Abstract
Rheumatoid arthritis is an autoimmune disease characterized as joint synovial inflammation. MicroRNA is a group of small noncoding RNA molecules discovered in recent years that can posttranscriptional regulate mRNA expression and involved in a variety processes of immune cell activation and differentiation. There is still lack of study about the role of miR-451 in rheumatoid arthritis. Synovial fibroblasts isolated from rheumatoid arthritis patients were cultured in vitro. Chemical synthesized miR-451 was lipo-transfected, real-time RT-PCR was applied to detect miR-451 expression level, and MTT method was used to detect the effect of miR-451 on synovial fibroblasts proliferation. Enzyme-linked immunosorbent assay was used to detect tumor necrosis factor TNF-α, IL-1β, and IL-6 level in the supernatant. Western blot was applied to test target protein p38 MAPK expression level. Our study found that synovial fibroblasts expressed higher miR-451 mRNA level in miR-451 treatment group. MiR-451 treatment significantly decreased cell proliferation ability (P < 0.05). Compared with the control, p38 MAPK protein expression reduced obviously in the miR-451 treatment group (P < 0.05). MiR-451 transfected synovial fibroblasts secreted lower levels of TNF-α (198 ± 12 pg/ml vs 124 ± 13 pg/ml, P < 0.01), IL-1β (352 ± 43 pg/ml vs 165 ± 87 pg/ml, P < 0.01), and IL-6 (487 ± 84 pg/ml vs 257 ± 92 pg/ml, P < 0.01). The results proved that miR-451 can down-regulate p38 MAPK protein expression, and reduce synovial fibroblasts proliferation and cytokine expression level.
Keywords: miR-451, rheumatoid arthritis, synovial fibroblast, cell proliferation
Introduction
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease characterized by multiple joints’ synovial chronic inflammation in clinic. Immune function abnormality is common among RA patients. Further elucidating the role of immune factors in RA incidence is beneficial to RA prevention and treatment [1-4].
MicroRNA (miRNA) is a noncoding single-stranded RNA molecule composed of 22 nucleotides. It is encoded by endogenous gene and involved in gene expression regulation [5]. It plays an important role in various kinds of physiological processes [6-8]. In recent years, following the rapid development of molecular biology technology, numerous clinical and basic scholars have reported a variety of microRNA abnormally expressed in RA patients and animal models [9-11].
MiR-451 mainly expressed in the plasma. It can regulate cell growth, apoptosis, and tumor development [12,13]. Recently, in vitro study showed that miR-451 could regulate cytokines secreted by dendritic cells under certain condition [14]. MiRNA abnormal expression and pathogenic role in RA becomes a hot spot in recent research. However, there is still no report about miRNA-451 expression and role in RA.
Specially, miRNA can only exert its biological effect by activating specific signaling pathway. p38 mitogen-activated protein kinases (p38 MAPK) is a main signaling pathway in the cell. It is not only related to cell apoptosis, cell movement, and a variety of pathophysiological process in stress [15], and but also play an important role in the pathological process in mediating inflammation and cytokine generation. p38MAPK pathway is thought to be an important regulating target during normal and disease states of RA.
Thus, this experiment mainly studied the effect and mechanism of miRNA-451 on synovial fibroblasts proliferation and inflammatory factors secretion in RA patients.
Materials and methods
The clinical trial was approved by the ethics committee in our hospital. All enrolled patients had signed the informed consent. Articular synovial tissue was collected from the RA patients in the department of joint surgery receiving joint replacement. RA diagnosis was performed according to the American rheumatism association standard. Patients were between 16 and 60 years old with no other complications and received no immune inhibitors or similar drugs.
Materials, reagents and instruments
Real time PCR, confocal microscope (Bio-Rad), ECL chemiluminescence reagent kit (Amersham), CO2 cell incubator and flow cytometry (BD), etc. RNA extraction kit was obtained from Takara; miR-451 was bought from Genepharma (Shanghai, China); TaqMan miRNA real-time RT-PCR Assays kit and TaqMan miRNA Reverse Transcription kit were got from Applied Biosystems (USA); Enzyme-linked immunosorbent Assay kit was purchased from BD company (USA); Lipofectamine 2000 was bought from Invitrogen (USA); Primary antibodies for p38 MAPK and β-actin were from Santa Cruz (USA).
Synovial fibroblasts culture
Knee joint synovial tissue was collected from the RA patient receiving joint replacement in the department of joint surgery. The tissue was cut into pieces in the sterile condition and digested by trypsin under the condition of 37°C for two hours. The synovial fibroblasts were collected after centrifugation and maintained in DMEM medium containing 5 mmol/L glucose and containing 10% fetal bovine serum in a humid atmosphere containing 5% CO2 at 37°C. The cells were passaged when they grow to fusion. 4-6th generation of cells were used for the experiment.
Cell grouping and transfection
Synovial fibroblasts were divided into the miR-451 treatment group and control group when grow to 80%. MiR-451 or control was transfected to the synovial fibroblasts mediated by Lipofectamine 2000, respectively and cultured for 6 hours. After changing the DMEM medium and continue cultured for 48 h, the synovial fibroblasts were harvested.
RT-PCR
Total RNA was extracted from the synovial fibroblasts and reverse transcripted to cDNA for PCR amplification. The PCR primers were designed by Primer 5.0 software as follows: miR-451 sense 5’-AAACCGUUACCAUUACUGAGUU-3’ anti-sense 5’-UAGUAAUGGUAAUGGUUCUC-3’ β-actin sense 5’-GGTGTGATGGTGGGTATGGGT-3’ anti-sense 5’-CTGGGTCATCTTTTCACGGT-3’. The cycling conditions consisted of an initial, single cycle of 5 min at 94°C, followed by 30 cycles of 60 s at 94°C, 60 s at 60°C, and 3 min at 72°C. PCR products were tested by agarose gel electrophoresis and analyzed by an optimized comparative Ct (ΔΔCt) value method.
MTT
Cells were seeded in 96-well plates at a density of 2 × 104 cells/well and incubated overnight at 37°C. The cells were incubated with miR-451 for 48 h at 37°C. After addition of 20 µL of MTT solution (5 g/L) to each well, plates were incubated for 4 h at 37°C. After removing the medium, 150 μL DMSO was added and oscillated for 10 min. Absorbance of each well at 570 nm was read using a spectrophotometer.
Western blot
Total protein was extracted and separated by denaturing 10% SDS-polyacrylamide gel electrophoresis. Detection was performed with ECL chemiluminescence system. Antibody dilutions were 1:200 for p38 MAPK, 1:500 for IL-1β, and 1:2000 for β-actin. Protein levels were normalized to β-actin and changes were determined.
Elisa
TNF-α, IL-1β, and IL-6 expression levels in the supernatant were determined by ELISA according to the specification in three duplicates for each sample.
Statistical analysis
All statistical analyses were performed using SPSS17.0 software (Chicago, IL). Numerical data were presented as means and standard deviation (± SD). Differences between means were analyzed using one-way ANOVA or paired t test when necessary. P < 0.05 was considered to indicate a statistically significant result.
Results
MiR-451 RNA expression in synovial fibroblasts
miR-451 level was 2.06 ± 0.12 and 0.93 ± 0.06 in the miR-451 treatment group and control, respectively. MiR-451 expression level was significantly higher in the treatment group than the control (P < 0.01, Figure 1).
Figure 1.
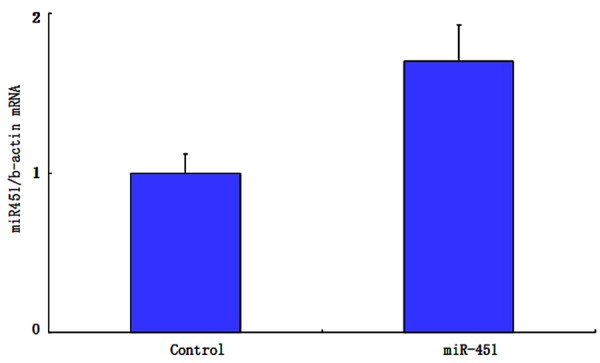
miR-451 expression level in two groups.
The effect of miR-451 on synovial fibroblasts proliferation MTT test results showed that compared with the control group, miR-451 treatment group grew obviously slower than in the 2nd and 3rd day (P < 0.05). It suggested that overexpressed miR-451 significantly decreased cell proliferation ability (P < 0.05) (Figure 2).
Figure 2.
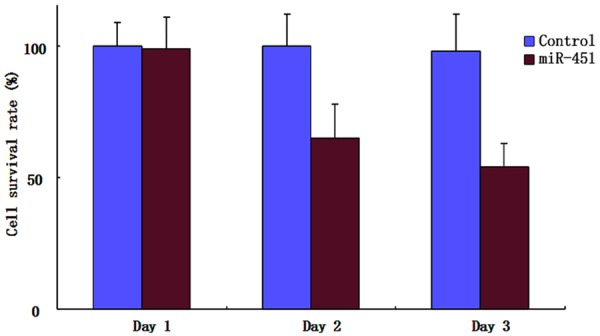
MTT test on cell proliferation.
P38 MAPK expression
Compared with the control, p38 MAPK protein expression reduced obviously in the miR-451 treatment group (P < 0.05, Figure 3).
Figure 3.
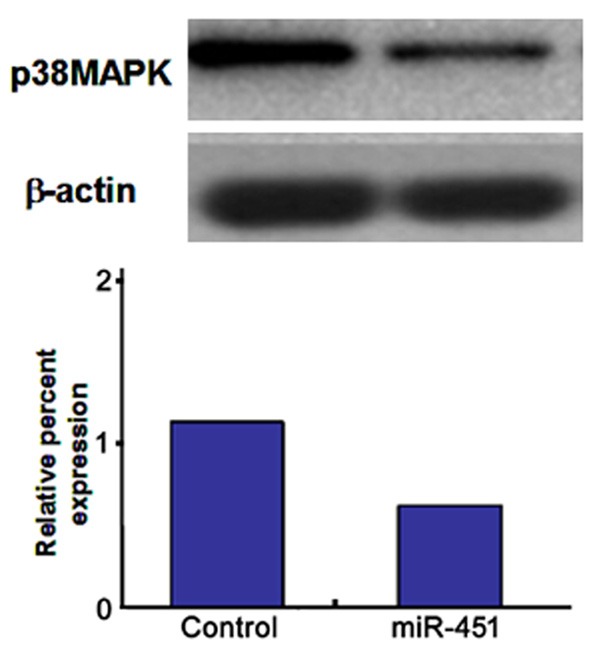
p38 MAPK protein expression.
MiR-451 down-regulated cytokines expression in synovial fibroblasts
ELISA results showed that compared with control, miR-451 transfected synovial fibroblasts secreted lower levels of TNF-α (198 ± 12 pg/ml vs 124 ± 13 pg/ml, P < 0.01), IL-1β (352 ± 43 pg/ml vs 165 ± 87 pg/ml, P < 0.01), and IL-6 (487 ± 84 pg/ml vs 257 ± 92 pg/ml, P < 0.01, Figure 4).
Figure 4.

Cytokines secretion.
Discussion
RA is an autoimmune disease with the pathological features of joint synovial inflammation. Studies have shown that there are many immune cells and cytokines in the synovial tissue and synovial fluid of RA patients. MiRNA, on the other hand, is a set of noncoding small RNA molecules found in recent years [16] that can posttranscriptional regulate mRNA expression and involve in a variety of immune cell activation and differentiation processes [17]. It has been reported that miRNA has influence on synovial fibroblasts proliferation [18]. However, there is still lack of report about the role of miR-451 in autoimmune diseases, especially in RA. Our results showed that miR-451 may affect RA occurrence through inhibiting synovial fibroblasts proliferation. As miRNA performed its biological function generally by inhibiting target gene expression, we speculated p38 MAPK may be the target gene of miR-451. MAPK is an important signaling pathway in mediating cell reaction, of which p38 MAPK is an important member of the MAPK family [19]. It can regulate cell growth, development and intercellular function by cytoskeleton related protein, transcription factors phosphorylation, and enzymes. Also, it plays an important role in the process of cell-mediated inflammatory response and cytokines generation. Thus, we thought p38MAPK might be an important target [15,20]. We transfected miR-451 to synovial fibroblasts and found that, compared with the control group, miR-451 can obviously reduce p38 MAPK protein expression in synovial fibroblasts. Therefore, we speculated that p38 MAPK activity is associated with miR-451 regulation, and involved in intracellular signal transduction process of miR-451.
In recent years, the role of miR-451 in autoimmune disease began to be attentioned by researchers [21]. For example, it was reported that miR-451 expression level in the diseased tissue is correlated with helper T lymphocytes 17 (Th17) subgroup expression changes in the viral myocarditis mice model. The author thought miR-451 involved in the pathogenesis of viral myocarditis, and related to Th17subgroup. Another experiment proved that miRNA-451 may inhibit glomerular mesangial cell proliferation in the diabetic nephropathy model, and can reduce p38 MAPK level. We also confirmed similar findings in RA synovial fibroblasts, and provided new evidence in investigating the role of miR-451 in autoimmune disease.
A large number of in vitro cells experiment and in vivo animal experiments found that cytokines is closely related to RA development. Currently, the cytokines thought to be associated with RA includes TNF-α, IL-1β, and IL-6. They are important inflammatory cytokines in RA. These cytokines can stimulate synovial fibroblasts hyperplasia resulting in chemokines and cytokines secretion and joint damage. In addition, there are some cytokines in synovial tissue and synovial fluid of RA, and these cytokines mainly produced by activated macrophages, monocytes, and dendritic cells. These cytokines, especially TNF-α, IL-1β, and IL-6, participate in the occurrence and development of the disease by inducing extensive chemokine expression and upregulating adhesion molecules in endothelial cells [18]. In this study, we found that miR-451 can down-regulate TNF-α, IL-1β, and IL-6 level in synovial fibroblasts. Our research proved that the key role of p38 MAPK in the incidence of RA. MiR-451 could inhibit p38 MAPK expression to suppress synovial fibroblasts proliferation and down-regulate corresponding cytokines levels, and potentially improve the disease clinical manifestation.
Up to now, though there is still no direct report about the role of miRNA-451 in the synovial fibroblasts of RA, it is reported that the miRNA-451 expression level is lower in the peripheral neutrophils of RA patients than normal control [22]. Further in vitro experiments proved that miR-451 can reduce the infiltration capacity of neutrophil in local inflammation model, while overexpressed miR-451 can obviously inhibit neutrophil activity [22]. Our results suggested that the miRNA-451 could inhibit synovial fibroblasts proliferation and cytokines production in RA. Thus, combined with the existing reports, miRNA-451 may have a variety of ways in RA by reducing the neutrophils infiltrating ability and inhibits synovial fibroblasts proliferation. Future study can further investigate the possible effect of miRNA-451 in animal model or clinical RA patients.
Our studies demonstrated that the expression of cytokines, including TNF-α, IL-1β, and IL-6, can be inhibited by miR-451. Previous in vitro experiments also proved that these cytokines can be regulated by different miRNAs. For example, miR-203 can regulate IL-6 production in synovial fibroblasts from RA through NF-κB signaling pathway. Other studies found miR-146a overexpressed in the peripheral blood mononuclear cells from RA, and its expression is associated with TNF-α expression level. The abovementioned results and our study showed that a number of different miRNAs can regulate different cytokines expression, while these cytokines actively involved in the occurrence and development of RA. The synergy of multiple miRNAs inhibited a variety of cytokines expression, and also provides a new strategy for RA even other autoimmune disease treatment. Furthermore, a variety of miRNAs were found abnormally expressed in the peripheral blood or synovial tissue of the RA patients, and some of miRNAs are closely related to the clinical symptoms and disease activity of RA. Some clinical scholars suggested that miRNA may be used for RA early diagnosis and disease activity judgment. On the other hand, as the great therapeutic effect and rare inflammation of miRNA in tumor, its role in RA treatment is also a new therapeutic direction [21,23,24]. Our experiments also suggested the potential treatment effect of miR-451 in RA.
Above all, miR-451 plays an important role in the pathogenesis of RA. It can both inhibit synovial fibroblasts proliferation and regulate p38 MAPK expression, resulting in corresponding cytokines downregulation. MiR-451 might be a new therapeutic drug for RA. Its specific mechanism still remains further research.
Disclosure of conflict of interest
None.
References
- 1.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 2.Zhao S, Wang Y, Liang Y, Zhao M, Long H, Ding S, Yin H, Lu Q. MicroRNA-126 regulates DNA methylation in CD4+ T cells and contributes to systemic lupus erythematosus by targeting DNA methyltransferase 1. Arthritis Rheum. 2011;63:1376–1386. doi: 10.1002/art.30196. [DOI] [PubMed] [Google Scholar]
- 3.Alevizos I, Alexander S, Turner RJ, Illei GG. MicroRNA expression profiles as biomarkers of minor salivary gland inflammation and dysfunction in Sjogren’s syndrome. Arthritis Rheum. 2011;63:535–544. doi: 10.1002/art.30131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Semaan N, Frenzel L, Alsaleh G, Suffert G, Gottenberg JE, Sibilia J, Pfeffer S, Wachsmann D. miR-346 controls release of TNF-alpha protein and stability of its mRNA in rheumatoid arthritis via tristetraprolin stabilization. PLoS One. 2011;6:e19827. doi: 10.1371/journal.pone.0019827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 6.Davalos V, Esteller M. MicroRNAs and cancer epigenetics: a macrorevolution. Curr Opin Oncol. 2010;22:35–45. doi: 10.1097/CCO.0b013e328333dcbb. [DOI] [PubMed] [Google Scholar]
- 7.Yang BF, Lu YJ, Wang ZG. MicroRNAs and apoptosis: implications in the molecular therapy of human disease. Clin Exp Pharmacol Physiol. 2009;36:951–960. doi: 10.1111/j.1440-1681.2009.05245.x. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Blelloch R. Cell cycle regulation by microRNAs in stem cells. Results Probl Cell Differ. 2011;53:459–472. doi: 10.1007/978-3-642-19065-0_19. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Peng W, Ouyang X, Li W, Dai Y. Circulating microRNAs as candidate biomarkers in patients with systemic lupus erythematosus. Transl Res. 2012;160:198–206. doi: 10.1016/j.trsl.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Gallo A, Tandon M, Illei G, Alevizos I. Discovery and validation of novel microRNAs in Sjogren’s syndrome salivary glands. Clin Exp Rheumatol. 2014;32:761–762. [PubMed] [Google Scholar]
- 11.Chatzikyriakidou A, Voulgari PV, Georgiou I, Drosos AA. miRNAs and related polymorphisms in rheumatoid arthritis susceptibility. Autoimmun Rev. 2012;11:636–641. doi: 10.1016/j.autrev.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Kirschner MB, Kao SC, Edelman JJ, Armstrong NJ, Vallely MP, van Zandwijk N, Reid G. Haemolysis during sample preparation alters microRNA content of plasma. PLoS One. 2011;6:e24145. doi: 10.1371/journal.pone.0024145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nan Y, Han L, Zhang A, Wang G, Jia Z, Yang Y, Yue X, Pu P, Zhong Y, Kang C. MiRNA-451 plays a role as tumor suppressor in human glioma cells. Brain Res. 2010;1359:14–21. doi: 10.1016/j.brainres.2010.08.074. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberger CM, Podyminogin RL, Navarro G, Zhao GW, Askovich PS, Weiss MJ, Aderem A. miR-451 regulates dendritic cell cytokine responses to influenza infection. J Immunol. 2012;189:5965–5975. doi: 10.4049/jimmunol.1201437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamachi Y, Kawano S, Takenokuchi M, Nishimura K, Sakai Y, Chin T, Saura R, Kurosaka M, Kumagai S. MicroRNA-124a is a key regulator of proliferation and monocyte chemoattractant protein 1 secretion in fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Rheum. 2009;60:1294–1304. doi: 10.1002/art.24475. [DOI] [PubMed] [Google Scholar]
- 16.Stanczyk J, Ospelt C, Karouzakis E, Filer A, Raza K, Kolling C, Gay R, Buckley CD, Tak PP, Gay S, Kyburz D. Altered expression of microRNA-203 in rheumatoid arthritis synovial fibroblasts and its role in fibroblast activation. Arthritis Rheum. 2011;63:373–381. doi: 10.1002/art.30115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shahrara S, Pickens SR, Dorfleutner A, Pope RM. IL-17 induces monocyte migration in rheumatoid arthritis. J Immunol. 2009;182:3884–3891. doi: 10.4049/jimmunol.0802246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berzat A, Hall A. Cellular responses to extracellular guidance cues. EMBO J. 2010;29:2734–2745. doi: 10.1038/emboj.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ondrouskova E, Slovackova J, Pelkova V, Prochazkova J, Soucek K, Benes P, Smarda J. Heavy metals induce phosphorylation of the Bcl-2 protein by Jun N-terminal kinase. Biol Chem. 2009;390:49–58. doi: 10.1515/BC.2009.007. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Wan Y, Guo Q, Zou L, Zhang J, Fang Y, Fu X, Liu H, Lu L, Wu Y. Altered microRNA expression profile with miR-146a upregulation in CD4+ T cells from patients with rheumatoid arthritis. Arthritis Res Ther. 2010;12:R81. doi: 10.1186/ar3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duroux-Richard I, Presumey J, Courties G, Gay S, Gordeladze J, Jorgensen C, Kyburz D, Apparailly F. MicroRNAs as new player in rheumatoid arthritis. Joint Bone Spine. 2011;78:17–22. doi: 10.1016/j.jbspin.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Murata K, Yoshitomi H, Furu M, Ishikawa M, Shibuya H, Ito H, Matsuda S. MicroRNA-451 down-regulates neutrophil chemotaxis via p38 MAPK. Arthritis Rheumatol. 2014;66:549–559. doi: 10.1002/art.38269. [DOI] [PubMed] [Google Scholar]
- 23.Long L, Yu P, Liu Y, Wang S, Li R, Shi J, Zhang X, Li Y, Sun X, Zhou B, Cui L, Li Z. Upregulated microRNA-155 expression in peripheral blood mononuclear cells and fibroblast-like synoviocytes in rheumatoid arthritis. Clin Dev Immunol. 2013;2013:296139. doi: 10.1155/2013/296139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu W, Xia LJ, Chen FH, Wu FR, Tang J, Chen CZ, Jiang S, Chen HH. Recombinant human endostatin inhibits adjuvant arthritis by down-regulating VEGF expression and suppression of TNF-alpha, IL-1beta production. Inflamm Res. 2012;61:827–835. doi: 10.1007/s00011-012-0477-z. [DOI] [PubMed] [Google Scholar]


