Abstract
Autologous implantation of bone mesenchymal stem cells (BMSCs) has achieved promising clinical efficacy for the treatment of early-stage osteonecrosis of the femoral head (ONFH). However, the underlying mechanisms are not completely elucidated. Here, we investigated the effect of BMSCs on the early ONFH in vitro and in vivo. In co-cultured system, primary BMSCs enhanced the activity and inhibited the apoptosis of primary OB. The concentrations of VEGF and BMP-2 in the co-cultured medium were significantly higher than those without co-culture. Importantly, BMSCs implantation increased OB, capillaries and VEGF and BMP-2 expressions of the necrotic areas of femoral head in the ONFH rabbits. In conclusion, our results indicated that BMSCs treated the early ONFH possibly through increasing OB and capillaries, as well as VEGF and BMP-2 expression in the femoral head. These results provided possible mechanisms for the treatment of early-stage ONFH with BMSCs transplantation.
Keywords: BMSCs, ONFH, OB, VEGF, BMP-2
Introduction
ONFH is a common ailment characterized by necrosis of bone trabecular and bone marrow [1]. Currently, many methods including conservative treatment and surgeries have no definitive effect [2,3]. Recently, BMSCs have been widely used in ONFH treatment, and the results of clinical studies indicated that this treatment was safe and effective [4-6].
BMSCs are regarded as potential seed cells for bone tissue engineering [7], and have differentiated into osteoblasts (OB) to restore trabecular bone [8]. Moreover, BMSCs can secrete large amounts of growth factors and angiogenic factors [9], such as VEGF and BMP-2. VEGF is essential for bone formation and repair during the osteogenesis process [10-12]. BMPs could induce osteogenesis in the femoral head with necrosis and stimulate the formation of new bones [13]. Additionally, BMSCs can be easily isolated from bone, and auto-implantation of BMSCs does not induce any immunological rejection.
Though treatment of early-stage ONFH with autologous BMSCs implantation achieved a promising outcome, its specific mechanisms remain elusive. Therefore, we explored the effect of BMSCs on OB in vitro and investigated the effect of BMSCs implanted into the ONFH rabbit. Our study will enhance the understanding of the mechanisms of early-stage ONFH treatment with BMSCs transplantation.
Materials and methods
Animals
Thirty New Zealand white rabbits were obtained from the Animal Center at Xi’an Jiaotong University. Animals were allowed to acclimatize to a new controlled environment. All animal work was carried out under protocols approved by the Institutional Animal Care and Use Committee of Xi’an Jiaotong University.
Isolation and culture of cells
Primary BMSCs were isolated from the bone marrow of tibiae and femurs of rabbits as previously described [14]. Cells were maintained in growth media of DMEM (Gibco BRL Life Technologies) containing 10% fetal bovine serum (FBS) (Gibco), 100 U/mL penicillin and streptomycin. Cells of passage 3 were used for the experiments.
Primary OB was isolated from the rabbits according to the previous method [15,16]. The femoral head fragments were treated with trypsin and digested with type II collagenase. Cells were collected and resuspended in growth media. Cells of passage 3 were used for the experiments.
Co-culture of BMSCs with OB
Noncontact co-culture was achieved by BMSCs (5×104/well) seeded onto the upper chamber of the transwell plate (Corning), while the lower chamber was filled with OB (5×104/well). Cells were suspended in growth media.
Determination of cell activity and apoptosis
BMSCs and OB were co-cultured for 3 days. Cell viability was determined using the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) (Sigma-Aldrich) assay (1×104 cells/well). Absorbance was determined on a spectrophotometric microplate reader (Bio-Rad, Hercules, CA).
Cell apoptosis were measured by flow cytometry. Treated OB were collected and resuspended in binding buffer containing Annexin V-fluoroisothiocyanate (FITC) and propidium iodide (PI) according to the manufacturer’s recommendations. The number of stained cells was quantified using a FACS can flow cytometer, and analyzed by FlowJo software.
Determination of VEGF and BMP-2 concentrations by ELISA
BMSCs and OB were co-cultured for 3 days. Culture supernatant was collected and centrifuged for 10 min (3,000 rpm). VEGF and BMP-2 concentrations were measured by ELISA (Cat. No. ABIN431314 and Cat. No. ABIN416053, Elabscience) according to the manufacturer’s recommendations.
Development of the ONFH rabbit model
ONFH models were established as previously described [17]. Rabbits were given 10 μg/kg Escherichia coli endotoxin LPS by ear vein injection, following 20 mg/kg methylprednisolone gluteal by muscle injection for three consecutive days and were breeding under conventional condition. The animal models were verified by MRI after 6 weeks. For the implantation, BMSCs were adjusted to 106/ml. Autologous BMSCs (1×106) suspension in PBS was implanted to decompression channel, and 1 ml PBS was injected in the control group.
Immunohistochemical assay
BMSCs implanted into the ONFH rabbits after 4 weeks. Rabbits were sacrificed by vein injection of air. The femoral head fragments were collected and sectioned. By using rabbit VEGF and BMP-2 primary antibody (BA0407 and BA0624, BOSTER, Wuhan) and anti-mouse HRP-conjugated secondary antibody, rabbit VEGF and BMP-2 was detected. The sections were visualized with motic digital slices scanning and application system.
Statistical analyses
All results were showed as mean ± SE. Statistical analysis was performed by using ANOVA, followed by Student’s t-test. P value <0.05 was considered to be statistically significant.
Results
The effect of BMSCs on OB in vitro
BMSCs enhanced the activity and inhibited the apoptosis of OB in co-cultured system
To determine the effect of BMSCs on OB in vitro, primary BMSCs and OB were isolated and co-cultured by transwell plate. We found that the cell activity of OB with BMSCs co-culture was significantly higher than that of the control (OB without co-culture) (P<0.05) by MTT assay (Figure 1A). Furthermore, the results of flow cytometry analysis showed that the apoptotic rates of OB were markedly decreased in the co-cultured group compared with the control group (P<0.05) (Figure 1B), and the results were quantified (Figure 1C). These results indicated that BMSCs enhanced the activity and inhibited the apoptosis of OB in noncontact co-cultured system.
Figure 1.
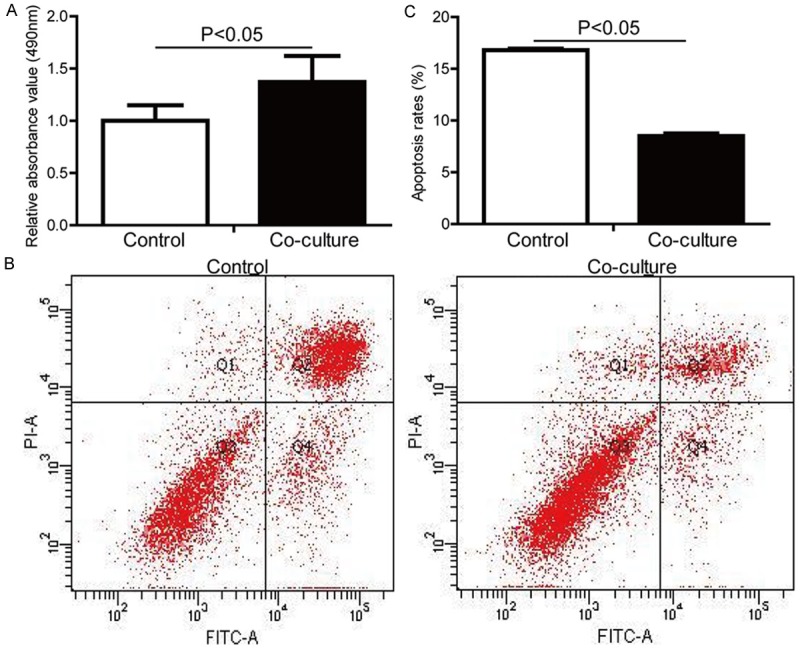
Primary BMSCs enhanced the activity and inhibited the apoptosis of primary OB in co-cultured system. Primary BMSCs and OB were co-cultured for 3 days in noncontact co-cultured system. A. The activity of OB was examined by MTT assay. B. The apoptosis of OB was measured by dual Annexin V-FITC and PI staining, following flow cytometry analysis. C. The result of flow cytometry analysis was quantified.
The concentrations of VEGF and BMP-2 in the medium of OB co-cultured with BMSCs were significantly increased
To illustrate cytokines secreted by BMSCs in co-cultured system, we quantified VEGF and BMP-2 in the co-culture medium by ELISA. The results showed that the concentrations of VEGF (Figure 2A) and BMP-2 (Figure 2B) in the co-cultured medium were higher than that of the control (OB without co-culture).
Figure 2.
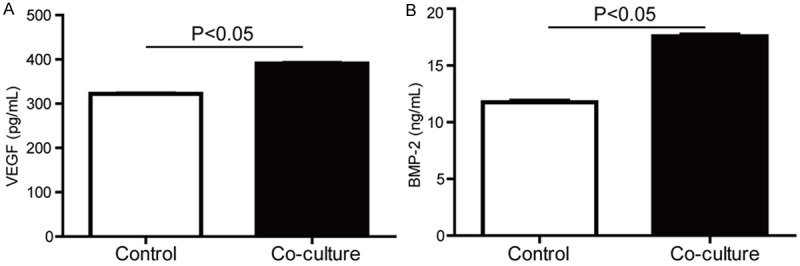
The concentrations of VEGF and BMP-2 in the medium of OB co-cultured with BMSCs were significantly increased. Primary BMSCs and OB were co-cultured for 3 days, and the supernatant was collected. (A) VEGF and (B) BMP-2 in the co-culture medium were quantified by ELISA.
The effect of BMSCs on OB in vivo
To further explore the effect of BMSCs on OB in vivo, we developed ONFH models in rabbit. Sagittal and Coronal MRI with T2-weighted fat suppression sequences showed that the blank group showed abnormal signal. However, femoral head of the ONFH rabbits exhibited inhomogeneous low signal area with visible articular fluid as black arrows depicted (Figure 3). MRI performance represented early avascular necrosis in ONFH rabbits, suggesting that the ONFH model was successfully developed.
Figure 3.
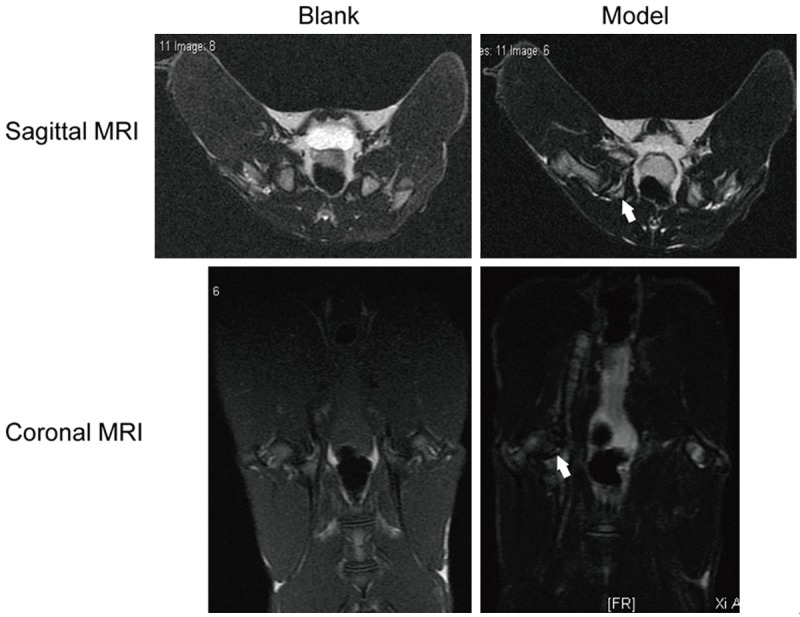
Development of ONFH models in rabbit. Rabbits were given 10 μg/kg Escherichia coli endotoxin LPS and 20 mg/kg methylprednisolone gluteal to induce ONFH model. Sagittal and Coronal MRI with T2-weighted fat suppression sequences showed the necrotic areas of femoral head. Black arrows depicted inhomogeneous low signal area in the ONFH model group.
Implantation of BMSCs increased OB and capillary in the ONFH rabbits
Next, BMSCs were implanted into the ONFH rabbits. The femoral head tissues were analyzed by H&E staining. We found that OB surrounding the trabecular bone and capillary were increased in BMSCs implantation group compared with the ONFH model group as black arrows depicted (Figure 4A). The ONFH model group developed necrosis of OB. Femoral trabecular was arranged regularly in the blank group.
Figure 4.
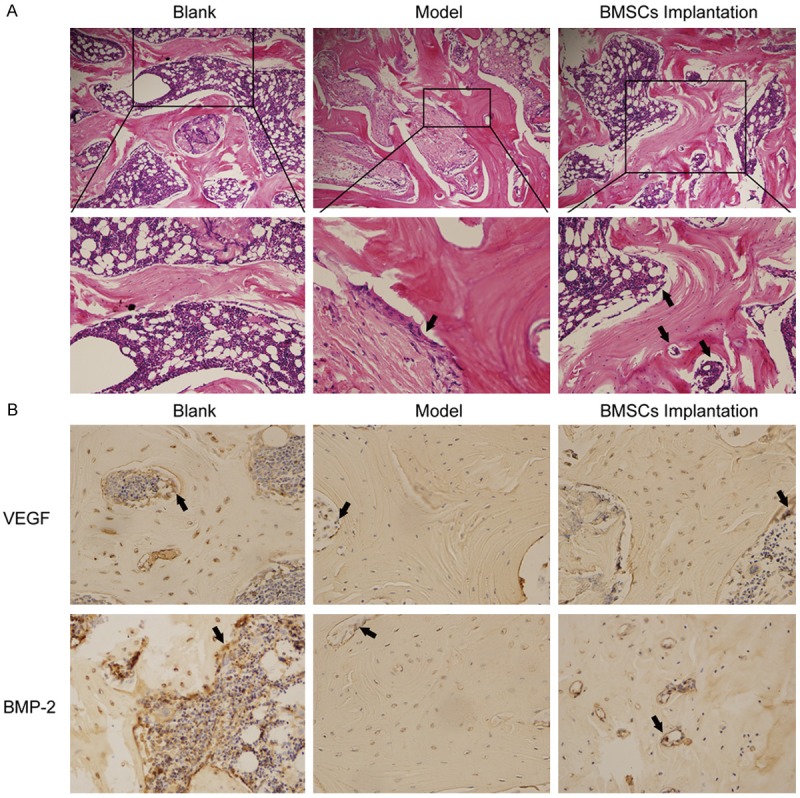
BMSCs implanted into the ONFH rabbits. BMSCs implanted into the ONFH rabbit for 4 weeks. The femoral head fragments were collected and sectioned. A. The femoral head tissues were analyzed by H&E staining. Black arrows depicted OB and capillary. B. VEGF and BMP-2 expressions were examined by immunohistochemistry.
VEGF and BMP-2 expressions in the necrotic areas of rabbits with BMSCs transplantation were increased
Similarly, VEGF and BMP-2 expressions were also examined by immunohistochemistry. We found that VEGF and BMP-2 expressions were increased in the BMSCs implantation group compared with the ONFH model group (Figure 4B). Expressions of VEGF and BMP-2 were positively stained in the blank group.
Discussion
ONFH is a common and severe disease with a high morbidity rate in the orthopedics field. Recent pioneer studies by Hernigou et al. [18] have demonstrated the efficacy of autologous BMSCs implantation into the femoral head during the early-stage of ONFH. Autologous BMSCs incurred neither an acute or chronic rejection response, nor a graft-versus-host disease. Thereby, autologous BMSCs implantation is a safe and effective treatment against early-stage ONFH. However, the underlying mechanisms remain unknown. Therefore, we aimed to investigate the effect of BMSCs on the early-stage ONFH.
Although the mechanisms of ONFH development remain elusive, the poor blood supply to the femoral head is mainly reason. Therefore, rebuilding and improving the blood supply has been proposed as an effective therapeutic measure for ONFH. Kinnaird et al. found that BMSCs could enhance new blood vessel growth both in vitro and in vivo models [19]. Here, we found that implantation of BMSCs increased capillary in the ONFH rabbits. The results indicated that BMSCs treated the ONFH possibly through improving blood supply.
Bone formation is a coordinated process involving BMPs and VEGF, orchestrating these factors may greatly enhance this process [20,21]. The combination of BMP-2 and VEGF induced significantly early bone formation, and VEGF might enhance BMP-2-induced bone formation [22]. Furthermore, local BMP-2 also affects the production and presence of VEGF [23]. In this study, we found that both VEGF and BMP-2 increased in the medium of co-culture. Importantly, the noncontact co-cultured system allows the passage of cells secreted molecules through the transwell hole, but does not allow direct cells contact, indicating that cytokines from BMSCs affected the proliferation and apoptosis of OB possibly by the paracrine way. Moreover, recent research also showed that BMSCs transplantation enhanced vascular regeneration mainly through a paracrine action of VEGF [24]. Therefore, our results supported that BMSCs may improve the cytokines of the microenvironment in the early stage ONFH.
In conclusion, BMSCs treated the early ONFH possibly through increasing OB and capillary, as well as VEGF and BMP-2 expressions in the femoral head. These results provided possible mechanisms for the treatment of early-stage ONFH with BMSCs transplantation.
Acknowledgements
This study was funded by Grants from the National Natural Science Foundation of China (No. 81172257) and the Science and Technology Project of Xi’an Municipality (HM1117(4)).
Disclosure of conflict of interest
None.
References
- 1.Ehlinger M, Moser T, Adam P, Bierry G, Gangi A, de Mathelin M, Bonnomet F. Early prediction of femoral head avascular necrosis following neck fracture. Orthop Traumatol Surg Res. 2011;97:79–88. doi: 10.1016/j.otsr.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Lee MS, Hsieh PH, Shih CH, Wang CJ. Non-traumatic osteonecrosis of the femoral head-from clinical to bench. Chang Gung Med J. 2010;33:351–360. [PubMed] [Google Scholar]
- 3.Hong JM, Kim TH, Kim HJ, Park EK, Yang EK, Kim SY. Genetic association of angiogenesisand hypoxia-related gene polymorphisms with osteonecrosis of the femoral head. Exp Mol Med. 2010;42:376–385. doi: 10.3858/emm.2010.42.5.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hernigou P, Beaujean F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res. 2002:14–23. doi: 10.1097/00003086-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Gangji V, Hauzeur JP, Matos C, De Maertelaer V, Toungouz M, Lambermont M. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. A pilot study. J Bone Joint Surg Am. 2004;86-A:1153–1160. doi: 10.2106/00004623-200406000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Yan ZQ, Chen YS, Li WJ, Yang Y, Huo JZ, Chen ZR, Shi JH, Ge JB. Treatment of osteonecrosis of the femoral head by percutaneous decompression and autologous bone marrow mononuclear cell infusion. Chin J Traumatol. 2006;9:3–7. [PubMed] [Google Scholar]
- 7.Deans RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol. 2000;28:875–884. doi: 10.1016/s0301-472x(00)00482-3. [DOI] [PubMed] [Google Scholar]
- 8.Niemeyer P, Szalay K, Luginbuhl R, Sudkamp NP, Kasten P. Transplantation of human mesenchymal stem cells in a non-autogenous setting for bone regeneration in a rabbit criticalsize defect model. Acta Biomater. 2010;6:900–908. doi: 10.1016/j.actbio.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 10.Wu X, Huang L, Zhou Q, Song Y, Li A, Jin J, Cui B. Mesenchymal stem cells participating in ex vivo endothelium repair and its effect on vascular smooth muscle cells growth. Int J Cardiol. 2005;105:274–282. doi: 10.1016/j.ijcard.2004.12.090. [DOI] [PubMed] [Google Scholar]
- 11.Al-Khaldi A, Eliopoulos N, Martineau D, Lejeune L, Lachapelle K, Galipeau J. Postnatal bone marrow stromal cells elicit a potent VEGF dependent neoangiogenic response in vivo. Gene Ther. 2003;10:621–629. doi: 10.1038/sj.gt.3301934. [DOI] [PubMed] [Google Scholar]
- 12.Annabi B, Naud E, Lee YT, Eliopoulos N, Galipeau J. Vascular progenitors derived from murine bone marrow stromal cells are regulated by fibroblast growth factor and are avidly recruited by vascularizing tumors. J Cell Biochem. 2004;91:1146–1158. doi: 10.1002/jcb.10763. [DOI] [PubMed] [Google Scholar]
- 13.Peng W, Wang L, Zhang J, Deng J, Gong Y, Li S, Hu Y. A novel tissue-engineered bone in repairing femoral head defect and necrosis. Int J Clin Exp Med. 2015;8:1087–1093. [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang W, Zhang F, Shi H, Tan R, Han S, Ye G, Pan S, Sun F, Liu X. Comparisons of rabbit bone marrow mesenchymal stem cell isolation and culture methods in vitro. PLoS One. 2014;9:e88794. doi: 10.1371/journal.pone.0088794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhat FA, Ramajayam G, Parameswari S, Vignesh RC, Karthikeyan S, Senthilkumar K, Karthikeyan GD, Balasubramanian K, Arunakaran J, Srinivasan N. Di 2-ethyl hexyl phthalate affects differentiation and matrix mineralization of rat calvarial osteoblasts--in vitro. Toxicol In Vitro. 2013;27:250–256. doi: 10.1016/j.tiv.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Cao XY, Yin MZ, Zhang LN, Li SP, Cao Y. Establishment of a new model for culturing rabbit osteoblasts in vitro. Biomed Mater. 2006;1:L16–19. doi: 10.1088/1748-6041/1/4/L02. [DOI] [PubMed] [Google Scholar]
- 17.Zhang C, Ma J, Li M, Li XH, Dang XQ, Wang KZ. Repair effect of coexpression of the hVEGF and hBMP genes via an adeno-associated virus vector in a rabbit model of early steroid-induced avascular necrosis of the femoral head. Transl Res. 2015;166:269–280. doi: 10.1016/j.trsl.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Hernigou P, Poignard A, Zilber S, Rouard H. Cell therapy of hip osteonecrosis with autologous bone marrow grafting. Indian J Orthop. 2009;43:40–45. doi: 10.4103/0019-5413.45322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, Epstein SE. Marrow derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 20.Peng H, Wright V, Usas A, Gearhart B, Shen HC, Cummins J, Huard J. Synergistic enhancement of bone formation and healing by stem cell expressed VEGF and bone morphogenetic protein-4. J Clin Invest. 2002;110:751–759. doi: 10.1172/JCI15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Street J, Bao M, deGuzman L, Bunting S, Peale FV Jr, Ferrara N, Steinmetz H, Hoeffel J, Cleland JL, Daugherty A, van Bruggen N, Redmond HP, Carano RA, Filvaroff EH. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A. 2002;99:9656–9661. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samee M, Kasugai S, Kondo H, Ohya K, Shimokawa H, Kuroda S. Bone morphogenetic protein-2 (BMP-2) and vascular endothelial growth factor (VEGF) transfection to human periosteal cells enhances osteoblast differentiation and bone formation. J Pharmacol Sci. 2008;108:18–31. doi: 10.1254/jphs.08036fp. [DOI] [PubMed] [Google Scholar]
- 23.Deckers MM, van Bezooijen RL, van der Horst G, Hoogendam J, van Der Bent C, Papapoulos SE, Löwik CW. Bone morphogenetic proteins stimulate angiogenesis through asteoblast-derived vascular endothelial growth factor A. Endocrinology. 2002;143:1545–1553. doi: 10.1210/endo.143.4.8719. [DOI] [PubMed] [Google Scholar]
- 24.Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5:623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]


