Abstract
Background and Purpose: Although recent studies have indicated that acid-sensing ion channels (ASICs) may play an important role in suppressing status epilepticus (SE) in rats, the precise mechanism is unclear. We attempted to investigate the antiepileptic effect of amiloride in SE rats and its mechanism. Methods: Rats with seizures induced by Li-pilocarpine were randomly divided into four groups, phosphate buffer saline (PBS) group, amiloride group, levetiracetam group and acidic liquid group, respectively. The electroencephalogram (EEG) of each group was recorded. Then rats treated with different drugs (2 h after amiloride or PBS injection or 1 h after PBS injection) and a normal control group was selected for reverse transcription-polymerase chain reaction (RT-PCR). The expression of ASIC1a, ASIC3 and sodium-hydrogen exchanger (NHE) in each group was detected. Results: Amiloride reduced the frequency of discharge in 60~90 min after injection significantly. In acidic liquid group, the epileptic discharge was increased in 0~30 min. Moreover, the expression of ASIC1a, ASIC3 and NHE was obviously increased in the SE groups. Compared with SE groups, the expression of ASIC1a and ASIC3 mRNA in amiloride group decreased significantly. While NHE mRNA expression in the SE groups showed no significant difference. Conclusion: Amiloride inhibited pilocarpine-induced SE and the anti-epileptic mechanism was associated with deactivation of the ASIC1a and ASIC3 instead of NHE in rats.
Keywords: Amiloride, epilepsy, ASICs, NHE, EEG, pilocarpine
Introduction
Epilepsy is the third most common chronic brain disorder affecting more than 50 million people worldwide, which is characterized by an enduring predisposition to generate seizures [1]. Drug therapy remains the main therapeutic choice of epilepsy despite of a variety of anti-epilepsy measures, such as surgery and transcranial magnetic stimulation (TMS). As seizures are caused by excessive discharge of neurons, pharmacological studies of epilepsy have primarily focused on modulation of voltage-gated ion channels, enhancement of GABAergic inhibition, and reduction of glutamatergic excitation [2]. However, according to clinical medicine, classic antiepileptic drug (AED) is neither universally effective nor invariably safe. It’s necessary to find potential therapeutic targets, such as acid-sensitive ion channels (ASICs), a hot area of anti-epilepsy research for several years.
ASICs, which belong to epithelial Na+ channel (ENaC) superfamily of ion channels, are typical voltage-independent proton sensors in the central nervous system and peripheral nervous system [3]. ASICs are coded by four genes with alternative splicing generate isoforms, and each has distinct biophysical properties (ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3 and ASIC4) [4]. ASICs are activated during acidic pH fluctuations. All ASIC subunits have different pH sensitivities that enable them to detect a wide range of physiological pH. ASIC1a and ASIC3 are sensitive to slight extracellular acidosis, whereas ASIC2a requires more acidic pH value for activation [5,6]. It is well known that seizure induces the decrease of pH levels in the brain [7]. Recently, investigators have discovered that ASICs are involved in activation and termination of seizures [8,9]. Some studies have demonstrated that ASICs are blockaded by amiloride and the selective ASIC1a blocker, Psalmotoxin 1 (PcTX1), significantly inhibits the increase of neuronal firing and the sustained membrane depolarization [8].
Accumulating evidence indicated that amiloride (a potassium-sparing diuretic agent) inhibited status epilepticus (SE) induced by pentylenetetrazole (PTZ), pilocarpine and maximal electroshock in vivo and vitro experiments [10,11]. However, the mechanisms underlying the potential anticonvulsant effects of amiloride are not yet fully understood. As we all know, amiloride is not only a non-specific blocker of ASICs, but also the blocker of sodium-hydrogen exchanger (NHE). It is unclear whether amiloride suppresses seizures via the ASICs and/or NHE pathway. Therefore, the present study aimed to investigate the antiepileptic effect of amiloride in pilocarpine-induced SE in rats and the regulation of their expression during SE.
Materials and methods
Materials
Sodium phenobarbital was from Shanghai. Amiloride and pilocarpine were purchased from Sigma-Aldrich, Inc. (St. Louis, MO, USA), and levetiracetam was purchased from UCB (Belgium). Lithium chloride was bought from Wuhan Yixin biotechnology Co., Ltd. Atropine sulfate was obtained from Wuhu Kangqi Pharmaceutical Co., Ltd. The aforementioned drugs were dissolved in distilled water, and solutions were injected at total volumes of 10 ml/kg. The acidic phosphate buffered saline (PBS) was made from PBS and hydrochloric acid, and the pH value was 1.57. Three pairs of primers for reverse transcription-polymerase chain reaction (RT-PCR) were designed and synthesized by Invertrigen, Wuhan Co., Ltd (Table 1). The RM-6240 multichannel physiologic recorder was made in Chengdu, China. The stereotactic instrument was provided from Second Military Medical University.
Table 1.
RT-PCR primer sequence and the size of magnification
| Gene | Primer | Sequence | Bp size |
|---|---|---|---|
| ASIC1a | Forward primer | CGGATCCATGGAATTGAAGACCGAGGA | 1593 |
| Reverse primer | CGATATCTGCAGGTAAAGTCCTCAAACG | ||
| ASIC3 | Forward primer | CGCGAATTCATGAAACCTCGCTCCGGACTG | 1617 |
| Reverse primer | GCGCTCGAGGAGCCTTGTGACGAGGTAAC | ||
| NHE | Forward primer | TCT GCC GTC TCA ACT GTC TCTA | 423 |
| Reverse primer | CCC TTC AAC TCC TCA TTC ACCA |
Animals and epilepsy models
Male SD rats weighted 150~200 g were used. The rats were housed in groups of 5-10 per cage and maintained at 20-30°C and 50-55% humidity in a natural light and dark cycle, with free access to food and water. The experiments were performed after the animals were adjusted to laboratory conditions. All animals were procured from animal laboratory of Wuhan University, Wuhan, China. All protocols of the animal experiments were approved by the Administrative Panel on Laboratory Animal Care of Wuhan University. This study was conducted in accordance with the International Association for the Study of Pain guidelines on the use of animals in experimental research [12]. All efforts were made in order to minimize the frequency of animals used and their suffering.
SE was induced by administration of pilocarpine hydrochloride (100 mg/kg, i.p.) 18-24 h after lithium chloride (127 mg/kg, i.p), and 30 min after atropina (1 mg/kg, i.p), which was injected to limit the peripheral effects of the convulsant [13,14]. Following pilocarpine administration, each rat was observed for behavioral signs. The animals which were identified as generalized seizures and stayed for 30 min were enrolled.
Grouping and drug treatment
SE rats were divided into five groups randomly, each having 6 rats. Another six normal rats were drawn into Gp.VI for RT-PCR, which were not performed with Li- pilocarpine. Only one type of treatment was offered to each rat and test was not reused. All drugs were given at a volume of 10 ml/Kg.
Gp. I: Levetiracetam (SE model); Gp. II: Acidic liquid (SE model); Gp. III: PBS (SE model); Gp. IV: Amiloride (SE model); Gp. V: SE for 1 h (SE model for RT-PCR); Gp. VI: Normal control (normal rats for PT-PCR).
EEG record
Gp. I~IV were elected for electroencephalogram (EEG) record, which were prepared as mentioned above, like levetiracetam group, acidic liquid group, PBS group and amiloride group. The rats were drugged by urethane (1.0 g/kg), and then fixed with tridimensional to record the cortex EEG. The record lasted at least 30 minutes as baseline for subsequent experiments. In amiloride group, the rats were then administered intraperitoneally with amiloride (100 mg/Kg). The EEG was recorded for 90 minutes after injection. The rats of other groups followed the same procedures except that they received alternative treatment individually, such as Levetiracetam (80 mg/Kg), PBS (10 ml/Kg) and acidic liquid (10 ml/Kg).
The EEG date was artificially divided into four phases (-30~0 min, 0~30 min, 30~60 min and 60~90 min), and the earliest phase (-30~0 min) was dated before drug injection. The data of 30 min EEG per rat before injection were set as baseline. The values were processed as the ratio of the data of different phase to those of 30 min EEG per rat before injection.
Tissue preparation
In order to learn the expression of ASIC1, ASIC3 and NHE mRNA in SE model, Gp. III, IV, V and VI rats were elected for RT-PCR. Gp. III, IV and VI rats were anaesthetized with sodium pentobarbital (50 mg/kg, i.p.) and decapitated at 2 h after drugs injection, while Gp. V rats were sacrificed at 1 h after PBS injection. Their brains were rapidly removed from the skull. On dry ice, the cerebral cortex was quickly isolated, and then stored at -80°C for RNA extraction.
RNA extraction, reverse transcription
Total RNA of these tissues was extracted by TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. Immediately following extraction, total RNA was concentrated and determined by spectrophotometric measurements by 260/280 nm. The OD260/280 absorption ratio was calculated during 2.0-2.2. Total RNA was reversely transcribed by SuperScriptTM First-Strand Synthesis system (Invitrogen, Carlsbad, CA, USA), with the thermal profile at 42°C for 60 min, at 70°C for 5 min and at 4°C for 5 min.
Semi quantitive RT-PCR
The expression of ASIC1a, ASIC3 and NHE was evaluated by semi-quantitative reverse transcription polymerase chain response (RT-PCR). Amplification was performed with a priming step of 5 min at 95°C, followed by 33 cycles of 30 s at 95°C for denaturation and 35 s at 55°C for annealing and 40 s at 72°C for extension. Lastly, amplification was conducted at 72°C for 10 min, and saved at 4°C. The primer sequences of Gp. III, IV, V and VI are listed in Table 1.
The PCR products of Gp. III, IV, V and VI rats were electrophoretically separated on 2% agarose gels, respectively. By staining with ethidium bromide, the cDNA bands were visualized and analyzed. The relative amount of ASIC1, ASIC3 or NHE mRNA was expressed as the ratio of the optical density (OD) of ASIC1, ASIC3 or NHE to that of GAPDH cDNA band.
Statistical analyses
All statistical analyses are expressed as the mean ± standard error of mean (SEM). Data were analyzed using a one-way analysis of variance (ANOVA) followed by Dunnett’s test for multiple comparisons. P<0.05 was chosen as the threshold for statistical significance.
Results
Behavior of epilepsy model
After pilocarpine injection, the animals entered stage I seizure activity after a few minutes (4±0.6 min) and rapidly progressed through stage II to IV, such as mouth and facial automatism, head nodding, forelimb clonus without rearing, etc. Then, they developed to generalized clonic-tonic seizures (stage V) within 35±4.0 min. Few animals (n=8) didn’t enter stage V were discarded.
Discharge of EEG in epilepsy model
The rats (Gp. I~IV, n=24) were divided into four groups, levetiracetam group, acidic liquid group, PBS group and amiloride group to investigate the effects of amiloride. Levetiracetam group was used as positive control, while the PBS group was used as negative control. In general, as showed in Figure 1, the frequency of EEG discharge decayed linearly in time. There were significant differences in the frequency of EEG discharge in 30~90 min after abdominal injection (P 30~60 min<0.05, P 60~90 min<0.01) (Figure 1). Furthermore, in the recording of EEG, animals treated with amiloride and levetiracetam exhibited a significant reduction in 60~90 min after injection compared with PBS group (P 60~90 min<0.05), respectively. There was no significant difference between amiloride group and levetiracetam group. On the contrary, in acidic liquid group, the frequency of EEG discharge in 0-30 min was moderately increased (P 0~30 min<0.05).
Figure 1.
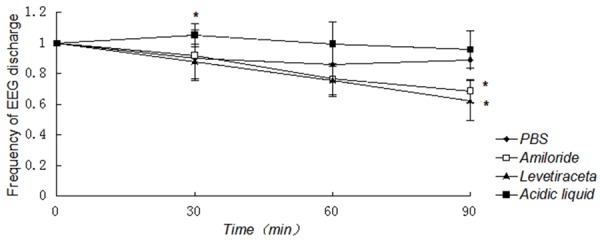
Effect of amiloride on the frequency of EEG discharge in rats. The difference of the frequency of rats EEG discharge in 30~90 min among the four groups after abdominal injection was statistically significant (P 30~60<0.05, P 60~90<0.01). Moreover, in both of amiloride group and levetiraceta group, the frequency of EGG discharge was significantly declined in 60~90 min after injection (P 60~90<0.05), compared with PBS group, respectively. There was no statistical difference between amiloride group and levetiraceta group. On the contrary, in acidic liquid group, the frequency of EGG discharge in 0~30 min (P 0~30 min<0.05) was moderately increased, while there was no statistically significant difference in 30~90 min between acidic liquid group and PBS group. *Compared with PBS group, P<0.05.
Alterations of ASIC and NHE expression induced by seizure and amiloride treatment
To investigate whether ASIC and NHE expression changes in the acute phase after SE, Gp.III, IV, V and VI rats were elected for the detection of ASIC and NHE expression as SE 2 h group, amiloride group, SE 1 h group and normal control group, respectively. mRNA levels of ASIC1a, ASIC3 and NHE were examined via RT-PCR. DNA fragment was successfully amplified from the cDNA template. For rats receiving different drugs treatment, the results were different. The gel images showed the clear bands of ASIC1a, ASIC3 and NHE in different groups (Figures 2, 3 and 4). IOD of semi quantitative analysis indicated that the expression of experimental groups was higher than that in normal control group (Figures 2, 3 and 4).
Figure 2.
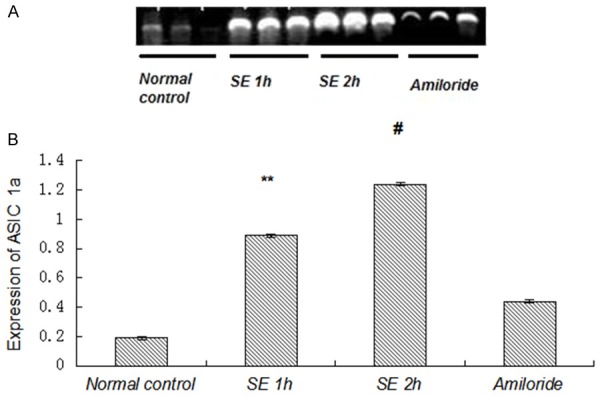
Expression of ASIC1a mRNA was increased by seizures. A: Photograph of an agarose gel showed ASIC1a mRNA expression. The expression of ASIC1a mRNA was decreased in amiloride group measured by RT-PCR. B: Compared with normal control group, the expression of ASIC1a mRNA was increased significantly in both SE 1 h group and SE 2 h group (P<0.01). Compared with other SE groups, the expression of ASIC1a mRNA in amiloride group was decreased significantly (P<0.01). **P<0.01, amiloride group vs. SE 1 h group, #P<0.01, amiloride group vs. SE 2 h group.
Figure 3.
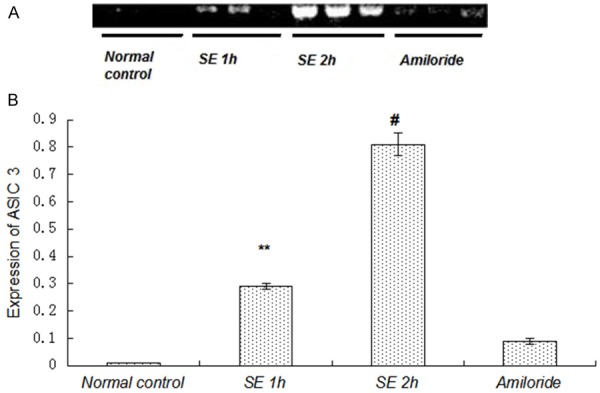
Expression of ASIC3 mRNA was increased by seizures. A: Photograph of an agarose gel showed ASIC3 mRNA expression. The expression of ASIC3 mRNA was decreased in the amiloride group measured by RT-PCR. B: Compared with normal control group, the expression of ASIC3 mRNA was increased significantly in SE 1 h group, SE 2 h group and amiloride group (P<0.01). Compared with other SE groups, the expression of ASIC3 mRNA in the amiloride group was decreased significantly (P<0.01). **P<0.01, amiloride group vs. SE 1 h group, #P<0.01, amiloride group vs. SE 2 h group.
Figure 4.
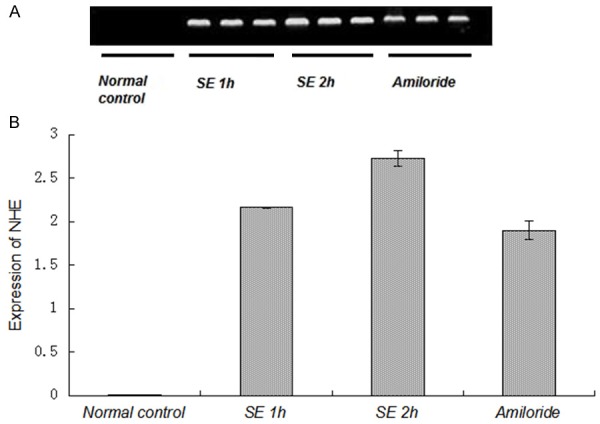
Expression of NHE mRNA was not affected by amiloride. A: Photograph of an agarose gel showed NHE mRNA expression. The decline of NHE mRNA was not evident in amiloride group measured by RT-PCR. B: Compared with the normal control group, the expression of NHE mRNA was increased significantly in SE 1 h group, SE 2 h group and amiloride group (P<0.01). On the contrary, compared with 1 h seizure group and 2 h seizure group, the expression of NHE mRNA in the amiloride-treated group was not statistically different.
Downregulation of ASIC1a and ASIC3 expression in brain of rats treated with amiloride
Alterations in ASIC1a mRNA levels were detected in the four groups mentioned above (Figure 2). ASIC1a was expressed in all of the four groups. The difference of ASIC1a expression in rats was statistically significant (P<0.01). Expression levels of ASIC1a in the three SE groups were higher than those in normal control groups (P<0.01). Compared with amiloride group, ASIC1a mRNA expression in SE 1 h group and SE 2 h group was increased significantly (P<0.01), which indicated the expression of ASIC1a mRNA could be decreased by amiloride.
The expression of ASIC3 in the four groups was also different (Figure 3). ASIC3 was lowly expressed in normal control group, while its expression was obviously increased in SE groups, indicating that the expression of ASIC3 played an important role in epilepsy. The difference of ASIC1a expression in rats was statistically significant (P<0.01). Expression levels of ASIC3 in the three SE groups were higher than those in normal control groups (P<0.01). Compared with SE 1 h group and SE 2 h group, ASIC3 mRNA expression in the amiloride-treated group was decreased significantly. (P<0.05), also indicating that the expression of ASIC3 mRNA could be decreased by amiloride.
NHE mRNA expression was not affected by amiloride
To investigate the effects of amiloride on NHE expression, NHE expression was examined among the four groups mentioned above. As shown in Figure 4, the expression of NHE in normal control group was close to zero, while it was obviously increased in the SE groups, indicating that SE may promote the expression of NHE. Expression levels of NHE in the three SE groups were higher than those in normal control groups (P<0.01). Compared with amiloride group, NHE mRNA expression in SE 1 h group and SE 2 h group was not statistically different (P>0.05), indicating the mechanism of the amiloride terminating the seizures was independent of NHE.
Discussion
Our study provided evidence that amiloride suppressed pilocarpine-induced seizures and SE in rats, and ASIC1a and ASIC3 may play an important role in the pathogenesis of the antiepileptic effect of amiloride. On the contrary, it was confirmed that amiloride had no influence on NHE expression in pilocarpine models.
According to the available evidence, it was suggested that amiloride exhibited anticonvulsant effects in epileptic animal models [10,14-18]. As widely recognized that levetiracetam suppressed seizures induced by pilocarpine, our research set levetiracetam as positive control group to evaluate the anticonvulsant effects of amiloride via EEG [19]. The EEG data showed pilocarpine-induced seizures were significantly suppressed by amiloride. And there was no statistical difference between amiloride group and levetiracetam group, meanwhile, either of which was significantly different compared with PBS group. However, the epileptic discharge was increased in acidic liquid group in a short time after acidic liquid injection, and there was a synchronous attenuation with the PBS group at the later stage of EEG record. It is confirmed that quick down-regulation of the pH in vivo may activate ASIC channels with the epileptic discharge increased before the compensation of the body. All the findings above suggest that amiloride modulates the occurrence of pilocarpine-induced SE, as is also associated with deactivation of the acidic channels.
Amiloride is a potent and non-selective blocker of ASICs and NHE [20]. In view of amiloride reducing the expression of ASICs or NHE, the expression of ASICs and NHE gene in pilocarpine-induced rats was examined. Our study supported that the expression of ASIC1a, ASIC3 and NHE was significantly increased in acute epilepsy rats. The expression of ASIC1a and ASIC3 was reduced in amiloride-treated rats obviously. On the contrary, the expression of NHE was declined insignificantly after amiloride treatment. Xiong et al. claimed that slices prepared from the brains of ASIC1a knockout mice demonstrated the reduction of stimulation-evoked seizure activities [8]. Biagini et al. also revealed the decrease of ASIC1a mRNA in amiloride treated pilocarpine-induced SE rats [18]. Cao et al. suggested that elevated levels of ASIC3 may serve as an anti-epileptic mechanism via postsynaptic mechanisms in interneurons [21]. Therefore, we concluded that blockade of ASIC1a and ASIC3, especially ASIC1a, rather than inhibition of NHE was primarily responsible for the mechanism of amiloride terminating seizures.
There were also certain limitations of the current study. Our results did not rule out the possibility that the observed neuroprotective effect of amiloride was due to inhibition of sodium-calcium exchanger. Growing evidence suggests that ethosuximide, as a clinical anticonvulsant and potent blocker of T-type calcium channels, failed to prevent the occurrence of pilocarpine-induced seizures [22,23]. Likewise, carbamazepine and phenytoin, clinically used as sodium channel blockers, also failed to prevent pilocarpine-induced seizures [24,25].
Furthermore, there are still some controversies. Several studies reported that extracellular acidosis, in turn, activated ASICs, which terminated seizure activity by increasing the inhibitory function [7,26,27]. It was demonstrated that severity of seizure could be enhanced by disrupting the ASIC1a and ASIC3 gene [9]. These results were contrary to our study. The mechanism of the high expression of ASICs and NHE in models with acute epilepsy is a novel direction for our future research.
It was reported that amiloride had low potential to be used as a future analgesic or neuroprotective agent in human subjects due to its nonspecificity for various ion channels and ion exchange systems [8]. However, the structure of amiloride may be chemically modified to produce a molecule that specifically blocks ASICs, or at least has more selectivity to these ion channels.
In conclusion, amiloride decreased ASIC1a and ASIC3 gene expression to suppress epileptic seizures, suggesting that ASICs played a more pivotal role than NHE in the mechanisms of amiloride against pilocarpine-induced seizures in rats. However, the mechanisms underlying the potential antiepileptogenic and anticonvulsant effects of amiloride are not yet fully understood, which remain to be further investigated.
Acknowledgements
We are highly appreciative to the National Natural Science Foundation of China (81471133, 30900459) and Natural Science Foundation of Hubei Province (2014CFB734) for funding the research. We are also grateful to the center of Wuhan University for providing laboratory for our research work. The authors had no conflicts of interest.
Disclosure of conflict of interest
None.
References
- 1.Vezzani A, Frech J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7:31–40. doi: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogawski MA, Löscher W. The neurobiology of antiepileptic drugs. Nat Rev Neurosci. 2004;5:553–564. doi: 10.1038/nrn1430. [DOI] [PubMed] [Google Scholar]
- 3.Krishtal OA, Pidoplichko VI. A receptor for protons in the nerve cell membrane. Neuroscience. 1980;5:2325–2327. doi: 10.1016/0306-4522(80)90149-9. [DOI] [PubMed] [Google Scholar]
- 4.Krishtal OA. The ASICs: signaling molecules? Modulator? Trends Neurosci. 2003;26:477–483. doi: 10.1016/S0166-2236(03)00210-8. [DOI] [PubMed] [Google Scholar]
- 5.Kweon HJ, Suh BC. Acid-sensing ion channels (ASICs): therapeutic targets for neurological diseases and their regulation. BMB Rep. 2013;46:295–304. doi: 10.5483/BMBRep.2013.46.6.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porirot O, Berta T, Decosterd I, Kellenberger S. Distinct ASIC currents are expressed in rat putative nociceptors and are modulated by nerve injury. J Physiol. 2006;576:215–234. doi: 10.1113/jphysiol.2006.113035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Somjen GG. Acidification of interstitial fluid in hippocampal formation caused by seizures and by spreading depression. Brain Res. 1984;311:186–188. doi: 10.1016/0006-8993(84)91416-1. [DOI] [PubMed] [Google Scholar]
- 8.Xiong ZG, Pignataro G, Li M, Chang SY, Simon RP. Acid-sensing ion channels (ASICs) as pharmacological targets for neurodegenerative diseases. Curr Opin Pharmacol. 2008;8:25–32. doi: 10.1016/j.coph.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ziemann AE, Schnizler MK, Albert GW, Severson MA, Howard MA. Seizure termination by acidosis depends on ASIC1a. Nat Neurosci. 2008;11:816–822. doi: 10.1038/nn.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.N’Gouemo P. Amiloride delays the onset of pilocarpine-induced seizures in rats. Brain Res. 2008;1222:230–232. doi: 10.1016/j.brainres.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhir A, Naidu PS, Kulkarni SK. Effect of cyclooxygenase inhibitors on pentylenetetrazol (PTZ)-induced convulsions: Possible mechanism of action. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1478–1485. doi: 10.1016/j.pnpbp.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 13.Ali A, Kolappa Pillai K, Jalees Ahmad F, Dua Y, Iqbal Khan Z, Vohora D. Comparative efficacy of liposome-entrapped amiloride and free amiloride in animal models of seizures and serum potassium in mice. Eur Neuropsychopharmacol. 2007;17:227–229. doi: 10.1016/j.euroneuro.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Ali A, Ahmad FJ, Pillai KK, Vohora D. Evidence of the antiepileptic potential of amiloride with neuropharmcological benefits in rodent models of epilepsy and behavior. Epilepsy Behav. 2004;5:322–328. doi: 10.1016/j.yebeh.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Tai KK, Truong DD. Amiloride but Not Memantine Reduces Neurodegeneration, Seizures and Myoclonic Jerks in Rats with Cardiac Arrest-Induced Global Cerebral Hypoxia and Reperfusion. PLoS One. 2013;8:e60309. doi: 10.1371/journal.pone.0060309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luszczki JJ, Sawicka KM, Kozinska J, Dudra-Jastrzebska M, Czuczwar SJ. Amiloride enhances the anticonvulsant action of various antiepileptic drugs in the mouse maximal electroshockseizure model. J Neural Transm. 2009;116:105–108. doi: 10.1007/s00702-008-0152-2. [DOI] [PubMed] [Google Scholar]
- 17.Quansah H, N’Gouemo P. Amiloride and SN-6 Suppress Audiogenic Seizure Susceptibility in Genetically Epilepsy-Prone Rats. CNS Neurosci Ther. 2014;20:860–866. doi: 10.1111/cns.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biagini G, Babinski K, Avoli M, Marcinkiewicz M, Séguéla P. Regional and Subunit-Specific Downregulation of Acid-Sensing Ion Channels in the Pilocarpine Model of Epilepsy. Neurobiol Dis. 2001;8:45–58. doi: 10.1006/nbdi.2000.0331. [DOI] [PubMed] [Google Scholar]
- 19.Al-Shorbagy MY, El Sayeh BM, Abdallah DM. Additional Antiepileptic Mechanisms of Levetiracetam in Lithium-Pilocarpine Treated Rats. PLoS One. 2013;8:e76735. doi: 10.1371/journal.pone.0076735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, Nolan BC, Yoder PG, Lamani E, Hoshi T, Freeman JH, Welsh MJ. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron. 2002;34:463–77. doi: 10.1016/s0896-6273(02)00661-x. [DOI] [PubMed] [Google Scholar]
- 21.Cao Q, Wang W, Gu J, Jiang G, Wang K, Xu Z, Li J, Chen G, Wang X. Elevated Expression of Acid-Sensing Ion Channel 3 Inhibits Epilepsy via Activation of Interneurons. Mol Neurobiol. 2014;53:485–98. doi: 10.1007/s12035-014-9014-0. [DOI] [PubMed] [Google Scholar]
- 22.Coulter DA, Huguenard JR, Prince DA. Characterization of ethosuximide reduction of lowthreshold calcium current in thalamic neurons. Ann Neurol. 1989;25:582–593. doi: 10.1002/ana.410250610. [DOI] [PubMed] [Google Scholar]
- 23.Turski WA, Cavalheiro EA, Coimbra C, da Penha Berzaghi M, Ikonomidou-Turski C, Turski L. Only certain antiepileptic drugs prevent seizures induced by pilocarpine. Brain Res. 1987;434:281–305. doi: 10.1016/0165-0173(87)90002-6. [DOI] [PubMed] [Google Scholar]
- 24.Turski L, Lkonomidou C, Turski WA, Bortolotto ZA, Cavalheiro EA. The seizures induced by pilocarpine: a novel experimental model of intractable epilepsy. Synapse. 1989;3:154–171. doi: 10.1002/syn.890030207. [DOI] [PubMed] [Google Scholar]
- 25.Leite JP, Cavalheiro EA. Effects of conventional antiepileptic drugs in a model of spontaneous recurrent seizures in rats. Epilepsy Res. 1995;20:93–104. doi: 10.1016/0920-1211(94)00070-d. [DOI] [PubMed] [Google Scholar]
- 26.Wemmie JA, Taugher RJ, Kreple GJ. Acidsensing ion channels in pain and disease. Nat Rev Neurosci. 2013;14:461–471. doi: 10.1038/nrn3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang RI, Sonnenschein RR. PH of Cerebral cortex during induced convulsions. J Neurophysiol. 1955;18:130–137. doi: 10.1152/jn.1955.18.2.130. [DOI] [PubMed] [Google Scholar]


