miR-141 mRNA level in a dose-dependent manner, with a maximal response at the 40 ng/ml (Figure 1D).
Figure 1.
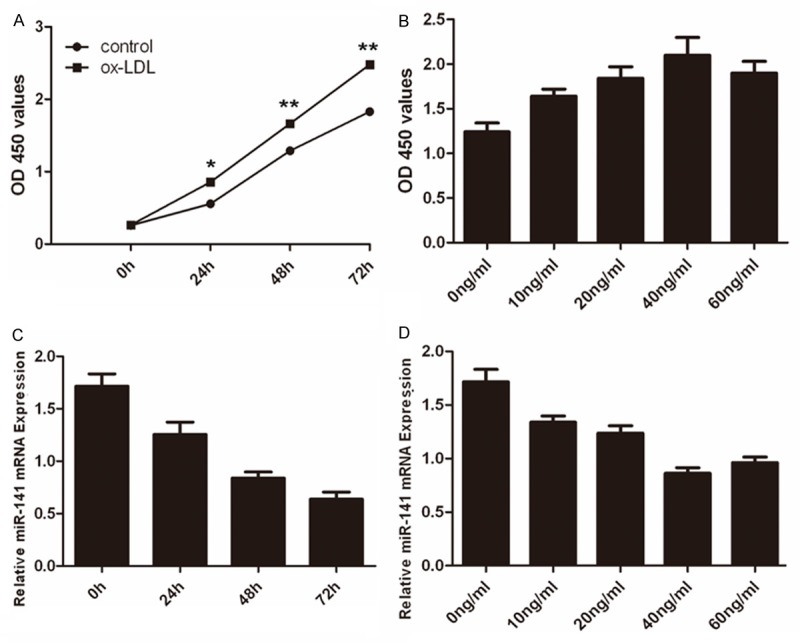
The expression of miR-141 was decreased in VSMCs induced by ox-LDL. A. CCK8 analysis showed that ox-LDL could induce VSMCs proliferation. B. For the dose-development studies, VSMCs were treated with either complete culture medium only or varying concentrations of ox-LDL (0-60 ng/ml) for 48 h. C. Real-time PCR analysis showed that ox-LDL inhibitedmiR-141 expression in the VSMCs. D. Treatment with ox-LDL significantly inhibited miR-141 mRNA level in a dose-dependent manner, with a maximal response at the 40 ng/ml using real-time PCR.
MiR-141 inhibited VSMCs proliferation
To examine the functional role of miR-141 in VSMCs, VSMCs were transfected with miR-141 mimics, inhibitors or scrambled control oligo, both of which showed high transfection efficiency (Figure 2A). Cell proliferation was increased in miR-141 inhibitors-transfected cells compared with control oligo-transfected cells or untreated cells (Figure 2B). In contrast, the miR-141 mimics inhibited the cell proliferation of the VSMCs. The proliferative effect of miR-141 was further confirmed by real-time PCR and western blot. As shown in Figure 2C and 2D, there was an increase in both the protein and mRNA levels of cyclin D1 in the group transfected with miR-141 inhibitor compared with the control group or untreated group. MiR-141 mimics inhibited the expression of cyclin D1 at both protein and mRNA levels.
Figure 2.
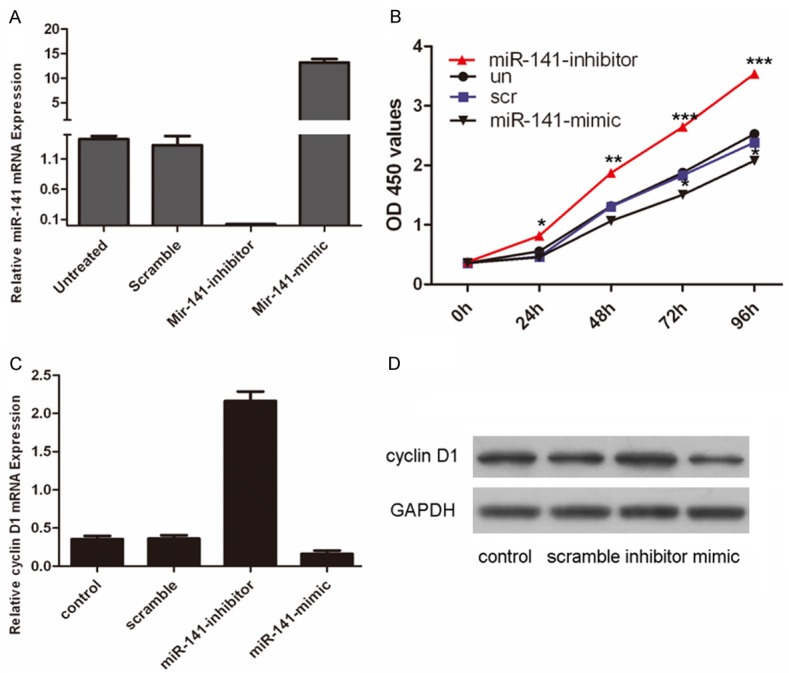
Inhibition of miR-141promoted VSMCs proliferation. A. Expression levels of miR-141 were examined by real-time PCR after transfection of miR-141 mimics or scramble or no transfection or miR-141 inhibitor. B. Growth of VSMCs was showed after transfection with miR-141 mimics or scramble or no transfection or miR-141 inhibitor. The growth index was assessed at 0, 24, 48, 72, and 96 hours. C. Inhibition of miR-141 promoted cyclin D1 mRNA expression. VSMCs were transfected with miR-141 mimics, scramble or not transfectedor miR-141 inhibitor. cyclin D1 was detected by real-time PCR. D. Inhibition of miR-141promotedcyclin D1 protein expression. VSMCs were transfected with miR-141 mimics, scramble or not transfectedor miR-141 inhibitor. cyclin D1 was detected by western blot. GAPDH was also detected for a loading control. Values are presented as mean ± SD. As compared with control, **P<0.01, *P<0.05, and ***P<0.001.
MiR-141 translationally repressed PAPPA
As predicted by PicTar, there was complementarily between has-miR-141 and the PAPPA 3’UTR (Figure 3A). The effect of miR-141 on the translation of PAPPA mRNA into protein was assessed by luciferase reporter assay in VSMCs (Figure 3B). Overexpression of miR-141 reduced luciferase activity of reporter gene with wild type, but not the mutant PAPPA 3’UTR, indicating that miR-141 directly targeted PAPPA 3’UTR. Furthermore, overexpression of miR-141 reduced the protein but not the mRNA levels of PAPPA in VSMCs (Figure 3C and 3D).
Figure 3.
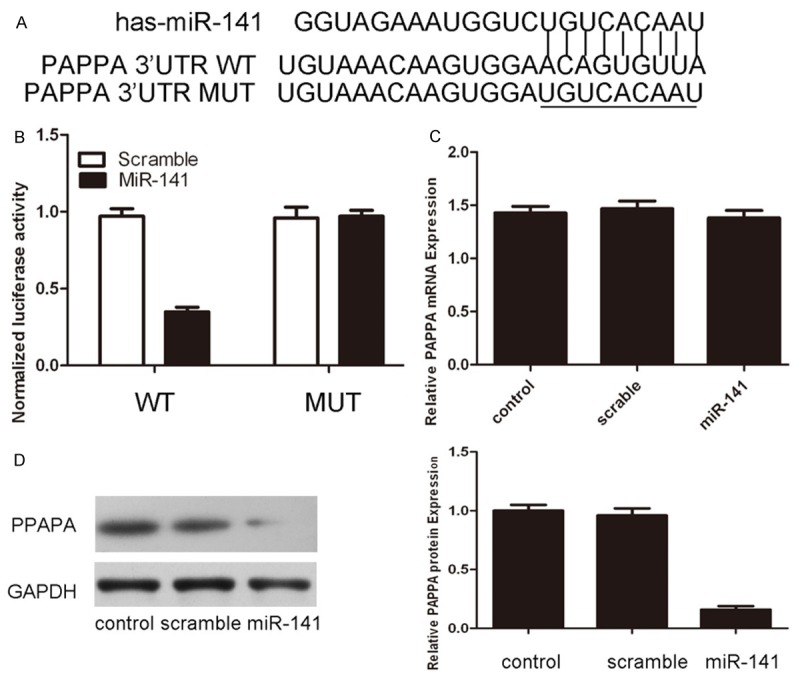
MiR-141 translationally repressed PAPPA expression. A. miR-141/PAPPA alignment by the Targetscan analysis. B. Relative luciferase activity in VSMCs 24 h post transfecting with miRNA control or miR-141 mimics. C. Overxpression of miR-141 did not suppress the mRNA expression of PAPPA using real-time PCR. D. Overxpression of miR-141 suppressed the protein expression of PAPPA using western blot.
Overexpression of PAPPA impaired the miR-141-induced inhibition of proliferation in VSMC
We then performed rescue experiments to further validate whether the PAPPA was involved in the role of miR-141 in VSMC. Western blot analysis confirmed the increased level of PAPPA using the PAPPA expression vector pcDNA3.1-PAPPA (Figure 4A). The inhibition of cell proliferation upon overexpression of miR-141 was attenuated by the re-introduction of PAPPA (Figure 4B). This effect of PAPPA was further confirmed by real-time PCR and western blot analysis (Figure 4C and 4D).
Figure 4.
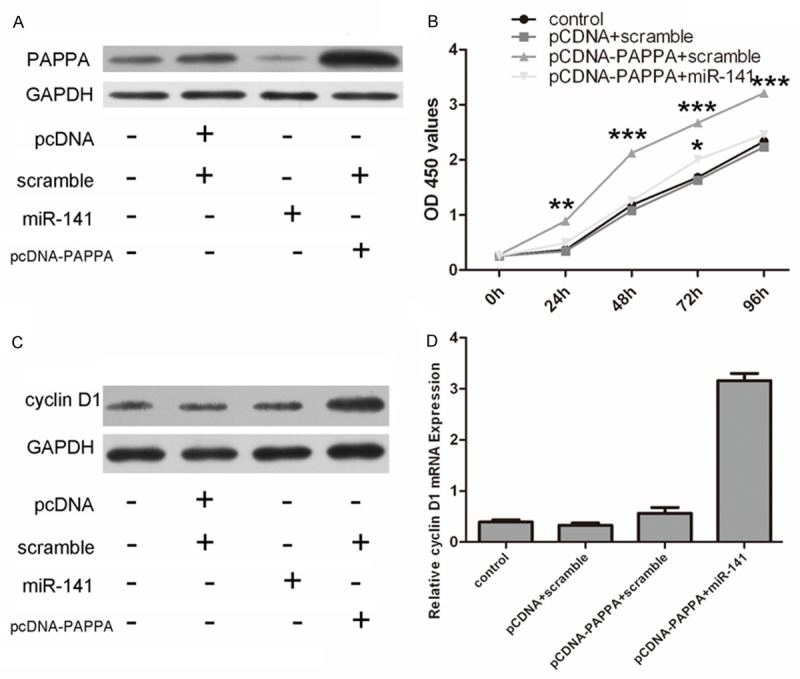
Overexpression of PAPPA impaired the miR-141-induced inhibition of proliferation in VSMCs. A. Western blot analysis of PAPPA expression in VSMCs co-transfected with either miR-141 mimics or scramble and pCDNA-PAPPA or pCDNA empty vector; GAPDH was also detected as a loading control. B. Cell growth curves in the VSMCs transfected with different combinations. C. The protein expression of cyclin D1 was detected in the VSMCs transfected with different combinations using western blot. D. The mRNA expression of cyclin D1 was detected in the VSMCs transfected with different combinations using real-time PCR.
Discussion
Accumulating evidences have suggested that ox-LDL is the major risk factor of atherosclerosis [20]. In the early stage of atherosclerosis, ox-LDL permeates into sub-endothelial layer through injured endothelium and triggers a series of pathological events [21]. As we know, VSMCs proliferation is the key events in the pathogenesis of atherosclerosis [22]. Ox-LDL plays a critical role in the development of atherosclerosis by stimulating the proliferation of VSMCs within the vessel wall [4,23]. However, the detailed mechanisms how ox-LDL regulate abnormal proliferation of the VSMC has not been fully elucidated. Our results showed that ox-LDL repressed miR-141 expression. Moreover, overexpression of miR-141 inhibited VSMCs proliferation and downregulation of miR-141 promoted the VSMCs proliferation. Furthermore, overexpression of PAPPA impaired the miR-141-induced inhibition of proliferation in VSMCs. These findings suggest that ox-LDL might regulate abnormal proliferation of the VSMC through inhibiting miR-141. MiR-141 might be potential novel therapeutic targets in treatment of vascular disease.
Previous studies using miRNA microarray showed that 25 miRNAs were changed with more than two-fold in the human coronary artery smooth muscle cells (hCASMC) treated ox-LDL, including miR-141 [3]. In addition, miR-141 was described to suppress renal cell carcinoma and human stromal (mesenchymal) stem cell proliferation [24,25]. However, the role and function of miR-141 in the VSMCs remain unknown. In our study, ox-LDL can inhibited the expression of miR-141 and inhibition of miR-141 promoted VSMCs proliferation. Thus, it is possible that ox-LDL induce VSMC proliferation partly through inhibiting the expression of miR-141.
We further explored the molecular mechanisms underlying the role of miR-141. TargetScan analysis showed a complementation of miR141 miRNA with the PAPPA 3’UTR (Figure 3A). In addition, the protein level but not the mRNA level of PAPPA was inhibited miR-141. Furthermore, the luciferase reporter constructs containing the 3’UTR of PAPPA mRNA cofirmed the suppression of PAPPA by miR-141 in VSMCs. Taken together, miR-141 downregulates PAPPA by targeting the 3’UTR and inducing translation repression of the gene.
PAPPA is a novel zinc metalloproteinase and played significant roles in cardiovascular disease [26,27]. PAPPA is mainly produced by the placental syncytiotrophoblast during pregnacy, as well as the fibroblasts, osteoblasts and VSMCs [28-31]. In chronic stable angina patients, high PAPPA levels correlate with the extent of coronary artery stenosis [32,33]. Moreover, PAPPA levels are increased in patients with ACS and represent a marker of adverse events in both ACS and CSA patients [33]. In the present study, miR-141 might also be involved in the regulation of atherosclerosis by targeting PAPPA. However, the association of miR-141 with PAPPA is yet to be explored. Nevertheless, such kind of studies might lead to the future development of an alternative strategy that targeting miR-141 in treatment of atherosclerosis.
In conclusion, our results indicated that ox-LDL could inhibit the expression of miR-141. And the inhibition of miR-141 promoted the proliferation of VSMC by targeting PAPPA. These results help us to understand the mechanisms of the pro-atherogenic effects of ox-LDL. MiR-141 may play an important role in the VSMCs proliferation and atherosclerosis induced by ox-LDL. However, the detailed mechanisms of regulation in the proliferation of VSMCs induced by ox-LDL need further investigation.
Acknowledgements
This work was supported by the Shandong Province ‘Taishan Scholar’ Construction Project Awards and Shandong Natural Science Foundation (Grant Numbers: ZR2012HL14).
Disclosure of conflict of interest
None.
References
- 1.Aghabozorg Afjeh SS, Ghaderian SM. The role of microRNAs in cardiovascular disease. Int J Mol Cell Med. 2013;2:50–57. [PMC free article] [PubMed] [Google Scholar]
- 2.Chen KC, Hank Juo SH. MicroRNAs in atherosclerosis. Kaohsiung J Med Sci. 2012;28:631–640. doi: 10.1016/j.kjms.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Sun Y, Chen D, Cao L, Zhang R, Zhou J, Chen H, Li Y, Li M, Cao J, Wang Z. MiR-490-3p modulates the proliferation of vascular smooth muscle cells induced by ox-LDL through targeting PAPP-A. Cardiovasc Res. 2013;100:272–279. doi: 10.1093/cvr/cvt172. [DOI] [PubMed] [Google Scholar]
- 4.Pirillo A, Norata GD, Catapano AL. LOX-1, OxLDL, and atherosclerosis. Mediators Inflamm. 2013;2013:152786. doi: 10.1155/2013/152786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang W, Gao B, Fu P, Xu S, Qian Y, Fu Q. The miRNAs in the pathgenesis of osteosarcoma. Front Biosci (Landmark Ed) 2013;18:788–794. doi: 10.2741/4142. [DOI] [PubMed] [Google Scholar]
- 6.Banaudha KK, Verma M. The role of microRNAs in the management of liver cancer. Methods Mol Biol. 2012;863:241–251. doi: 10.1007/978-1-61779-612-8_14. [DOI] [PubMed] [Google Scholar]
- 7.Ueda T, Volinia S, Okumura H, Shimizu M, Taccioli C, Rossi S, Alder H, Liu CG, Oue N, Yasui W, Yoshida K, Sasaki H, Nomura S, Seto Y, Kaminishi M, Calin GA, Croce CM. Relation between microRNA expression and progression and prognosis of gastric cancer: a microRNA expression analysis. Lancet Oncol. 2010;11:136–146. doi: 10.1016/S1470-2045(09)70343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han M, Toli J, Abdellatif M. MicroRNAs in the cardiovascular system. Curr Opin Cardiol. 2011;26:181–189. doi: 10.1097/HCO.0b013e328345983d. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Zhao L, He X, Yang T, Yang K. MiR-21 inhibits c-Ski signaling to promote the proliferation of rat vascular smooth muscle cells. Cell Signal. 2014;26:724–729. doi: 10.1016/j.cellsig.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Mackenzie NC, Staines KA, Zhu D, Genever P, Macrae VE. miRNA-221 and miRNA-222 synergistically function to promote vascular calcification. Cell Biochem Funct. 2014;32:209–216. doi: 10.1002/cbf.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X, Cheng Y, Zhang S, Lin Y, Yang J, Zhang C. A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res. 2009;104:476–487. doi: 10.1161/CIRCRESAHA.108.185363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang YS, Li SH, Guo J, Mihic A, Wu J, Sun L, Davis K, Weisel RD, Li RK. Role of miR-145 in cardiac myofibroblast differentiation. J Mol Cell Cardiol. 2014;66:94–105. doi: 10.1016/j.yjmcc.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Boucher JM, Peterson SM, Urs S, Zhang C, Liaw L. The miR-143/145 cluster is a novel transcriptional target of Jagged-1/Notch signaling in vascular smooth muscle cells. J Biol Chem. 2011;286:28312–28321. doi: 10.1074/jbc.M111.221945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lovren F, Pan Y, Quan A, Singh KK, Shukla PC, Gupta N, Steer BM, Ingram AJ, Gupta M, Al-Omran M, Teoh H, Marsden PA, Verma S. MicroRNA-145 targeted therapy reduces atherosclerosis. Circulation. 2012;126:S81–90. doi: 10.1161/CIRCULATIONAHA.111.084186. [DOI] [PubMed] [Google Scholar]
- 15.Zhang C. MicroRNA-145 in vascular smooth muscle cell biology: a new therapeutic target for vascular disease. Cell Cycle. 2009;8:3469–3473. doi: 10.4161/cc.8.21.9837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elia L, Quintavalle M, Zhang J, Contu R, Cossu L, Latronico MV, Peterson KL, Indolfi C, Catalucci D, Chen J, Courtneidge SA, Condorelli G. The knockout of miR-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: correlates with human disease. Cell Death Differ. 2009;16:1590–1598. doi: 10.1038/cdd.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quintavalle M, Elia L, Condorelli G, Courtneidge SA. MicroRNA control of podosome formation in vascular smooth muscle cells in vivo and in vitro. J Cell Biol. 2010;189:13–22. doi: 10.1083/jcb.200912096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding Z, Wang X, Schnackenberg L, Khaidakov M, Liu S, Singla S, Dai Y, Mehta JL. Regulation of autophagy and apoptosis in response to ox-LDL in vascular smooth muscle cells, and the modulatory effects of the microRNA hsa-let-7 g. Int J Cardiol. 2013;168:1378–1385. doi: 10.1016/j.ijcard.2012.12.045. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Chen N, Zhang J, Tong Y. Hsa-let-7g miRNA targets caspase-3 and inhibits the apoptosis induced by ox-LDL in endothelial cells. Int J Mol Sci. 2013;14:22708–22720. doi: 10.3390/ijms141122708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin B, Xiao B, Liang D, Li Y, Jiang T, Yang H. MicroRNA let-7c inhibits Bcl-xl expression and regulates ox-LDL-induced endothelial apoptosis. BMB Rep. 2012;45:464–469. doi: 10.5483/BMBRep.2012.45.8.033. [DOI] [PubMed] [Google Scholar]
- 21.Maiolino G, Rossitto G, Caielli P, Bisogni V, Rossi GP, Calo LA. The role of oxidized lowdensity lipoproteins in atherosclerosis: the myths and the facts. Mediators Inflamm. 2013;2013:714653. doi: 10.1155/2013/714653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Wang Y, Wang X, Eisner GM, Asico LD, Jose PA, Zeng C. Insulin promotes vascular smooth muscle cell proliferation via microRNA-208-mediated downregulation of p21. J Hypertens. 2011;29:1560–1568. doi: 10.1097/HJH.0b013e328348ef8e. [DOI] [PubMed] [Google Scholar]
- 23.Khaidakov M, Mehta JL. Do atherosclerosis and obesity-associated susceptibility to cancer share causative link to oxLDL and LOX-1? Cardiovasc Drugs Ther. 2011;25:477–487. doi: 10.1007/s10557-011-6330-8. [DOI] [PubMed] [Google Scholar]
- 24.Chen X, Wang X, Ruan A, Han W, Zhao Y, Lu X, Xiao P, Shi H, Wang R, Chen L, Chen S, Du Q, Yang H, Zhang X. miR-141 is a key regulator of renal cell carcinoma proliferation and metastasis by controlling EphA2 expression. Clin Cancer Res. 2014;20:2617–2630. doi: 10.1158/1078-0432.CCR-13-3224. [DOI] [PubMed] [Google Scholar]
- 25.Qiu W, Kassem M. miR-141-3p inhibits human stromal (mesenchymal) stem cell proliferation and differentiation. Biochim Biophys Acta. 2014;1843:2114–2121. doi: 10.1016/j.bbamcr.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Consuegra-Sanchez L, Fredericks S, Kaski JC. Pregnancy-associated plasma protein-A (PAPP-A) and cardiovascular risk. Atherosclerosis. 2009;203:346–352. doi: 10.1016/j.atherosclerosis.2008.07.042. [DOI] [PubMed] [Google Scholar]
- 27.Consuegra-Sanchez L, Fredericks S, Kaski JC. Pregnancy-associated plasma protein A: Has this biomarker crossed the boundary from research to clinical practice? Drug News Perspect. 2009;22:341–348. doi: 10.1358/dnp.2009.22.6.1395257. [DOI] [PubMed] [Google Scholar]
- 28.Gruber HE, Hoelscher G, Ingram JA, Hanley EN Jr. Immunolocalization and biochemical evidence of pregnancy-associated plasma protein A in the intervertebral disc. Spine (Phila Pa 1976) 2008;33:E447–454. doi: 10.1097/BRS.0b013e318178e642. [DOI] [PubMed] [Google Scholar]
- 29.Thorn EM, Khan IA. Pregnancy-associated plasma protein-A: an emerging cardiac biomarker. Int J Cardiol. 2007;117:370–372. doi: 10.1016/j.ijcard.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Resch ZT, Oxvig C, Bale LK, Conover CA. Stress-activated signaling pathways mediate the stimulation of pregnancy-associated plasma protein-A expression in cultured human fibroblasts. Endocrinology. 2006;147:885–890. doi: 10.1210/en.2005-0908. [DOI] [PubMed] [Google Scholar]
- 31.Bayes-Genis A, Schwartz RS, Lewis DA, Overgaard MT, Christiansen M, Oxvig C, Ashai K, Holmes DR Jr, Conover CA. Insulin-like growth factor binding protein-4 protease produced by smooth muscle cells increases in the coronary artery after angioplasty. Arterioscler Thromb Vasc Biol. 2001;21:335–341. doi: 10.1161/01.atv.21.3.335. [DOI] [PubMed] [Google Scholar]
- 32.Liu ZY, Zhang JY, Sun TW, Zhang YJ, Zhang L, Wang LX. Levels of pregnancy-associated plasma protein A in patients with coronary artery disease. Clin Invest Med. 2008;31:E85–89. doi: 10.25011/cim.v31i2.3368. [DOI] [PubMed] [Google Scholar]
- 33.You L, Li L, Zhang F, Xu Q, Ren J. A pilot study of the clinical relevance of the relationship between the serum level of pregnancyassociated plasma protein A and the degree of acute coronary syndrome. J Int Med Res. 2010;38:625–632. doi: 10.1177/147323001003800225. [DOI] [PubMed] [Google Scholar]


