Abstract
Congenital thyroid hypofunction can cause a variety of developmental disorders. Hippocampus is an important structure participating in the cognitive activities. Neural function damage is able to induce hippocampal neuron apoptosis. As a miRNA expressed specifically and abundantly in brain tissue, miR-124 has protective effect to neuron apoptosis caused by cerebral apoplexy. However, its role in neuron apoptosis caused by thyroid hypofunction is still unclear. The rats were divided into four groups including normal group, thyroid hypofunction group, miR-124 negative control group, and miR-124 mimics group. Propylthiouracil (50 mg/d) was injected to the stomach to the rats with 15 d pregnancy till the newborn rats were born. Inducing the thyroid hypofunction rat model and then injecting miR-124 mimics to ventricle. Serum TSH, FT3 and FT4 were detected to confirm the model. Immunohistochemistry was carried out to calculate neuron number. Tunel assay was used to detect neuron apoptosis. Western blot was applied to detect apoptosis related protein Caspase-3, Bcl-2 and Bax expression. After brain injection miR-124 mimics, hippocampal neuron number and morphology both improved in 15 d newborn mice compared with thyroid hypofunction group. Tunel staining found positive neurons reduced, which indicated that miR-124 can inhibit hippocampal neuron apoptosis in thyroid hypofunction rats. Further Western blot results revealed that apoptosis inhibition might be related to down-regulating activated Caspase-3 and Bax levels, and up-regulating tumor-suppressor gene Bcl-2 expression. MiR-124 can protect neuron apoptosis in thyroid hypofunction rat.
Keywords: miR-124, thyroid hypofunction, hippocampal neuron, apoptosis
Introduction
MicroRNAs is a kind of non-coding single stranded small RNAs about 18-25 nucleotides in length [1]. Mature miRNA has regulating effect to mRNA by complementary binding with target mRNAs [2]. Recent research suggested that miRNA widely participated in various systems growth regulation including nervous system. Thyroid hormone plays an important role in the mammalian brain development. Hypothyroidism is a type of general endocrine disease caused by a variety of reasons that lead to thyroid hormone dyssynthesis, paracrisis or lack of biological effects. Among them, congenital thyroid hypofunction can cause a variety of developmental disorders [3], while hippocampal structure is an important center participating cognitive activities. Previous preliminary experiment data and other research results showed that thyroid hypofunction in the perinatal period may induce hippocampal neuron apoptosis in rats. As a miRNA expressed specifically and abundantly in brain tissue, miR-124 expression in central nervous system and nerve cells was 100 times higher than in other parts [4]. It has been confirmed that miR-124 could protect neuron apoptosis caused by cerebral apoplexy [5]. However, its role in neuron apoptosis caused by thyroid hypofunction is still unclear. At present, apoptosis regulation is often closely related to Caspase-3, Bcl-2 and Bax. In the preliminary experiment, we observed miR-124 down-regulation and neuron apoptosis in thyroid hypofunction of rats in the perinatal period but the relationship is unclear. This study aimed to clarify mechanism of miR-124 on protecting hippocampal neuron apoptosis on thyroid hypofunction rat model.
Materials and methods
Main reagents
MiR-124 mimics and negative control were both bought from Genepharma Biological co., LTD. (Shanghai, China). Brain stereotaxic apparatus was purchased from Stoelting Company (Shanghai, China). Rat thyroid stimulating hormone (TSH), FT3 and FT4 chemiluminescence immunoassay kits were got from Baoman Biological Technology co., LTD. (Shanghai, China). MiRNAs qPCR Quantitation Kit was bought in Invitrogen (CA). RNA extraction reagent was bought in Takara (Dalian, China). PVDF membrane and neuN antibody were got from Milipore (Hong Kong, China). Total protein extraction kit, BCA quantitative kit and other western blot reagents were all purchased from Beyotime co., LTD. (Shanghai, China). Immunohistochemistry kit was from Zsbio co., LTD. (Beijing, China). Tunel kit was bought from Roche (Shanghai, China). Caspase-3, Bcl-2 and Bax antibodies were purchased from Proteintech Company (Wuhan, China).
Modeling
Pregnant SD rats were provided by Harbin Medical University experimental animal center. Research were approved by the Animal Ethics Committee of the Second Affiliated Hospital of Harbin Medical University. The rats were divided into four groups including normal group, thyroid hypofunction group, miR-124 negative control group, and miR-124 mimics group. Propy-lthiouracil (50 mg/d) was injected once a day to the stomach to the rats with 15 d pregnancy till the newborn rats were born. Inducing the thyroid hypofunction rat model and then injecting miR-124 mimics to ventricle [6]. Newborn rats’ eye open time, weight and activity were observed.
Serum TSH, FT3 and FT4 detection
Serum TSH, FT3 and FT4 were detected after birth, 1st, 5th, 10th, and 15th day to confirm the model.
Real-time PCR
After anesthetized by 2% pentobarbital sodium abdominal injection, the rats were fixed on the brain stereotaxic apparatus. The skull was opened at 1.0 mm after the former fontanelle and 1.7 mm near the midline. A micro syringe was injected vertically for 3.8 mm at 15 μm/s, and 5 μL 1 nmol/L miR-124 mimics solution was injected [7]. Newborn rats were killed and hippocampus was collected at 15th after the birth. Total RNA was extracted from the tissue according to the manual. The cDNA was synthesized through reverse transcription. The primers used for reverse transcription were as follows: MiR-124’-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACGGCATT-3’; U6, 5’-AAAATATGGAACGCTTCACGAATTTG-3’. The primers used for PCR were as follows: miR-124, forward 5’-GCTAAGGCACGCGGTG-3’, reverse 5’-GTGCAGGGTCCGAGGT-3’; U6, forward 5’-CTCGCTTCGGCAGCACATATACT-3’, reverse 5’-ACGCTTCACGAATTTGCGTGTC-3’. The cycling conditions for PCR consisted of an initial, single cycle of 5 min at 95°C, followed by 40 cycles of 30 s at 95°C, 30 s at 60°C, and 30 s at 72°C. PCR amplifications were performed in three duplicates for each sample. Gene expression levels were quantified relative to the expression of U6 using an optimized comparative Ct (ΔΔCt) value method.
Immunohistochemistry
After conventional paraffin embedding, sectioning, dewaxing, and hydrating steps, hippocampal tissue received antigen repair by citric acid buffer (pH = 10.0). After natural cooling, neuN antibody was added for incubation. Brown represented positive staining. Neuron number was calculated.
Tunel assay
After conventional paraffin embedding, sectioning, dewaxing, and hydrating steps, hippocampal tissue section was treated with 0.3% Triton for 10 min and incubated with Tunel at 37°C for 1 h. After washed by PBS, the section was observed under fluorescence microscope.
Western blot
After transfection, the cells were digested with lysis buffer. Total protein was separated by denaturing 10% SDS-polyacrylamide gel electrophoresis. After incubated with Caspase-3, Bcl-2, and Bax primary antibodies, the membrane was detected with chemiluminiscence. Protein levels were normalized to β-actin and changes were determined.
Statistical analysis
All statistical analyses were performed using SPSS10.0 software. Numerical data were presented as means and standard deviation. Differences between multiple groups were analyzed by one-way ANOVA and SNK-Q test.
Results
Animal model
Newborn rats in normal group presented good activity with rapid weight gain and normal fur developing. Rats in other groups grew slower than the control with small figure, short tail, less activity, and sparse fur. Serum TSH, FT3, and FT4 expression in newborn rats at 1st, 5th, 10th, 15th day were listed in group 1, 2, and 3. Groups of young rats after the birth of one day, five days, ten days, fifteen days of serum TSH (Table 1), FT3 (Table 2) and FT4 (Table 3) are shown in Tables 1, 2 and 3. Serum FT4 levels in each day was lower in experimental groups than in control (2.8 pmol/L); serum FT3 also decreased in different days; while TSH level increased significantly in experimental groups than in control.
Table 1.
Serum TSH level (mU/L)
| 1st day | 5th day | 10th day | 15th day | |
|---|---|---|---|---|
| Normal control | 1.27±0.62 | 1.36±0.45 | 1.24±0.82 | 1.42±0.33 |
| Model | 3.88±0.23** | 3.25±0.30** | 3.73±0.71** | 3.88±0.42** |
| miR-124 negative | 3.17±0.52** | 3.22±1.21** | 4.56±1.23** | 4.52±1.09** |
| miR-124 mimics | 4.13±0.46** | 4.21±0.62** | 4.25±0.59** | 4.71±0.79** |
P<0.05, compared with control;
##P<0.05, compared with thyroid hypofunction group.
Table 2.
Serum FT3 level (pmol/L)
| 1st day | 5th day | 10th day | 15th day | |
|---|---|---|---|---|
| Normal control | 4.12±0.33 | 6.13±0.45 | 7.54±0.47 | 8.42±0.73 |
| Model | 2.11±0.54** | 3.25±0.47** | 4.73±0.72** | 4.21±0.33** |
| miR-124 negative | 2.17±0.43** | 3.42±0.54** | 4.56±0.57** | 4.52±0.44** |
| miR-124 mimics | 2.23±0.36** | 3.12±0.72** | 4.82±0.49** | 4.71±0.72** |
P<0.05, compared with control;
##P<0.05, compared with thyroid hypofunction group.
Table 3.
Serum FT4 level (pmol/L)
| 1st day | 5th day | 10th day | 15th day | |
|---|---|---|---|---|
| Normal control | 5.12±0.42 | 10.23±0.75 | 17.53±1.23 | 19.26±1.34 |
| Model | 2.52±0.56** | 2.64±0.65** | 4.23±0.74** | 2.54±0.56** |
| miR-124 negative | 2.46±0.64** | 2.78±0.48** | 4.56±1.22** | 2.64±0.74** |
| miR-124 mimics | 2.56±0.85** | 2.85±0.64** | 4.69±1.24** | 2.85±1.24** |
P<0.05, compared with control.
MiR-124 expression in hippocampus tissue
MiR-124 is highly expressed in the brain and has been confirmed to regulate a variety of diseases. It is called the decision factor of ventricle region neurons [8]. To observe its correlation with neuron apoptosis in the rats with thyroid hypofunction, we used real-time PCR to detect its expression in newborn rats at 15 days (Figure 1). MiR-124 level decreased obviously in rats with thyroid hypofunction. After treated with miR-124 mimics, its expression increased significantly.
Figure 1.
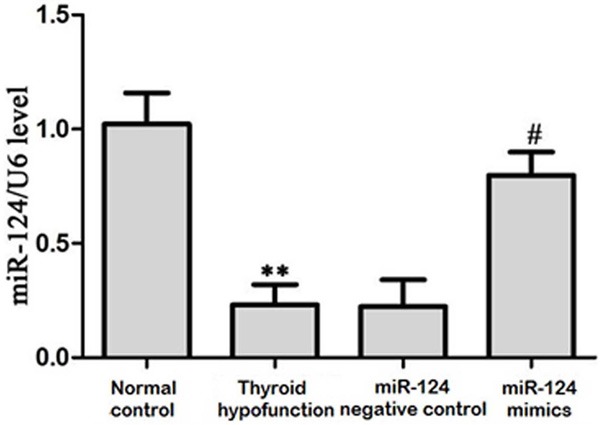
MiR-124 expression in hippocampus tissue. **P<0.05, compared with control; ##P<0.05, compared with thyroid hypofunction group.
NeuN staining in rats at 15 days old
Hippocampus neuN staining clearly revealed the number and morphology of neuron. Compared with normal control, the positive neuron number reduced significantly in rats with thyroid hypofunction group, and the cell contour was unclear. After treated with miR-124 mimics, neuron number and morphology improved obviously (Figure 2).
Figure 2.
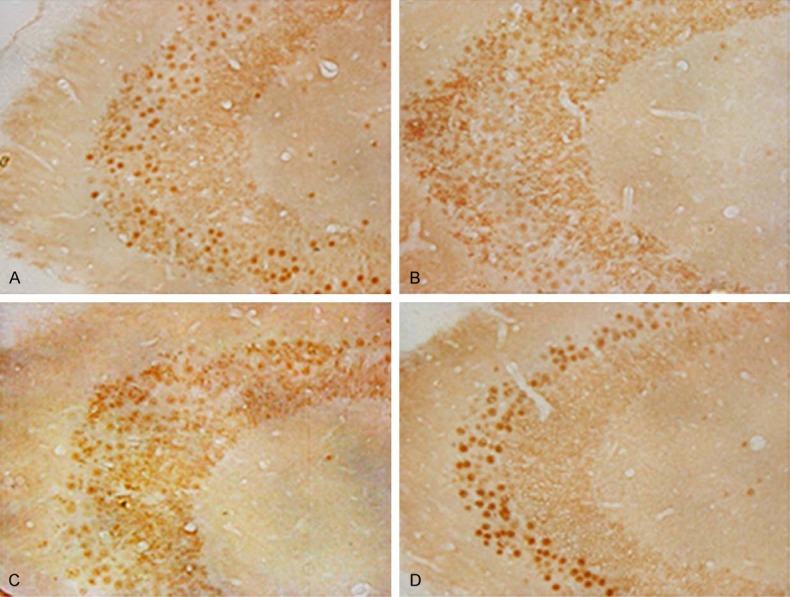
Hippocampus neuN staining in rats at 15 days old. A. Normal control; B. Thyroid hypofunction group; C. miR-124 negative control group; D. miR-124 mimics group.
Hippocampus Tunel staining in rats at 15 days old
Tunel staining revealed rat hippocampus neuron shrivel and positive cells scattered distribution. Granulosa cell apoptosis number at CA1 area was obviously larger in thyroid hypofunction group and miR-124 negative control group when compared with control, while apoptotic cell number declined after treated with miR-124 mimics (Figure 3).
Figure 3.
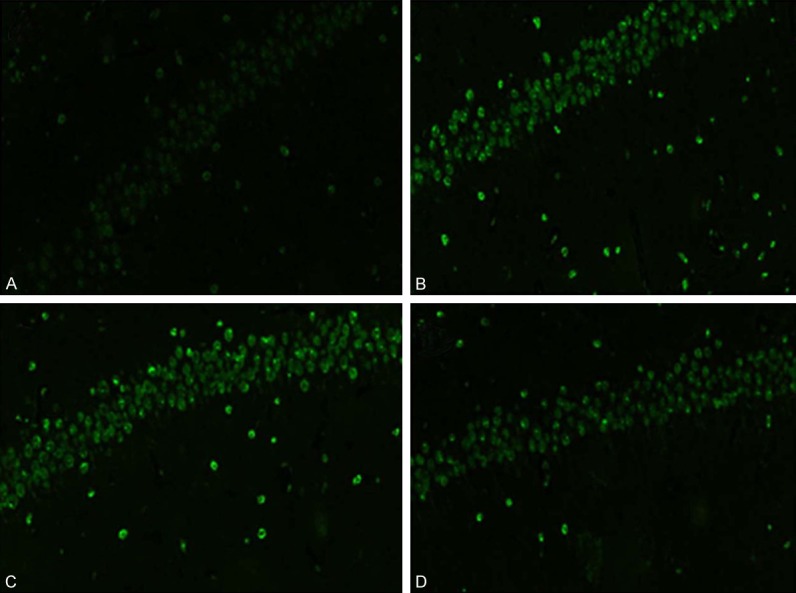
Hippocampus Tunel staining in rats at 15 days old. A. Normal control; B. Thyroid hypofunction group; C. miR-124 negative control group; D. miR-124 mimics group.
Caspase-3, Bcl-2, Bax protein expression change
Activated caspase-3 expression increased significantly in thyroid hypofunction group and miR-124 negative control group compared with normal control, while it declined after treated with miR-124 mimics. MiR-124 mimics could elevate Bcl-2 expression in model group and miR-124 negative control group. MiR-124 mimics also can reduce Bax expression (Figure 4).
Figure 4.
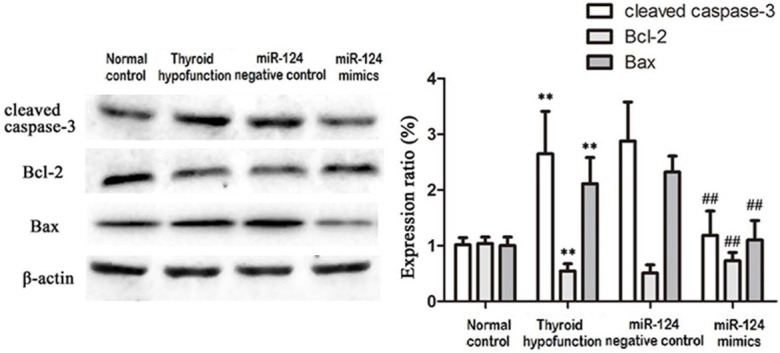
Caspase-3, Bcl-2, Bax protein expression in rats at 15 days old. **P<0.05, compared with control; ##P<0.05, compared with thyroid hypofunction group.
Discussion
Iodine deficiency in lactation is one of the important reasons that cause offspring irreversible central nerve damage. Thyroid hormone can directly act on neural base layer in chromosome and transcription level, which lead to brain morphological and biochemical changes, and brain function damage [9]. Iodine deficiency in fetal period or thyroid hypofunction after the birth is an important reason causing language, cognition, behavior, and movement disorder. Clinical phenomenon indicated that a lot of developmental delay and nervous system defects were caused by thyroid dysfunction [10]. At the critical moment of neural development, even a short time of thyroid hormone deficiency can lead to on hippocampal neurons function damage or irreversible injury [11], which often characterized by hippocampal neuron apoptotic number elevation [12].
MiRNAs are small, non-coding RNA that can regulate mRNA expression [13]. MiR-124 is highly expressed in nerve cells [14]. Numerous in vitro studies showed that miR-124 has a very important role in deciding neuronal fate [15,16]. Experiments revealed that miR-124 has different roles in different tissue or physiological condition. For example, miR-124 showed inhibitory effect on colorectal cancer [17] and osteosarcoma [18], suggesting to be a widespread anticancer agent [19]. On the contrary, it showed opposite effect in many pathological conditions leading to apoptosis. For instance, it can protect liver cell apoptosis caused by stroke [20] or oxidative stress [21]. Previous results found that hippocampal neurons apoptosis appeared in rats with thyroid hypofunction in rats, while the role of miR-124 is still unclear.
Multiple methods could detect cell apoptosis, including morphology, biochemistry and flow cytometry, etc. The mainstream method is specific neuN staining observed by light or electron microscope. Terminal-deoxynucleoitidyl Transferase Mediated Nick End Labeling (Tunel) assay is a common method to detect apoptosis specifically. Furthermore, we applied Western blot to detect apoptosis related proteins Caspase 3, Bcl-2, and Bax.
In this experiment, we found that miR-124 down-regulated in rats with thyroid hypofunction, along with neuron apoptosis number elevation. Combining with a variety of microRNAs protective role to nerve apoptosis in the literature [22] and miR-124 protective effect on neuron apoptosis caused by stroke [5], we speculated that miR-124 also plays a protective role in rats with thyroid hypofunction. Neurons neuN staining revealed neuron number reduction and cell contour changes in the thyroid hypofunction group, while miR-124 mimics can reverse such change to some extent. Similarly, Tunel assay showed that neuron apoptosis could be improved by miR-124 mimics in rats with thyroid hypofunction. Caspase-3 is the main terminal enzyme in the process of cell apoptosis, and its upregulation could be reversed by miR-124 mimics. MiR-124 mimics intervention can increase Bcl-2 and reduce Bax. We believed that miR-124 can up-regulate antiapoptotic proteins expression, which may be the molecular mechanism of miR-124 protection on the neural apoptosis.
Taken together, our results suggested that miR-124 can protect neuron apoptosis in rats caused by thyroid hypofunction. As a new kind of antiapoptotic regulator, miR-124 could be treated as nerve protection material in thyroid hypofunction caused nerve damage. Similarly, it is still unclear about the role of miRNA in thyroid hypofunction and need further investigation.
Acknowledgements
Research supported by The Education Department of Heilongjiang province science and technology projects 12541286 and Postdoctoral Science Foundation funded project of Heilongjiang Province LBH-Z13140.
Disclosure of conflict of interest
None.
References
- 1.Zaravinos A. The Regulatory Role of MicroRNAs in EMT and Cancer. J Oncol. 2015;2015:865816. doi: 10.1155/2015/865816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shukla GC, Singh J, Barik S. MicroRNAs: Processing, Maturation, Target Recognition and Regulatory Functions. Mol Cell Pharmacol. 2011;3:83–92. [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrara AM, Onigata K, Ercan O, Woodhead H, Weiss RE, Refetoff S. Homozygous thyroid hormone receptor beta-gene mutations in resistance to thyroid hormone: three new cases and review of the literature. J Clin Endocrinol Metab. 2012;97:1328–1336. doi: 10.1210/jc.2011-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mishima T, Mizuguchi Y, Kawahigashi Y, Takizawa T, Takizawa T. RT-PCR-based analysis of microRNA (miR-1 and -124) expression in mouse CNS. Brain Res. 2007;1131:37–43. doi: 10.1016/j.brainres.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 5.Sun Y, Gui H, Li Q, Luo ZM, Zheng MJ, Duan JL, Liu X. MicroRNA-124 protects neurons against apoptosis in cerebral ischemic stroke. CNS Neurosci Ther. 2013;19:813–819. doi: 10.1111/cns.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fabian ID, Rosner M, Fabian I, Vishnevskia-Dai V, Zloto O, Maman ES, Cohen K, Ellis M, Lin HY, Hercbergs A, Davis PJ, Ashur-Fabian O. Low thyroid hormone levels improve survival in murine model for ocular melanoma. Oncotarget. 2015;6:11038–46. doi: 10.18632/oncotarget.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu LJ, Ouyang YB, Xiong X, Stary CM, Giffard RG. Post-stroke treatment with miR-181 antagomir reduces injury and improves long-term behavioral recovery in mice after focal cerebral ischemia. Exp Neurol. 2015;264:1–7. doi: 10.1016/j.expneurol.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akerblom M, Sachdeva R, Barde I, Verp S, Gentner B, Trono D, Jakobsson J. MicroRNA-124 is a subventricular zone neuronal fate determinant. J Neurosci. 2012;32:8879–8889. doi: 10.1523/JNEUROSCI.0558-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kapoor R, Fanibunda SE, Desouza LA, Guha SK, Vaidya VA. Perspectives on thyroid hormone action in adult neurogenesis. J Neurochem. 2015;133:599–616. doi: 10.1111/jnc.13093. [DOI] [PubMed] [Google Scholar]
- 10.Mastorakos G, Karoutsou EI, Mizamtsidi M, Creatsas G. The menace of endocrine disruptors on thyroid hormone physiology and their impact on intrauterine development. Endocrine. 2007;31:219–237. doi: 10.1007/s12020-007-0030-y. [DOI] [PubMed] [Google Scholar]
- 11.Guo Y, Wan SY, Zhong X, Zhong MK, Pan TR. Levothyroxine replacement therapy with vitamin E supplementation prevents the oxidative stress and apoptosis in hippocampus of hypothyroid rats. Neuro Endocrinol Lett. 2014;35:684–690. [PubMed] [Google Scholar]
- 12.Fu AL, Zhou CY, Chen X. Thyroid hormone prevents cognitive deficit in a mouse model of Alzheimer’s disease. Neuropharmacology. 2010;58:722–729. doi: 10.1016/j.neuropharm.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 13.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissuespecific microRNAs from mouse. Curr Biol. 2002;12:735–739. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 15.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 16.Silber J, Lim DA, Petritsch C, Persson AI, Maunakea AK, Yu M, Vandenberg SR, Ginzinger DG, James CD, Costello JF, Bergers G, Weiss WA, Alvarez-Buylla A, Hodgson JG. miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med. 2008;6:14. doi: 10.1186/1741-7015-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taniguchi K, Sugito N, Kumazaki M, Shinohara H, Yamada N, Nakagawa Y, Ito Y, Otsuki Y, Uno B, Uchiyama K, Akao Y. MicroRNA-124 inhibits cancer cell growth through PTB1/PKM1/PKM2 feedback cascade in colorectal cancer. Cancer Lett. 2015;363:17–27. doi: 10.1016/j.canlet.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 18.Geng S, Zhang X, Chen J, Liu X, Zhang H, Xu X, Ma Y, Li B, Zhang Y, Bi Z, Yang C. The tumor suppressor role of miR-124 in osteosarcoma. PLoS One. 2014;9:e91566. doi: 10.1371/journal.pone.0091566. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Li X, Yu Z, Li Y, Liu S, Gao C, Hou X, Yao R, Cui L. The tumor suppressor miR-124 inhibits cell proliferation by targeting STAT3 and functions as a prognostic marker for postoperative NSCLC patients. Int J Oncol. 2015;46:798–808. doi: 10.3892/ijo.2014.2786. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Li F, Zhao S, Luo Y, Kang J, Zhao H, Yan F, Li S, Ji X. MicroRNA-124-mediated regulation of inhibitory member of apoptosis-stimulating protein of p53 family in experimental stroke. Stroke. 2013;44:1973–1980. doi: 10.1161/STROKEAHA.111.000613. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Yi S, Deng Y, Cheng J, Wu X, Liu W, Tai Y, Chen G, Zhang Q, Yang Y. MiR-124 protects human hepatic L02 cells from H2O2-induced apoptosis by targeting Rab38 gene. Biochem Biophys Res Commun. 2014;450:148–153. doi: 10.1016/j.bbrc.2014.05.085. [DOI] [PubMed] [Google Scholar]
- 22.Yin KJ, Deng Z, Huang H, Hamblin M, Xie C, Zhang J, Chen YE. miR-497 regulates neuronal death in mouse brain after transient focal cerebral ischemia. Neurobiol Dis. 2010;38:17–26. doi: 10.1016/j.nbd.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]


