Abstract
In recent years, the incidence of type 1 diabetes mellitus (T1DM) has been increasing. The role of CXCL10 and its receptor, CXCR3, in the occurrence of T1DM has drawn lots of research interests, as the disease incidence was correlated with their expression levels. We thus used an antagonist of CXCR3, NBI-74330, to block the specific binding, for further observation of islet cell apoptosis in a T1MD rat model. A total of 80 SD rats were given STZ intraperitoneally for generating T1DM model. Different concentrations of NBI-74330 were then applied, followed by the collection of blood and pancreatic tissue samples. CXCL10 and CXCR3 levels were detected by enzyme linked immunosorbent assay (ELISA), followed by expressional assays in pancreatic tissues by real-time PCR, Western blotting and flow cytometry. Compared to control group, model rats had significantly elevated blood glucose level (>16.7 mmol/L), with depressed CXCL10 and CXCR3 levels compared to model group (P<0.05). After NBI-74330 treatment, mRNA and protein levels of CXCL10 and CXCR3 were significantly lowered, with significantly decreased apoptotic cell ratios compared to model group (P<0.05). CXCL10 receptor antagonist NBI-74330 can inhibit the apoptosis of pancreatic islet cells in T1DM rats.
Keywords: Type 1 diabetes mellitus, NBI-74330, CXCL10, CXCR3
Introduction
Type 1 diabetes mellitus (T1DM), also called insulin-dependent diabetes, is caused by insufficient insulin secretion due to genetic and environmental factors, and is mostly occurred in children and adolescents [1]. T1DM has relatively fast disease progression and high incidence of ketoacidosis, thus severely affecting patient’s life span [2]. It has been suggested that the incidence of T1DM will continuously increase, with transition of lifestyles [3]. Pathological studies have revealed the infiltration of lymphocytes into pancreatic islet cells, following genetic, environmental and viral infections [4]. The precise mechanism underlying pathogenesis, however, remained unclear. Recent studies have focused on the role of chemokines and their receptors in T1DM pathogenesis. During the early stage of pancreatitis, CXC chemokine ligand-10 (CXCL10) has been expressed in islet β cells, following by elevated expression with disease aggravation [5]. In one T1DM-targeted study, CXCL10 contents in serum started to increase during early stage [6], while individuals with intact islet tissues had barely any CXCL10 expression [7]. An animal study has shown the decrease of diabetic incidence in mice after inhibiting endogenous expression of CXCL10 [8]. The in vivo biological function of CXCL10 requires specific bindings onto several receptors, among which CXCR3 plays a crucial role. In newly diagnosed T1DM patients, both CXCL10 and CXCR3 were up-regulated [9]. The incidence of diabetes in CXCR3-deficient mice was significantly depressed, even with de novo up-regulation of CXCL10 expression [10]. All those studies suggested the occurrence of islet cell injury by β cells-derived CXCL10, which can bind with CXCR3 to activate downstream signal pathways for modulating diabetes pathogenesis. NBI-74330 has been developed as one antagonist for CXCR3 for blocking their specific interactions [11]. This study thus applied NBI-74330 on T1DM model rats, for observing the cell apoptosis of islet tissues and expressional profiles of CXCL10 and CXCR3, thus investigating the role of CXCL10 receptor antagonist on islet cells during T1DM pathogenesis.
Materials and methods
Animals
A total of 80 male SD rats (Fujian Medical University Animal Center, China) were kept in a facility with fixed temperature (22°C ± 2°C), humidity (50% ± 5%) and 12/12-hour light cycle. Animals were randomly assigned to either of control or diabetic model group, which received saline or 30 mg/kg STZ via intraperitoneal injection, respectively. Blood glucose level was monitored before and 1, 2, 3, and 4 weeks after injection. The successful generation of T1DM model was identified by higher blood glucose level (<16.7 mmol/L) in two consecutive measures, accompanied with diabetic symptoms including polyuria, polydipsia, polyphagia and body weight lost. Those rats with T1DM symptoms were further sub-divided into model, low-, moderate- and high-NBI-74330 group, which received saline, 2 μg/(kg.d), 4 μg/(kg.d) and 8 μg/(kg.d) drugs via subcutaneous injection for 8 weeks. 4 hours after the last injection, artery blood samples were collected from rats, which were then decapitated for collecting islet tissues.
Rats were used for all experiments, and all procedures were approved by the Animal Ethics Committee of Zhangzhou Hospital Affiliated to Fujian Medical University.
Enzyme linked immunosorbent assay (ELISA)
Rat blood samples (1 mL) was centrifuged at 4°C and 12000 g for 5 min. The upper aqueous layer was collected and diluted into 96-well plate in triplicates, along with parallel standards in serial dilutions. After 37°C incubation for 30 min, supernatants were discarded, followed by washing (in 0.2 mL washing buffer) for 5 times. Enzyme-linked reagents were then added to each well, followed by 37°C incubation for 30 min. Chromogenic substrates (0.1 mL) were then added to develop the signal (37°C for 15 min). A microplate reader was used to quantify absorbance value at 450 nm. A standard curve was firstly plotted using standard samples, followed by the deduction of CXCL10 and CXCR3 concentrations.
Real-time PCR
Total RNA was extracted from pancreatic tissues by Trizol reagents (Invitrogen, US). 1 μg RNA was used as the template to synthesize cDNA using reverse transcription kit (Toyobo, Japan) by incubation at 37°C (15 min) and 95°C (10 min). Real-time PCR was carried out using cDNA as the template and specific primers (Table 1) in a VIIA 7 cycler (ABI, US) under following conditions: 95°C pre-denature for 5 min, followed by 40 cycles each containing 95°C denature for 15 sec, 60°C annealing for 60 sec and 72°C elongation for 60 sec. Semi-quantitative analysis was performed using 2-ΔΔCt method.
Table 1.
PCR primer list
| Gene | Forward (5’-3’) | Reverse (5’-3’) |
|---|---|---|
| β-actin | GAGGGAAATCGTGCGTGAC | CTGGAAGGTGGACAGTGAG |
| CXCL10 | GGACAAATCGTATCTCGG | GAAACCAACTCTACGCTG |
| CXCR3 | GAAGAAGGACCCCAGATC | TGGAAGGCAGTGCTCTAGTC |
Western blotting
About 1 g of pancreatic tissues was homogenized for mixing with 1 mL RIPA lysis buffer (Beyotime, China) containing proteinase inhibitor. The mixture was then kept on ice and ruptured by ultrasonic. Proteins were extracted by 12000 g centrifugation for 10 min, and were quantified by BCA method. Proteins were firstly separated in a vertical electrical field, and were transferred to NC membrane (Life Science, US). The membrane was firstly blocked in 5% defatted milk powder for 2 hours, followed by 1:1 000 primary antibody (Abcam, US) incubation overnight at 4°C. On the next day, the membrane was rinsed in PBST for three times, followed by adding 1:10000 secondary antibody for 1-hour incubation. Chromogenic substrates were added to develop the membrane, which was exposed in a dark room. GIS-2020D gel imaging analysis system was used to analyze the optical density of protein bands, for further deduction of protein expression level.
Flow cytometry
Fresh pancreatic tissues were firstly fixed in 70% ethanol for 24 hours, and were then cut into small pieces for homogenization in saline. Cell mixture was collected and centrifuged at 1 000 g for 5 min. After discarding the supernatants, 5 mL blocking buffer was added to rinse the cells for 10 min at room temperature. The supernatants were discarded after 1 000 g centrifugation (5 min), followed by adding 0.3 mL anti-insulin-FITC dye and PI dye. Samples were loaded on flow cytometry (BD, US) for detecting the percentage of double-positive cells.
Statistical analysis
SPSS 19.0 software was used to process all collected data, of which measurement data were presented as mean ± standard deviation (SD) and were tested by student t-test or analysis of variance (ANOVA). While enumeration data were presented as percentage and compared by chi-square test. A statistical significance was defined when P<0.05.
Results
Blood glucose level
Before STZ injection, blood glucose levels in control and model rats were 4.45 ± 1.81 mmol/L and 4.38 ± 1.75 mmol/L, respectively. Those rats with successful T1DM model had significantly elevated blood glucose level (maintaining above 16.7 mmol/L, Table 2, P<0.05).
Table 2.
Blood glucose level of rats before andafter STZ injection (in mmol/L)
| Group | Before | After STZ | |||
|---|---|---|---|---|---|
|
| |||||
| 1 w | 2 w | 3 w | 4 w | ||
| Control | 4.45 ± 1.81 | 4.51 ± 1.64 | 4.62 ± 1.28 | 4.71 ± 1.79 | 4.75 ± 1.93 |
| Model | 4.38 ± 1.75 | 17.85 ± 2.74* | 18.64 ± 3.08* | 19.31 ± 3.52* | 19.91 ± 3.58* |
P<0.05 compared to control group.
Serum CXCL10 and CXCR3 levels
Using ELISA approach, we found significantly depressed CXCL10 and CXCR3 levels in NBI-74330 treatment group compared to control group (CXCL10: 9.61 ± 2.15 μg/mL, 6.87 ± 1.82 μg/mL, 5.51 ± 1.54 μg/mL and 4.37 ± 1.48 μg/mL for control, low-, moderate- and high-dosage of drugs, respectively, F=9.658, P<0.05, Figure 1A; CXCR3: 15.19 ± 3.72 μg/mL, 11.76 ± 2.61 μg/mL, 9.34 ± 2.31 μg/mL and 8.02 ± 1.82 μg/mL for control, low-, moderate- and high-dosage of drugs, respectively, F=18.216, P<0.05, Figure 1B). NBI-74330 inhibited expression of those factors in a dose-dependent manner.
Figure 1.
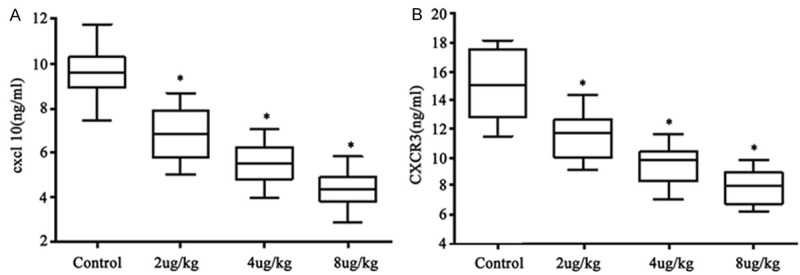
CXCL10 (A) and CXCR3 (B) expression level in serum. *P<0.05 compared to control group.
CXCL10 and CXCR3 expression in pancreatic tissues
Using real-time fluorescent PCR, we detected mRNA levels of CXCL10 and CXCR3 in pancreatic tissues and found significantly depressed expressions in drug-treating group (CXCL10: 65%, 57% and 42% against control group for low-, moderate- and high-dosage of drugs, respectively, F=11.715, P<0.05, Figure 2A; CXCR3: 59%, 47% and 38% against control group for low-, moderate- and high-dosage of drugs, respectively, F=13.351, P<0.05, Figure 2B).
Figure 2.
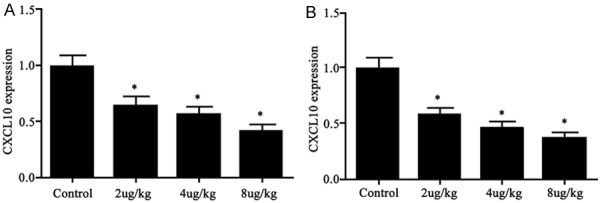
CXCL10 (A) and CXCR3 (B) mRNA levels. *P<0.05 compared to control group.
CXCL10 and CXCR3 protein level
Western blotting obtained consistent results as those in RT-PCR. As shown in Figure 3, CXCL10 protein expression level was decreased to 63% of the control group in average after drug treatment (F=9.724, P<0.05, Figure 3B). Meanwhile, CXCR3 levels were decreased to 41% against control (F=12.837, P<0.05, Figure 3C). Both protein levels in pancreatic tissues were decreased with higher concentrations of NBI-74330.
Figure 3.
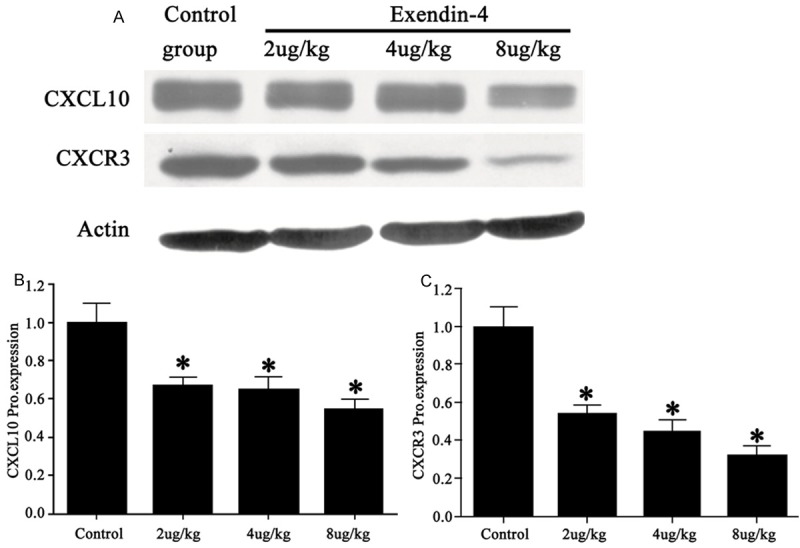
CXCL10 (B) and CXCR3 (C) protein levels in pancreatic tissues of rats. (A) representative protein bands. *P<0.05 compared to control group.
Apoptosis of pancreatic cells
To further investigate the protective function of NBI-74330 on pancreatic cells, we also employed flow cytometry to observe the effect of NBI-74330 on apoptosis of pancreatic cells. As shown in Figure 4, about 14.15% of primary cultured islet cells had apoptosis (Figure 4A). Such ratio, however, was decreased to 10.35% (Figure 4B), 9.65% (Figure 4C) and 6.45% (Figure 4D) for low-, moderate- and high-dosage of drugs. Compared to control group, NBI-74330 had significantly decreased apoptotic cells in pancreatic tissues (F=10.824, P<0.05).
Figure 4.
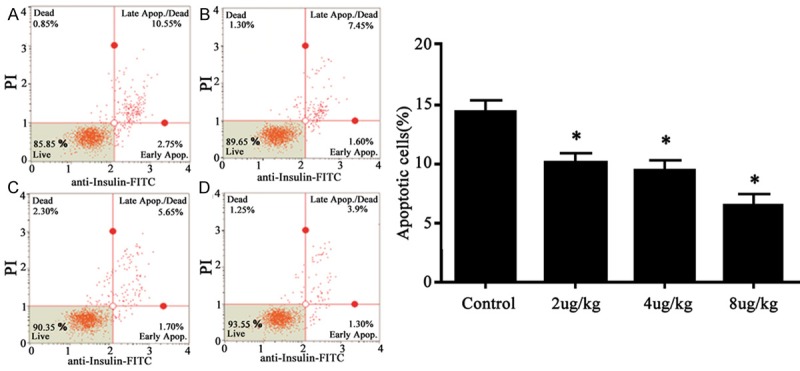
Effect of NBI-74330 on apoptosis of pancreatic tissues. (A) Control; (B) Low-dosage drugs; (C) Moderate-dosage drugs; (D) High-dosage drugs. Right panel, quantitative analysis of (A) to (D). *P<0.05 compared to control group.
Discussion
More than 6 million people in China are T1DM patients, with an increasing trend of incidence [12]. T1DM is now believed to be one auto-immune disease featured with insulin deficiency and hyperglycemia, and is caused by interaction of multiple endogenous and exogenous factors. As one DNA alkylating agent, STZ exerts certain selective injury on islet B cells, thus inducing T1DM in rodents [13]. As one antagonist for CXCR3, NBI-74330 can block the specific binding between CXCL10 and CXCR3 [14]. Other studies also identified the critical importance of CXCL10 and its receptor CXCR3 in pathogenesis of T1DM [15]. As one chemokine family, CXC includes various factors such as CXCL9, CXCL10 and CXCL11. Mainly derived from activated fibroblasts, mononuclear macrophage and endothelial cells [16], CXCL10 requires the interaction with its receptor CXCR3, for further activating downstream signals regarding cell growth, proliferation, differentiation, apoptosis and carcinogenesis [16,17]. Early study has confirmed the up-regulation of CXCL10 and CXCR3 in diabetic patients [18]. The inhibition of endogenous expression of CXCL10 and CXCR3 can decrease the incidence of diabetes [19], suggesting the correlation between CXCL10 and diabetes. Based on these information, we thus treated T1DM rats with NBI-74330, in an attempt to investigate the effect of drugs on islet cell apoptosis.
Our results showed significant tissue injury on rat’s islet by STZ, thus inducing diabetes, as shown by elevated blood glucose level with typical symptoms of diabetes. We further treated those diabetic rats with NBI-74330 and found lower serum levels of chemokines CXCL10 and its receptor CXCR3. Therefore the drug can inhibit the expression of those factors in a dose-dependent manner. Consequent RT-PCR and Western blotting assays obtained consistent results in pancreatic tissues, which had significantly depressed expression of CXCL10 and CXCR3 after treating with NBI-74330. These results collectively suggested the inhibition of chemokine expression by NBI-74330 in T1DM rats. Early study has established the modulation of CXCL10 on cell activity via specific binding onto receptor CXCR3, for activating downstream pathway and inducing cell proliferation/apoptosis [20]. This study showed the inhibition of NBI-74330 on CXCL10 and CXCR3 expression in islet cells. As previous study has established the role of NBI-74330 in decreasing diabetes incidence in rats, we thus investigated the potential mechanism of drugs by observing the apoptosis condition in pancreatic tissues of T1DM rats after drug exposure. Our results showed a dose-dependent decrease of apoptotic cell percentage in islet tissues after drug application, suggesting the inhibition of cell apoptosis by NBI-74330.
In summary, our study showed the inhibition of expression of CXCL10 and its receptor CXCR3 in islet tissues by NBI-74330, for further suppressing of cell apoptosis and protection, thus decreasing diabetes incidence. However, due to the inherent complication of pathogenesis mechanism underlying diabetes, further experiments are required to elucidate the precise biological functions of NBI-74330 in diabetes.
Acknowledgements
Research supported by the Medical innovation of Fujian Province (2015-CX-38).
Disclosure of conflict of interest
None.
References
- 1.Bendas A, Rothe U, Kiess W, Kapellen TM, Stange T, Manuwald U, Salzsieder E, Holl RW, Schoffer O, Stahl-Pehe A, Giani G, Ehehalt S, Neu A, Rosenbauer J. Trends in Incidence Rates during 1999-2008 and Prevalence in 2008 of Childhood Type 1 Diabetes Mellitus in GERMANY-Model-Based National Estimates. PLoS One. 2015;10:e0132716. doi: 10.1371/journal.pone.0132716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patton SR, Goggin K, Clements MA. The Cost of a Healthier Diet for Young Children With Type 1 Diabetes Mellitus. J Nutr Educ Behav. 2015;47:361–366. e1. doi: 10.1016/j.jneb.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kakleas K, Soldatou A, Karachaliou F, Karavanaki K. Associated autoimmune diseases in children and adolescents with type 1 diabetes mellitus (T1DM) Autoimmun Rev. 2015;14:781–97. doi: 10.1016/j.autrev.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Mohamad IL, Saad K, Abdel-Azeem A, Mohamed SA, Othman HA, Abdel Baseer KA, Thabet AF, El-Houfey AA. Evaluation of pulmonary function changes in children with type 1 diabetes mellitus in Upper Egypt. Ther Adv Endocrinol Metab. 2015;6:87–91. doi: 10.1177/2042018815580514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wightman SC, Uppal A, Pitroda SP, Ganai S, Burnette B, Stack M, Oshima G, Khan S, Huang X, Posner MC, Weichselbaum RR, Khodarev NN. Oncogenic CXCL10 signalling drives metastasis development and poor clinical outcome. Br J Cancer. 2015;113:327–35. doi: 10.1038/bjc.2015.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosa JS, Mitsuhashi M, Oliver SR, Ogura M, Flores RL, Pontello AM, Galassetti PR. Ex vivo TCR-induced leukocyte gene expression of inflammatory mediators is increased in type 1 diabetic patients but not in overweight children. Diabetes Metab Res Rev. 2010;26:33–9. doi: 10.1002/dmrr.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cossu A, Ferrannini E, Fallahi P, Antonelli A, Sechi LA. Antibodies recognizing specific Mycobacterium avium subsp. paratuberculosis’s MAP3738c protein in type 1 diabetes mellitus children are associated with serum Th1 (CXCL10) chemokine. Cytokine. 2013;61:337–9. doi: 10.1016/j.cyto.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Ahmadi Z, Arababadi MK, Hassanshahi G. CXCL10 activities, biological structure, and source along with its significant role played in pathophysiology of type I diabetes mellitus. Inflammation. 2013;36:364–71. doi: 10.1007/s10753-012-9555-1. [DOI] [PubMed] [Google Scholar]
- 9.Schulthess FT, Paroni F, Sauter NS, Shu L, Ribaux P, Haataja L, Strieter RM, Oberholzer J, King CC, Maedler K. CXCL10 impairs beta cell function and viability in diabetes through TLR4 signaling. Cell Metab. 2009;9:125–39. doi: 10.1016/j.cmet.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Dabelea D. The accelerating epidemic of childhood diabetes. Lancet. 2009;373:1999–2000. doi: 10.1016/S0140-6736(09)60874-6. [DOI] [PubMed] [Google Scholar]
- 11.Scholten DJ, Roumen L, Wijtmans M, Verkade-Vreeker MC, Custers H, Lai M, de Hooge D, Canals M, de Esch IJ, Smit MJ, de Graaf C, Leurs R. Identification of overlapping but differential binding sites for the high-affinity CXCR3 antagonists NBI-74330 and VUF11211. Mol Pharmacol. 2014;85:116–26. doi: 10.1124/mol.113.088633. [DOI] [PubMed] [Google Scholar]
- 12.Roep BO, Kleijwegt FS, van Halteren AG, Bonato V, Boggi U, Vendrame F, Marchetti P, Dotta F. Islet inflammation and CXCL10 in recent-onset type 1 diabetes. Clin Exp Immunol. 2010;159:338–43. doi: 10.1111/j.1365-2249.2009.04087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bortolin RH, da Graça Azevedo Abreu BJ, Abbott Galvão Ururahy M, Costa de Souza KS, Bezerra JF, Loureiro MB, da Silva FS, Marques DE, Batista AA, Oliveira G, Luchessi AD, Lima VM, Miranda CE, Lia Fook MV, Almeida Md, de Rezende LA, de Rezende AA. Protection against T1DM-Induced Bone Loss by Zinc Supplementation: Biomechanical, Histomorphometric, and Molecular Analyses in STZ-Induced Diabetic Rats. PLoS One. 2015;10:e0125349. doi: 10.1371/journal.pone.0125349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Wanrooij EJ, de Jager SC, van Es T, de Vos P, Birch HL, Owen DA, Watson RJ, Biessen EA, Chapman GA, van Berkel TJ, Kuiper J. CXCR3 antagonist NBI-74330 attenuates atherosclerotic plaque formation in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:251–7. doi: 10.1161/ATVBAHA.107.147827. [DOI] [PubMed] [Google Scholar]
- 15.Korniejewska A, McKnight AJ, Johnson Z, Watson ML, Ward SG. Expression and agonist responsiveness of CXCR3 variants in human T lymphocytes. Immunology. 2011;132:503–15. doi: 10.1111/j.1365-2567.2010.03384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu C, Luo D, Reynolds BA, Meher G, Katritzky AR, Lu B, Gerard CJ, Bhadha CP, Harrison JK. Chemokine receptor CXCR3 promotes growth of glioma. Carcinogenesis. 2011;32:129–37. doi: 10.1093/carcin/bgq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yue C, Shen S, Deng J, Priceman SJ, Li W, Huang A, Yu H. STAT3 in CD8+ T Cells Inhibits Their Tumor Accumulation by Downregulating CXCR3/CXCL10 Axis. Cancer Immunol Res. 2015;3:864–70. doi: 10.1158/2326-6066.CIR-15-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruffilli I, Ferrari SM, Colaci M, Ferri C, Politti U, Antonelli A, Fallahi P. CXCR3 and CXCL10 in autoimmune thyroiditis. Clin Ter. 2014;165:e237–42. doi: 10.7417/CT.2014.1727. [DOI] [PubMed] [Google Scholar]
- 19.Saxena A, Bujak M, Frunza O, Dobaczewski M, Gonzalez-Quesada C, Lu B, Gerard C, Frangogiannis NG. CXCR3-independent actions of the CXC chemokine CXCL10 in the infarcted myocardium and in isolated cardiac fibroblasts are mediated through proteoglycans. Cardiovasc Res. 2014;103:217–27. doi: 10.1093/cvr/cvu138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antonelli A, Ferrari SM, Corrado A, Ferrannini E, Fallahi P. CXCR3, CXCL10 and type 1 diabetes. Cytokine Growth Factor Rev. 2014;25:57–65. doi: 10.1016/j.cytogfr.2014.01.006. [DOI] [PubMed] [Google Scholar]


