Abstract
As a main cause of cardiac hypertrophy, myocardial hypertrophy includes the proliferation and enlongation of myocardial cell, resulting in abnormally cardiac enlargement. However, the pathogenesis and the molecular mechanism that regulate gene expression of myocardial hypertrophy remain incompletely understood. MiRNAs were deemed as an important molecules involved in a variety of pathological processes. MiR-96 has been reported being associated with the tumor proliferation, but whether miR-96 is involved in cardiac hypertrophy remains uncertain. In this study, we have confirmed that, as the myocardial hypertrophy gene, mTOR was a target gene of miR-96, who would promote the occurrence of myocardial hypertrophy. Thus, we got the conclusion that miR-96 could promote myocardial hypertrophy by inhibiting mTOR, miR-96 and mTOR were negatively correlated.
Keywords: MiR-96, myocardial hypertrophy, mTOR, target gene
Introduction
Cardiac hypertrophy is a common pathological changes of various kinds of cardiovascular diseases [1,2]. The major identifying characteristic of cardiac hypertrophy is the abnormal hypertrophic growth, involving the proliferation of cardiac myocytes and the expansion of cell size [3]. The understanding its mechanism may help with the prevention and treatment of this disease. Although tremendous efforts have been made to unravel the molecular mechanism of physiologic hypertrophy, the molecular mechanisms that regulate gene expression in cardiac hypertrophy remain incompletely understood.
MiRs are very small non-coding post transcription regulating RNAs, and have been shown to exert a critical role in regulating mRNA in heart diseases and cell apoptosis [4]. Growing evidences regarding microRNA expression profiles in a myocardial hypertrophy model suggest that altered expression of micro-RNAs are related to normal heart tissue [5-10]. Zhang et al published that miR-26 regulates pathological structural changes in the rat heart, and implicate the potential application of miR-26 in diagnosis and therapy of cardiac hypertrophy [11].
MiR-96 has been reported being associated with the tumor proliferation, including a variety of cancers, such as urothelial carcinoma, colorectal cancer, bladder cancer and so on [12-15]. Meanwhile, mTOR was reported as a kind of signaling molecules, the regulation and functions of mTOR had revealed the crucial involvement of this signaling pathway in the onset and progression of diabetes, cancer and ageing, as well as the cardiac growth and function [16-19].
In this study, we would investigate the expression of miR-96 in the mice cardiac hypertrophy model. Furthermore, to further understand the molecular mechanism, we sought to determine the target gene and analysis the correlation between them.
Material and methods
Animal model
Sixteen male Wistar rats (100-120 g) were purchased from the Guangdong Medical Laboratory Animal Center (Guangzhou, China) and were randomly divided into control and transverse abdominal aortic constriction (TAAC) groups. Rats in the TAAC group were anesthetized with ketamine (80 mg/kg, IP) and xylazine (5 mg/kg, IP). All the surgical procedures were performed as previously described. 14 Electro cardiogram and respiratory rate were recorded on a data acquisition system (MP 150; Biopac System). The suprarenal abdominal aorta was isolated and tightened with a 4-0 nylon suture against a 5-gauge needle (external diameter = 0.5 mm). After removing the needle, the incision was closed. Rats in the control group underwent a sham operation as the surgical procedures in TAAC group except aortic constriction. All the experimental protocols followed the Guidelines of the Care and Use of Laboratory Animals. The experiment was approved by the Guangzhou Medical University Ethics Review Board.
Cell culture
Primary cultures of cardiac myocytes were prepared as previously described. 11 Cardiomyocytes isolated from 1- to 2-day-old Sprague-Dawley rats were seeded at a density of 5×105 cells per well onto 6-well culture plates in plating medium that consisted of high glucose Dulbecco’s minimum essential medium (DMEM) supplemented with 10% fetal calf serum (FCS). The fibroblasts and other proliferation cells were removed through preplating for 90 minutes at 378C. Bromo deoxyuridine of 0.1 mM was added to the culture media for the first 72 hours to further minimize contamination from fibro blasts. These methods resulted in a cardiac myocyte purity greater than 95%, as demonstrated by immunocytochemical staining with an anticardiac a-myosin heavy chain antibody. HEK293 (human embryonic kidney cell line) used in this study was purchased from American Type Culture Collection (ATCC, Manassas, VA) and cultured in high glucose DMEM supplemented with 10% FCS.
Leucine incorporation
Incorporation of radioactive-labeled [3H] leucine was quantified as a parameter of CM protein synthesis. The cells were serum starved overnight and incubated with Angiotensin II (AngII). A 1-mCi/mL aliquot of L-4, 5-3H–labelled leucine was then added, and the cells were incubated for additional 12 hours at 37°C. The cells were washed with phosphate buffered saline and harvested in ice-cold lysis buffer. Aliquots were collected for liquid scintillation counting.
Transfection and fluorescence analysis
The miRNA mimics and inhibitors were synthesized by Dharmacon (Lafayette, CO). The cells were transfected with the miRNA mimics (50 nM), inhibitors, or plasmid (200 ng) using the Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s instructions. The assays were performed 48 hours after transfection. The 3’-UTR of the mTOR was cloned into the KpnI and BglII restriction sites downstream of the open reading frame of luciferase in the pGL3-promoter luciferase vector. HEK293 cells were seeded in a 24-well plate (1.5×105 per well) and transfected with miRNA mimics (50 nM). Twenty-four hours later, RNA-transfected cells were cotransfected with 200 ng of firefly luciferase reporter containing the wild-type or mutant 3’UTR of mTOR and 20 ng of the Renilla luciferase reporter plasmid pRL-RSV (Promega). Forty-eight hours after transfection, the luciferase activity was analyzed using dual luciferase assays (Promega) normalized against the activity of the Renilla luciferase gene.
RNA extraction and qRT-PCR
Total RNA was isolated from tissues, plasma, or cultured cells using Trizol reagent according to the manufacturer’s protocol (Invitrogen, Shanghai, China). Complementary DNA was synthesized with M-MLV reverse transcriptase. Each quantitative real-time polymerase chain reaction (qRT-PCR) contained 1 mL of RT product, 10 mL of SYBR Green PCR Master Mix (Invitrogen), and 500 nM forward and reversed primers. qRT-PCR was performed on a MyiQ Single Colour Real-time PCR Detection System (Bio-Rad Laboratories, Hercules, CA). The data were analyzed with the 22DDCT method, 17 and the fold changes in gene expression were normalized to U6 small nuclear RNA as an endogenous control.
Western blot
The heart tissue and cells were lysed on ice with RIPA lysis buffer containing 150 mM NaCl, 1% NP40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris (pH 7.9), 10 mM NaF, PMSF, and a protease inhibitor cocktail (Sigma, St. Louis, MO). The protein concentrations were determined using a BioRad protein assay kit (Bio-Rad, Hercules, CA). The protein sample (50 mg per lane) was separated by SDS-PAGE and transferred onto polyvinylidene fluoride membranes. The blots were probed with antibody (final dilution 1:1000; Santa Cruz Biotechnology, Dallas, TX). GAPDH antibody (final dilution 1:1000; Sigma) was used as a control. The bands were visualized with the ChemiDoc XRS system (Bio-Rad).
Statistics analysis
The results are expressed as the mean ± SD. Statistical significance was determined with an unpaired 2-tailed Student’s t test or an analysis of variance. Each experiment included triplicate measurements for each condition tested, unless otherwise indicated. P < 0.05 was considered statistically significant.
Results
MiR-96 promotes myocardial hypertrophy in vitro
To identify the function of miR-96 in the myocardial hypertrophy, we used AngII to stimulate rat myocardial cells and created the rat model of cardiac hypertrophy successfully. Next, we tested the related cells area, the expression levels of hypertrophic genes and the leucine incorporation. As is shown in (Figure 1A-C), the quantitative real-time polymerase chain reaction analysis demonstrated that the AngII stimulated miR-96 can significantly promoted the cell areas, the expression levels of hypertrophic gene and the incorporation of leucine.
Figure 1.
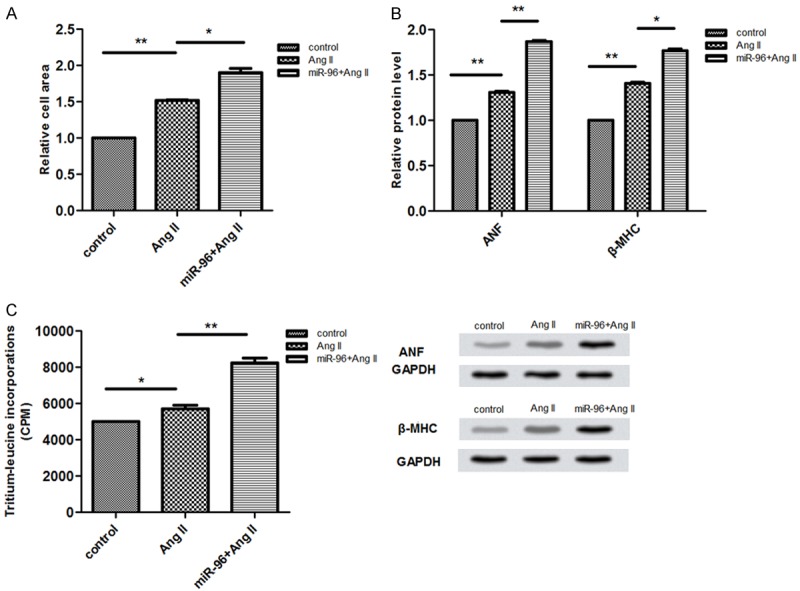
A-C. Represent ANF and b-MHC mRNA levels, protein synthesis rate, and relative cell area in Vitro, respectively, that were transfected with AngII.
MTOR is the direct target genes of miR-96
To identify the mechanism of miR-96 promoting the myocardial hypertrophy, we predicted and finally confirmed that mTOR was the target gene of miR-96 by using bioinformatics method (Figure 2A). Then, after separately overexpressed and inhibited miR-96, we detected the mRNA and protein expression levels of mTOR. Our dates showed that the inhibiting miR-96 can promote both the mRNA and the protein expression levels of mTOR (Figure 2B, 2C). Next, we implemented Fluorescent Report Carrier Assay for deep analysis and finally confirmed that miR-96 was directly binding to 3’UTR of mTOR (Figure 2D). Overall, we can conclude that mTOR is the direct target gene of miR-96.
Figure 2.
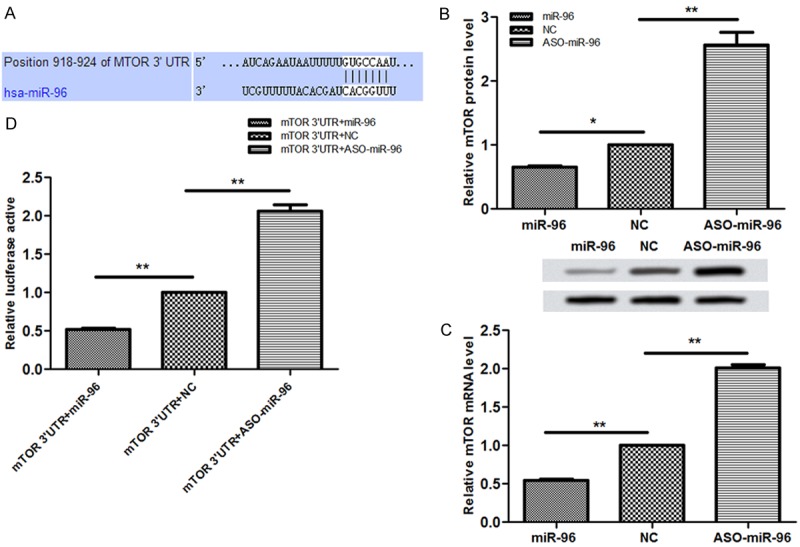
Prediction of target gene for miR-96. A. Investigate the underlying mechanisms, we predicted the potential targeted genes of miR-96 by bioinformatics algorithms. Among all predicted genes, mTOR was found to be a target for miR-96. B. We found that miR-96 significantly reduced the luciferase activity of reporter for mTOR, while aso miR-96 markblly increased the luciferase activity of 3’UTR reporter for mTOR. C. mTOR mRNA and protein expression levels transfected with NC, miR-96, and ASO- miR-96 were analyzed by using western blotting. D. The relative luciferase active of the 3’UTR of target gene for miR-96 in different group. All experiments were performed in triplicate the significance in change in protein expression was analyzed by using statistical tests.
MTOR promotes the occurrence of myocardial hypertrophy
We used RNA interference to make siRNA for mTOR, after the validity testing, the cell area, the expression of hypertrophic genes and the leucine incorporation.were detected. The rusults suggested that siRNA observably increased their expression (Figure 3A-C). Thus, we can confirm that mTOR obvioursly promotes myccardial hypertrophy.
Figure 3.
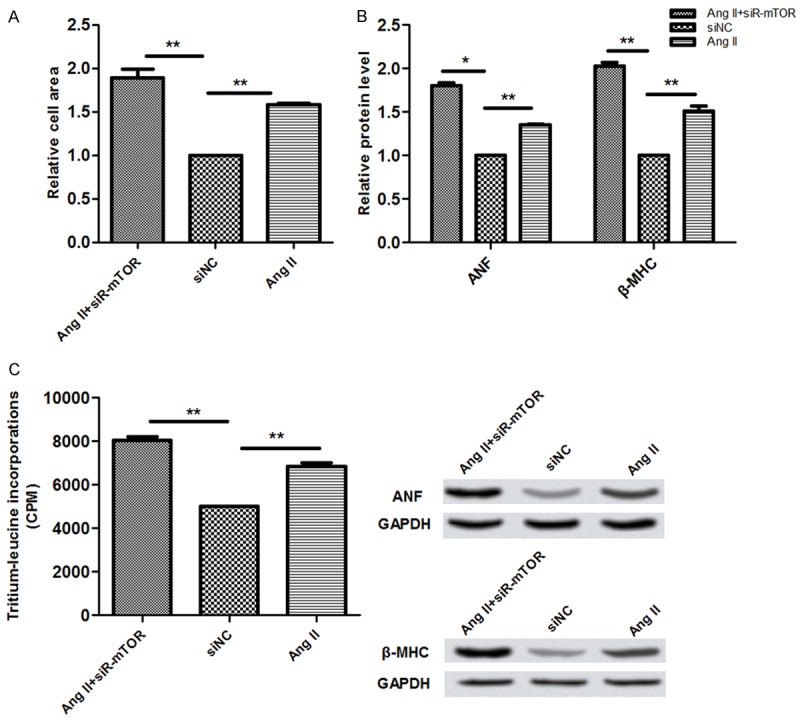
A-C. Represent ANF and b-MHC mRNA levels, protein synthesis rate, and relative cell area that were transfected with AngII, AngII+siR-mTOR, and siNC.
Expression of miR-96 and mTOR
In order to determine the expression levels of miR-96 and mTOR in the myocardial hypertrophy mice model, we launched transverse abdominal aortic constriction (TAAC) on mice. Four weeks later, we tested the expression levels of miR-96 and mTOR. Compared with the control group, the expression level of miR-96 was significantly elevated in the TAAC group, while the result of mTOR was inversed (Figure 4A-C).
Figure 4.
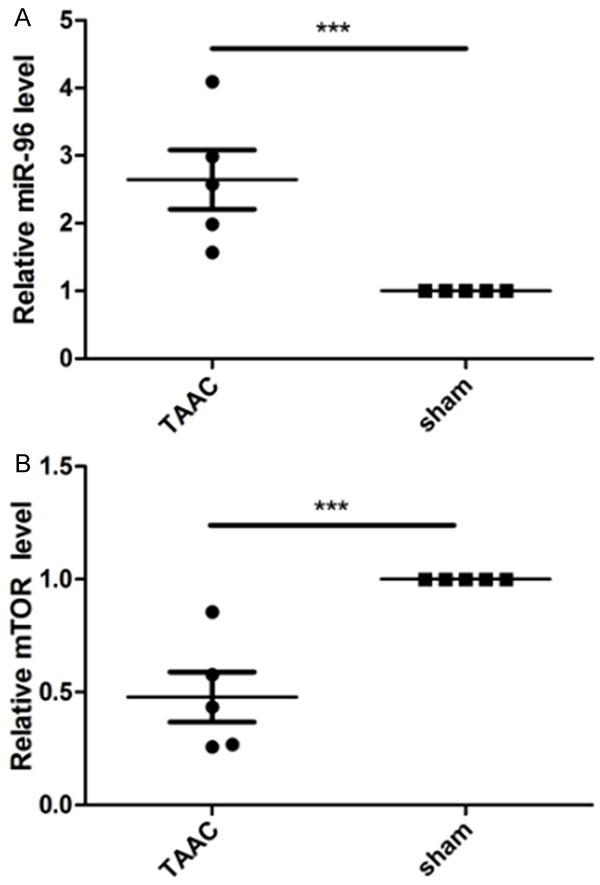
The expression levels of miR-96 and mTOR were measured by quantitative real-time polymerase chain reaction.
Discussion
Cardiac hypertrophy is a common pathological response to a number of cardiovascular diseases, including hypertension, vascular disease, endocrine disorder, as well as ischemic heart disease. Pathological cardiac hypertrophy is an important predecessor of heart failure that is a major determinant of mortality and morbidity in cardiovascular diseases. Cardiac hypertrophy is known as the heart’s response to a variety of extrinsic and intrinsic stimuli that impose increased biomechanical stress. While hypertrophy can eventually normalize wall tension, it is associated with an unfavorable outcome and threatens affected patients with sudden death or progression to overt heart failure, thus, pathological cardiac hypertrophy is progressive process and irreversible. Though there exist some insights into it, the potential molecular events underlying the transition from compensated cardiac hypertrophy to failure still need deeply researched.
MiRNAs participate in a variety of pathophysiological processes, including cellular differentiation, apoptosis, proliferation, and organ development by targeting different genes [20]. Accumulating researches supports that micro-RNAs are important gene expression regulators that play a fundamental role in disease development. The specific role of miRNAs in the genesis of cardiac hypertrophy has received great attention in the last decade. The study by Wei et al [7] proved that miR-101 was a critical regulator of cardiomyocyte hypertrophy. That showed the implication of the potential application of miRNA in the therapy of cardiac hypertrophy. There is a real prospect that miRNA could be useful in a therapeutic setting [21-28]. However, the promise of miR-directed therapeutics requires clear functional understanding of individual miRNA in both normal and pathological circumstances.
In this study, we created a rat model to elucidate a role for miR-96 in cardiac hypertrophy, the result told that by influencing on the related cells area, the expression levels of hypertrophic genes and the leucine incorporation, MiR-96 can obviously promotes myocardial hypertrophy in vitro. As target genes play a typical role for the miRNA expression, miRNAs directly regulate the target genes by binding their 3’-UTRs [29], we researched the mechanism of miR-96 promoting the myocardial hypertrophy. By using bioinformatics method and the Fluorescent Report Carrier Assay, we finally identified that mTOR was the target gene of miR-96. Furthermore, by launching transverse abdominal aortic constriction (TAAC) on mice, we found the correlation between miR-96 and mTOR and successfully domonstrated that miR-96 promotes the occurrence of myocardial hypertrophy by suppressing the expression of mTOR.
In conclusion, our study revealed a previously unrecognized role and correlation of miR-96 and mtor in myocardial hypertrophy. Our study strongly supports the notion that miR-96 is a critical regulator of cardiomyocyte hypertrophy. However, we cannot eliminate the possibility that mTOR was not the only target of miR-96 that involved in cardiac hypertrophy and that miR-96 may, therefore, regulate cardiac hypertrophy through multiple pathways. All of this suggests miR-96 may be a potential therapeutic target for myocardial hypertrophy and offers a new strategy for the treatment of it.
Disclosure of conflict of interest
None.
References
- 1.Loffredo FS, Steinhauser ML, Jay SM GJ, Pancoast JR, Yalamanchi P, Sinha M, Dall’Osso C, Khong D, Shadrach JL, Miller CM, Singer BS, Stewart A, Psychogios N, Gerszten RE, Hartigan AJ, Kim MJ, Serwold T, Wagers AJ, RT L. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–839. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McPherron AC. Through thick and thin: a circulating growth factor inhibits age-related cardiac hypertrophy. Circ Res. 2013;113:487–491. doi: 10.1161/CIRCRESAHA.113.302239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei L, Yuan M, Zhou R, Bai Q, Zhang W, Zhang M, Huang Y, Shi L. MicroRNA-101 Inhibits Rat Cardiac Hypertrophy by Targeting Rab1a. J Cardiovasc Pharmacol. 2015;65:357–363. doi: 10.1097/FJC.0000000000000203. [DOI] [PubMed] [Google Scholar]
- 4.Yu B, Gong M, Wang Y, Millard RW, Pasha Z, Yang Y, Ashraf M, Xu M. Cardiomyocyte protection by GATA-4 gene engineered mesenchymal stem cells is partially mediated by translocation of miR-221 in microvesicles. PLoS One. 2013;8:e73304. doi: 10.1371/journal.pone.0073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katare R, Riu F, Mitchell K, Gubernator M, Campagnolo P, Cui Y, Fortunato O, Avolio E, Cesselli D, Beltrami AP, Angelini G, Emanueli C, Madeddu P. Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132. Circ Res. 2011;109:894–906. doi: 10.1161/CIRCRESAHA.111.251546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikeda S, He A, Kong SW, Lu J, Bejar R, Bodyak N, Lee KH, Ma Q, Kang PM, Golub TR, Pu WT. MicroRNA-1 negatively regulates expression of the hypertrophy-associated calmodulin and Mef2a genes. Mol Cell Biol. 2009;29:2193–2204. doi: 10.1128/MCB.01222-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang X, Liu Y, Zeng L, Yu C, Hu Z, Zhou Q, Yang Z. MiR-101 inhibits the G1-to-s Phase transition of cervical cancer cells by targeting Fos. Int J Gynecol Cancer. 2014;24:1165–1172. doi: 10.1097/IGC.0000000000000187. [DOI] [PubMed] [Google Scholar]
- 8.Xue Z, Zhao J, Niu L, An G, Guo Y, Ni L. Up-Regulation of MiR-300 Promotes Proliferation and Invasion of Osteosarcoma by Targeting BRD7. PLoS One. 2015;10:e0127682. doi: 10.1371/journal.pone.0127682. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Kuppusamy KT, Sperber H, Ruohola-Baker H. MicroRNA regulation and role in stem cell maintenance, cardiac differentiation and hypertrophy. Curr Mol Med. 2013;13:757–764. doi: 10.2174/1566524011313050007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang D, Li J. MiRNA-204 drives cardiomyocyte proliferation via targeting Jarid2. Int J Cardiol. 2015;201:38–48. doi: 10.1016/j.ijcard.2015.06.163. [DOI] [PubMed] [Google Scholar]
- 11.Zhang ZH, Li J, Liu BR, Luo CF, Dong Q, Zhao LN, Zhong Y, Chen WY, Chen MS, Liu SM. MicroRNA-26 Was Decreased in Rat Cardiac Hypertrophy Model and May Be a Promising Therapeutic Target. J Cardiovasc Pharmacol. 2013;62:312–319. doi: 10.1097/FJC.0b013e31829b82e6. [DOI] [PubMed] [Google Scholar]
- 12.Yamada Y, Enokida H, Kojima S, Kawakami K, Chiyomaru T, Tatarano S, Yoshino H, Kawahara K, Nishiyama K, Seki N, Nakagawa M. MiR-96 and miR-183 detection in urine serve as potential tumor markers of urothelial carcinoma: correlation withstage and grade, and comparison with urinary cytology. Cancer Sci. 2011;102:522–529. doi: 10.1111/j.1349-7006.2010.01816.x. [DOI] [PubMed] [Google Scholar]
- 13.Xu XM, Qian JC, Deng ZL, Cai Z, Tang T, Wang P, Zhang KH, Cai JP. Expression of miR-21,miR-31, miR-96 and miR-135b is correlated with the clinical parameters of colorectal cancer. Oncol Lett. 2012;4:339–345. doi: 10.3892/ol.2012.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Han Y, Zhang H, Nie L, Jiang Z, Fa P, Gui Y, Cai Z. Synthetic miRNA mowers targeting miR-183-96-182 cluster or miR-210 inhibit growth and migration and induceapoptosis in bladder cancer cells. PLoS One. 2012;7:e52280. doi: 10.1371/journal.pone.0052280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang W, Qian P, Zhang X, Zhang M, Wang H, Wu M, Kong X, Tan S, Ding K, Perry JK, Wu Z, Cao Y, Lobie PE, Zhu T. Autocrine/Paracrine Human Growth Hormone-stimulated MicroRNA 96-182-183 Cluster Promotes pithelial-Mesenchymal Transition and Invasion in Breast Cancer. J Bio Chem. 2015;290:13812–13829. doi: 10.1074/jbc.M115.653261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laplante M, DM S. MTOR signaling in growth control and disease. Cell. 2011;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Yang F, Ma W, Sun Q. Metformin inhibits proliferation and proinflammatory cytokines of human keratinocytes in vitro via mTOR-signaling pathway. Pharm Biol. 2015;25:1–6. doi: 10.3109/13880209.2015.1057652. [DOI] [PubMed] [Google Scholar]
- 19.Huang TQ, Zou MX, Pasek DA, Meissner G. MTOR signaling in mice with dysfunctional cardiac ryanodine receptor ion channel. J Receptor Ligand Channel Res. 2015;8:43–51. doi: 10.2147/JRLCR.S78410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 21.Murray MY, Rushworth SA, MacEwan DJ. MicroRNAs as a new therapeutic target towards leukaemia signalling. Cell Signal. 2012;24:363–368. doi: 10.1016/j.cellsig.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 22.Rushworth SA. Targeting the oncogenic role of miRNA in human cancer using naturally occurring compounds. Br J Pharmacol. 2011;162:346–348. doi: 10.1111/j.1476-5381.2010.01075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schrauder MG, Strick R, Schulz-Wendtland R, Strissel PL, Kahmann L, Loehberg CR, Lux MP, Jud SM, Hartmann A, Hein A, Bayer CM, Bani MR, Richter S, Adamietz BR, Wenkel E, Rauh C, Beckmann MW, Fasching PA. Circulating micro-RNAs as potential blood-based markers for early stage breast cancer detection. PLoS One. 2012;7:e29770. doi: 10.1371/journal.pone.0029770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng HJ, Ouyang W, Liu JH, Sun YG, Hu R, Huang LH, Xian JL, Jing CF, Zhou MJ. Global microRNA profiles and signaling pathways in the development of cardiac hypertrophy. Braz J Med Biol Res. 2014;47:361–368. doi: 10.1590/1414-431X20142937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yongchun Z, Linwei T, Xicai W, Lianhua Y, Guangqiang Z, Ming Y, Guanjian L, Yujie L, Yunchao H. Microrna-195 inhibits non-small cellung cancer cell proliferation, migration and invasion by targeting myb. Cancer Lett. 2014;347:65–74. doi: 10.1016/j.canlet.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 26.Cheng J, Deng H, Xiao B, Zhou H, Zhou F, Shen Z, Guo J. piR-823, a novel non-coding small RNA, demonstrates in vitro and in vivo tumor suppressive activity in human gastric cancer cells. Cancer Lett. 2012;315:12–17. doi: 10.1016/j.canlet.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Youn JI, Kumar V, Collazo M, Nefedova Y, Condamine T, Cheng P, Villagra A, Antonia S, McCaffrey JC, Fishman M. Epigenetic silencing of retinoblastoma gene regulates pathologic differentiation of myeloid cells in cancer. Nat Immunol. 2013;14:211–220. doi: 10.1038/ni.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lundstrom K. Micro-RNA in disease and gene therapy. Curr Drug Discov Technol. 2011;8:76–86. doi: 10.2174/157016311795563857. [DOI] [PubMed] [Google Scholar]
- 29.Xie Z, Cai L, Li R, Zheng J, Wu H, Yang X, Li H, Wang Z. Down-regulation of miR-489 contributes into NSCLC cell invasion through targeting SUZ12. Tumour Biol. 2015;36:6497–505. doi: 10.1007/s13277-015-3340-3. [DOI] [PubMed] [Google Scholar]


