Abstract
Objectives: Periodontal ligament stem cells (PDLSCs) are characterized by having multipotential differentiation and immunoregulatory properties, which are the main mechanisms of PDLSCs-mediated periodontal regeneration. Periodontal or bone regeneration requires coordination of osteoblast and osteoclast, however, very little is known about the interactions between PDLSCs and osteoblast-like cells or osteoclast precursors. In this study, the indirect co-culture approach was introduced to preliminarily elucidate the effects of PDLSCs on differentiation of osteoblast-like cells and osteoclast precursors in vitro. Materials and methods: Human PDLSCs were obtained from premolars extracted and their stemness was identified in terms of their colony-forming ability, proliferative capacity, cell surface epitopes and multi-lineage differentiation potentials. A noncontact co-culture system of PDLSCs and preosteoblastic cell line MC3T3-E1 or osteoclast precursor cell line RAW264.7 was established, and osteoblastic differentiation of MC3T3-E1 and osteoclastic differentiation of RAW264.7 were evaluated. Results: PDLSCs exhibited features of mesenchymal stem cells. Further investigation through indirect co-culture system showed that PDLSCs enhanced ALP activity, expressions of ALP, Runx2, BSP, OPN mRNA and BSP, OPN proteins and mineralization matrix deposition in MC3T3-E1. Meanwhile, they improved maturation of osteoclasts and expressions of TRAP, CSTK, TRAF6 mRNA and TRAP, TRAF6 proteins in RAW264.7. Conclusions: PDLSCs stimulates osteoblastic differentiation of osteoblast precursors and osteoclastic differentiation of osteoclast precursors, at least partially, in a paracrine fasion.
Keywords: Periodontal ligament stem cells, co-culture, osteoblastic differentiation, osteoclastic differentiation
Introduction
Periodontal diseases are highly prevalent and characterized by the destruction of tooth-supporting alveolar bone. They are the major cause of tooth loss in adults as the result of loss of connective tissue and alveolar bone [1]. The ideal goal of the periodontal treatment is the reconstruction of the lost or injured periodontal tissue, for which the stem cell-based tissue engineering method provides a reliable and promising option [2]. Three basic biological parts are involved in tissue engineering, which are seed cells, suitable matrical materials and effective inducing factors. Mesenchymal stem cells (MSCs) as the main seed cells in tissue engineering have been widely studied and demonstrated to have the capacities of self-renewal and multipotential differentiation into multiple cell lineages, including osteoblasts, adipocytes and chondrocytes [3]. Although MSCs are traditionally isolated from bone marrow, adipose tissue and umbilical cord blood, MSC-like cell populations can also been isolated from mature or developing periodontal tissue, dental follicle, root apical papilla and gingival tissue [4-12]. Periodontal ligament stem cells (PDLSCs) have long been considered as a promising candidate for periodontal tissue regeneration [13]. MSCs are believed to not only directly differentiate into different cell types including osteoblasts, adipocytes and chondroblasts, but also secrete trophic factors that exert chemotactic, mitotic, and differentiation-modulating effects [14], which have been interpreted as the major mechanisms of MSCs in tissue repair [15]. The process of periodontal or bone regeneration involves coordination of osteoblasts and osteoclasts, however, very little is known about the possible effect of PDLSCs on differentiation of their neighbouring osteoblast-like cells and osteoclast precursors during periodontal tissue regeneration. In this study, the indirect co-culture approach was introduced to preliminarily elucidate the effects of PDLSCs on mature differentiation of osteoblast-like cells and osteoclast precursors.
Materials and methods
Culture and characterization of PDLSCs
Human periodontal ligament tissues were obtained from premolars extracted for orthodontic reason from twelve healthy individuals (four males and eight females, aged 14 to 27 years). All procedures were approved by the Institutional Review Board of China and performed at the Department of Stomatology, Qingdao Municipal Hospital (Affiliated Hospital of Medical College, Qingdao University), and written informed consent was obtained from each participant. Human periodontal ligament tissues were scraped from the middle third of the root surfaces as previously described [16]. In brief, human periodontal ligament tissues were minced into 1 mm3 fragments, and then digested with 3 mg/ml collagenase I (Invitrogen, Carlsbad, CA) and 4 mg/ml dispase II (Invitrogen, Carlsbad, CA) for 40 minutes at 37°C. The reaction solution was centrifugated and 10 ml alpha-minimal essential medium (α-MEM, Hyclone, Logan, UT) with 10% fetal bovine serum (FBS, Hyclone, Logan, UT), 100 U/mL penicillin G and 100 mg/mL streptomycin (JRH Biosciences, Lenexa, KS) was added to produce a cell suspension, which was then passed through a cell strainer (70-μm pore size) (BD Falcon, BD Biosciences, Bedford, MA). After the cells were counted, the single-cell suspension was plated at a concentration of 60 cells/cm2 in 10 cm Petri dishes (Falcon Labware; BD, Franklin Lakes, N.J., USA). Only cells forming single cell-derived colonies were isolated with colony rings and passaged (passage 1) as PDLSCs. PDLSCs of passage < 5 were used for all experiments.
Immunophenotype characterization of PDLSCs
Immunophenotype of PDLSCs was determined by flow cytometry (Beckman Coulter, Brea, CA, USA). A total of 1×106 cells at passage 3 were incubated for 40 min on ice with phycoerythrin or fluorescein isothiocyanate-conjugated mouse monoclonal antibodies (5 μg/ml) specific for human CD29, CD90, CD105, Stro-1, CD45, CD34, CD44 (BioLegend, San Diego, Calif., USA). After being washed with phosphate buffer saline (PBS), cells were fixed in fluorescence-activated cell sorting fix solution and then analyzed using flow cytometry.
Colony-forming unit-fibroblast assays of PDLSCs
To assess colony-forming efficiency of PDLSCs, colony-forming unit-fibroblast assays were performed as described previously [17]. Briefly, cells at a density of 103 were plated in 10 cm Petri dishes. After 14 days, the cells were fixed with 4% paraformaldehyde, stained with 0.1% crystal violet, washed with distilled water and dried. A colony-forming unit was defined as a cluster of at least 50 cells.
In vitro multipotent differentiation of PDLSCs
For osteogenic differentiation, PDLSCs were plated in 24-well plates and cultured in osteogenic medium (α-MEM containing 5% FBS, 0.1 μM dexamethasone, 10 mM β-glycerophosphate and 50 mg/ml ascorbate-2-phosphate; Sigma-Aldrich, St. Louis, Mo., USA). Cells cultured in α-MEM containing only 5% FBS served as a control group. The medium was changed twice a week. The mineralized nodules were characterized by 2% alizarin red S (Sigma-Aldrich, St. Louis, Mo., USA) staining 4 weeks later.
For adipogenic differentiation, PDLSCs were incubated in 96-well plates (5×103 cells/cm2) in α-MEM growth medium (Hyclone, Logan, Utah, USA). Upon reaching 80% confluence, cells were cultured in adipogenic medium (α-MEM containing 10% FBS, 0.1 μM dexamethasone, 60 μM indomethacin and 50 mg/ml ascorbate-2-phosphate; Sigma-Aldrich, St. Louis, Mo., USA). Cells cultured in α-MEM containing 10% FBS served as a control group. The medium was changed twice a week. Oil red O (Sigma-Aldrich, St. Louis, Mo., USA) staining was performed to identify the oil globules 2 weeks later.
Cell cultures of MC3T3-E1 and RAW264.7 cells
MC3T3-E1, a preosteoblast cell line was maintained in α-MEM supplemented with 5% FBS, 2 mM L-glutamine, 100 U/mL penicillin G and 100 mg/mL streptomycin. RAW264.7, an osteoclast precursor cell line was cultured in α-MEM containing 10% FBS, 100 U/mL penicillin G and 100 mg/mL streptomycin at 37°C in a humidified atmosphere of 95% air and 5% CO2 with the medium changed every 2 days.
Co-culture of MC3T3-E1or RAW264.7 cells with PDLSCs
Transwell cell culture inserts (Corning Costar, Cambridge, MA) with 0.4-μm pore-size filters were placed in individual wells of six-well plates. In PDLSCs coculture (PDLSCs CC) group, MC3T3-E1 (5×104 cells/ml) or RAW264.7 (5×104 cells/ml) cells were seeded in six-well plates and PDLSCs (3×104 cells/ml) were grown on the transwell inserts on top. While in control group, MC3T3-E1 or RAW264.7 cells were seeded in six-well plates with empty transwell inserts on top. Osteogenic medium and osteoclast culture medium with 10 ng/ml macrophage colony stimulating factor (M-CSF) and 20 ng/ml receptor activator of nuclear factor-kappaB ligand (RANKL) were used respectively to induce osteoblastic and osteoclastic differentiation.
After different periods of culture, the transwell inserts with PDLSCs were discarded and the osteoblastic differentiation of MC3T3-E1 cells or osteoclastic differentiation of RAW264.7 cells in the six-well plated was analyzed. Alkaline phosphatase (ALP) activity staining and quantitation, mRNA expressions of ALP, bone sialoprotein (BSP), osteopontin (OPN) by RT-PCR, protein expressions of BSP, OPN by Western-blotting and mineral matrix deposition by alizarin red staining were performed on MC3T3-E1 cells. While tartrate-resistant acid phosphatase (TRAP) positive cell counting and quantification, mRNA expressions of TRAP, tumor necrosis factor receptor-associated factor 6 (TRAF6) and Cathepsin K (CTSK) by RT-PCR and protein expressions of TRAP, TRAF6 by Western-blotting were conducted on RAW264.7 cells.
Alkaline phosphatase activity
After 7 and 14 days of incubation, MC3T3-E1 cells were washed with 0.01 M PBS and scraped into 100 μl 0.2% TritonX-100. Then cells were sonicated and cell lysates were obtained after centrifuging at 14,000× g for 10 min at 4°C. ALP activity in the supernatant was assayed according to the instruction of the manufacturer (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). In brief, 30 μl supernatant, 50 μl of buffer solution and 50 μl matrix liquid were added to each well of a 96-well plate and mixed. The plate was incubated for 15 min at 37°C. Next, 150 μl coloration solution was added to each well and the absorbance was measured at 520 nm wavelength with a spectrophotometer. ALP activity can be calculated according to the concentrations of the phenol in standard wells, and results were normalized to the protein concentrations detected by bicinchoninic acid (BCA) method.
Alizarin red staining
After 21 days of incubation, MC3T3-E1 cells were rinsed with 1×PBS (pH 7.4) five times, fixed with 10% formalin, washed with deionized water and stained with alizarin red (pH 4.2) at 4°C overnight. Stained cells were photographed.
TRAP staining
After 3 and 7 days of incubation, RAW264.7 cells were fixed and stained for TRAP using a leukocyte acid phosphatase staining kit (387A, Sigma, St. Louis, MO, USA) according to the manufacturer’s instructions. Red and multinucleated (≥3 nuclei) cells were considered as differentiated osteoclast-like cells. The number of TRAP positive cells was counted blindly by two persons.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA was isolated from MC3T3-E1 or RAW264.7 cells by adding Trizol reagent (Sigma, St. Louis, MO, USA) after the medium was removed. First strand cDNA was synthesized from 0.7 μg total RNA using the reverse transcript kit (TaKaRa, Dalian, China), and real-time PCR was performed using Rotor-Gene 6000 (Corbett Life Science) with SYBR Premix Ex Taq (Takara, Dalian, China) according to manufacturer’s instructions. The relative expression levels of ALP, BSP, OPN, TRAP, CTSK and TRAF6 were normalized to the expression of house-keeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Primers for the selected genes are listed in Table 1.
Table 1.
Primer sequences for osteogenic and osteoclastogenic markers
| Gene | GenBank number | Forward | Reverse |
|---|---|---|---|
| ALP | NM_007431.3 | CTGATGTGGAATACGAACTGGA | AGTGGGAATGCTTGTGTCTGG |
| BSP | NM_008318.3 | CAGGGAGGCAGTGACTCTTC | AGTGTGGAAAGTGTGGAGTT |
| OPN | NM_001204203.1 | ACACTTTCACTCCAATCGTC | TGCCCTTTCCGTTGTTGTCC |
| TRAP | NM_001102405.1 | GGGTCACTGCCTACCTGTGT | TCATTTCTTTGGGGCTTATCTC |
| TRAF6 | NM_009424.3 | AGCCCACGAAAGCCAGAAGAA | CCCTTATGGATTTGATGATGC |
| Cathepsin K | NM_007802.4 | CTGAAGATGCTTACCCATATGTGGG | GCAGGCGTTGTTCTTATTCCGAGC |
| GAPDH | NM_008084.3 | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
Western blot analysis
Total protein was extracted from MC3T3-E1 cells or RAW264.7 cells using RIPA buffer (10 mM Tris-HCl, 1 mM EDTA, 1% sodium dodecyl sulfate, 1% Nonidet P-40, 1:100 proteinase inhibitor cocktail, 50 mM b-glycerophosphate and 50 mM sodium fluoride). Protein concentrations of the lysates were determined using a protein assay solution (Bio-Rad, Hercules, CA, USA) based on the absorbance at 595 nm. Afterwards, 20 to 50 μg of protein samples were separated by 10% SDS-PAGE gel and then transferred to a polyvinylidene fluoride (PVDF) membranes (Bio-Rad, Hercules, CA, USA). The membranes were blocked with 5% milk for 2 hours and then incubated with anti-BSP, OPN, anti-TRAP, TRAF6 (Abcam, Cambridge, UK) and anti-GAPDH (Cell Signaling Technology, Beverly, MA, USA) primary antibodies. The immune complexes were then incubated with horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG (Boshide, Beijing, China). Immunodetection was then performed using the Western-Light Chemiluminescent Detection System (Peiqing, Shanghai, China).
Statistical analysis
All data were expressed as means ± SD from at least three replicates for each experiment and one-way ANOVA with Bonferroni correction was used to test significance using SPSS 12.0 software (SPSS Inc., IL, USA). P values less than 0.05 were considered statistically significant.
Results
Characterization of PDLSCs
To identify the PDLSCs, single-cell colonies were generated from human periodontal ligament-derived cells which formed adherent clonogenic cell clusters of fibroblast-like cells (Figure 1A). After 4 weeks of culture, extensive amounts of mineralized nodules were found in the experimental group, whereas no mineralized nodules were observed in the control group (Figure 1B). Moreover, after a 2-week culture in the adipogenic medium, PDLSCs were found to differentiate towards adipocytes, as indicated by the accumulation of lipid droplets. In contrast, no lipid droplets were detected in the control group (Figure 1C). Flow-cytometric analysis revealed that PDLSCs were uniformly positive for CD29, CD44, CD90, CD105 and Stro-1, and did not express hematopoietic stem cell markers CD34 and CD45 (Figure 1D). These findings indicated that the single colony-derived PDLSCs had the basic characteristics of MSCs.
Figure 1.
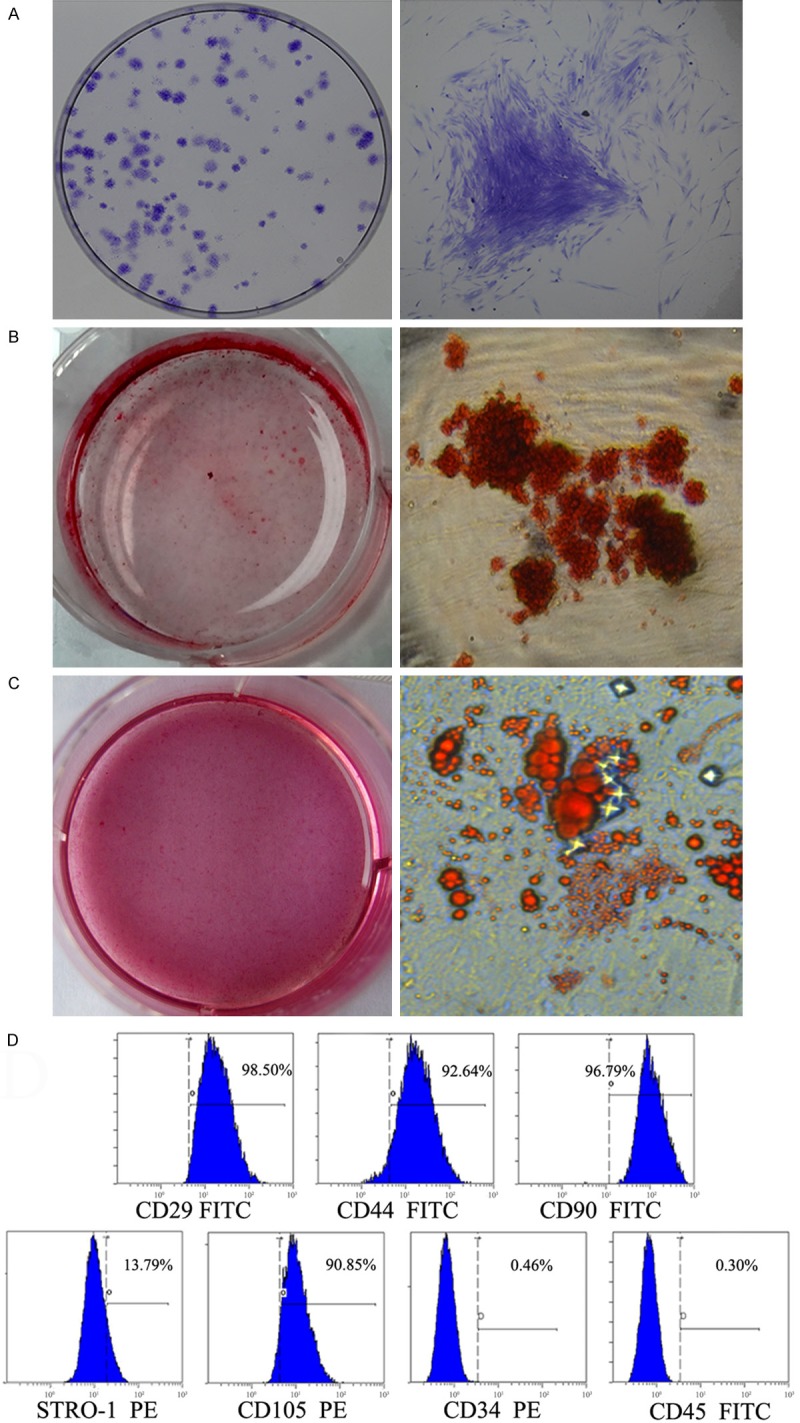
Identification and characters of PDLSCs. (A) Representative images of colony-forming units from PDLSCs at 14 days. (B, C) Multipotent differentiation of PDLSCs. Osteogenic differentiation of PDLSCs was demonstrated by the presence of alizarin red S-positive mineralized nodules (B). Adipogenic differentiation PDLSCs was demonstrated by the formation of oil red O-positive lipid globules (C). (D) Flow-cytometric analysis of surface markers expressed on PDLSCs.
Effects of PDLSCs on osteogenic differentiation of MC3T3-E1 cells
PDLSCs increased ALP activity in MC3T3-E1 cells
ALP activity has been widely used as a marker of the early differentiation of osteoblast-like cells [18]. In our research, ALP activity of MC3T3-E1 was measured at day 7 and day 14 of incubation with or without PDLSCs CC and the result showed that ALP activity increased in the presence of PDLSCs (Figure 2A).
Figure 2.
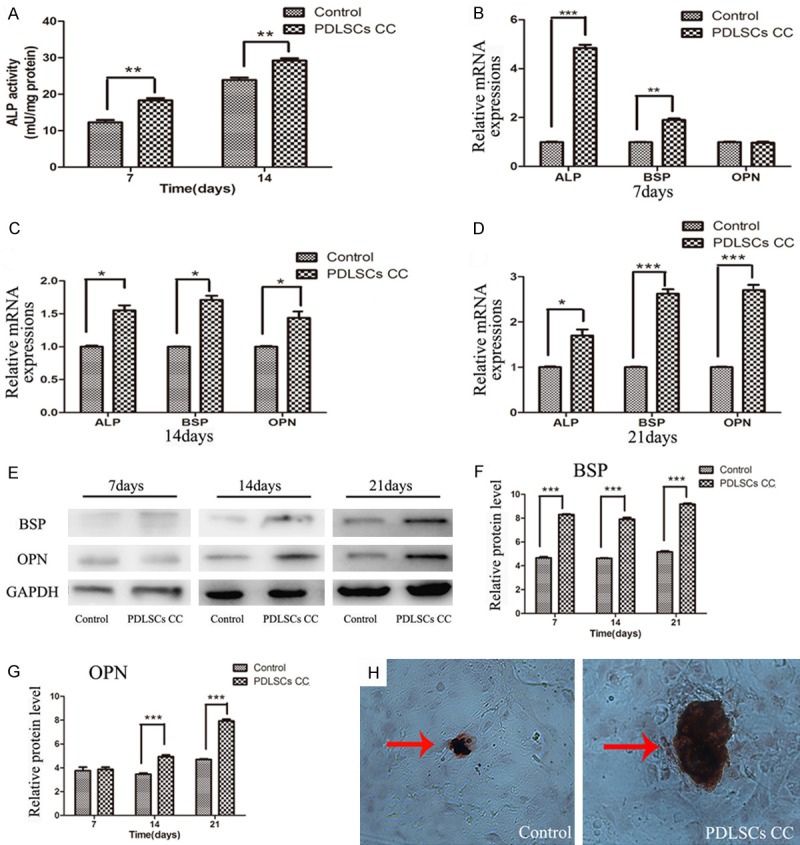
Effects of PDLSCs on the osteogenenesis in MC3T3-E1 cells in the co-culture system. A. ALP activity in MC3T3-E1 cells co-cultured with PDLSCs. ALP activity increased in the presence of PDLSCs at day 7 and day 14. B-D. Expressions of ALP, BSP, and OPN genes in MC3T3-E1 cells co-cultured with PDLSCs analyzed by real time-PCR. B. Gene expression after 7 days of induction. C. Gene expression after 14 days of induction. D. Gene expression after 21 days of induction. E. Time-related expression of BSP and OPN in MC3T3-E1 cells analyzed by Western blot. F. Quantitative analysis of the protein expression of BSP. G. Quantitative analysis of the protein expression of OPN. H. Mineral matrix deposition (Alizarin red staining) when MC3T3-E1 cells were co-cultured with PDLSCs for 21 days (original magnification 200×). *P < 0.05; **P < 0.01; ***P < 0.001 vs. control group.
PDLSCs increased the mRNA expressions of osteogenic parameters in MC3T3-E1 cells
To further determine the effects of PDLSCs on osteogenic differentiation of MC3T3-E1, RT-PCR was performed to measure the gene expressions in MC3T3-E1 cells after different time of incubation with or without PDLSCs CC. It was found that the gene expression levels of ALP and BSP were significantly up-regulated but no significant difference in the gene expression of OPN was detected when cells were co-cultured with PDLSCs for 7 days (Figure 2B). At day 14 and day 21 the gene expression levels of ALP, BSP and OPN were all significantly higher in the co-culture group than those in the control group (Figure 2C, 2D). In addition, the results also indicated that the highest expression level of ALP was on day 14 while the highest expression levels of BSP and OPN were on day 21 in the co-culture group.
PDLSCs increased the protein expressions of BSP & OPN in MC3T3-E1 cells
Western blotting was used to detect the protein expressions of osteogenic markers in MC3T3-E1 cells after different time of incubation with or without PDLSCs. The results demonstrated that the protein expression of BSP increased at day 7, day 14 and day 21, while that of OPN, an osteogenic marker in the later stage, increased at day 14 and day 21 in co-culture group compared with the control group (Figure 2E-G).
PDLSCs increased the mineral deposition in MC3T3-E1 cells
Some studies indicated mineralization by MC3T3-E1 cells occurred in a time-dependent manner and can be easily observed after 3 weeks of culture [19]. PDLSCs significantly enhanced the mineralization in MC3T3-E1 cells when compared with the control group on day 21. As shown in Figure 2H, the size of mineralization nodule was increased in co-culture group by alizarin red staining.
Effects of PDLSCs on osteoclastic differentiation of RAW264.7 cells
PDLSCs improved the osteoclast-like cell formation from RAW264.7 cells
The effect of PDLSCs on osteoclastic differentiation of RAW264.7 cells in co-culture system was examined using TRAP staining. We studied whether PDLSCs had any effect on multinucleated osteoclast-like cell formation in RANKL-stimulated RAW264.7 cells. Result of TRAP staining revealed that PDLSCs markedly increased the TRAP positive osteoclast-like cell formation in RANKL-stimulated RAW264.7 cells (Figure 3A, 3B).
Figure 3.
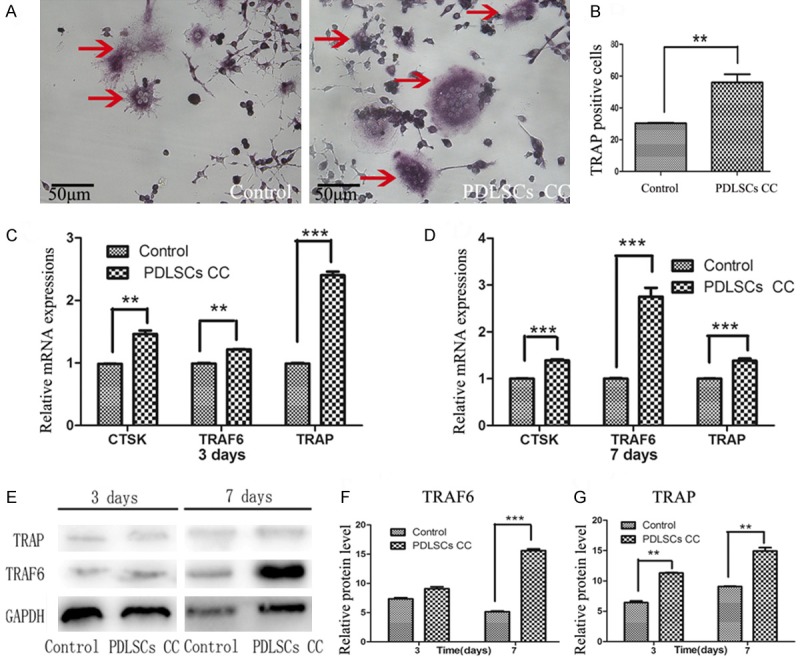
Effects of PDLSCs on the osteoclastogenesis in RAW264.7 cells in the co-culture system. The formation of TRAP-positive, multinuclear cells (arrows) was increased in the presence of PDLSCs vs. control group. A. TRAP-positive cells were identified with microscope (original magnification 200×). B. The number of TRAP-positive multinucleated cells was counted in ten 200× fields. C, D. Expressions of TRAP, TRAF6 and CTSK in RAW264.7 cells co-cultured with PDLSCs analyzed by real time-PCR. C. Gene expression after 3 days of induction. D. Gene expression after 7 days of induction. E. Time-related protein expressions of TRAP and TRAF6 in RAW264.7 cells analyzed by Western blot. F. Quantitative analysis of the protein expression of TRACP. G. Quantitative analysis of the protein expression of TRAF6. *P < 0.05; **P < 0.01; ***P < 0.001 vs. control group.
PDLSCs increased the gene expressions of osteoclastic parameters in RAW264.7 cells
To further elucidate the role of PDLSCs play in osteoclastic differentiation, the expressions of osteoclastic marker genes of RAW264.7 cells with or without PDLSCs co-culture were detected. Result showed that expressions of TRAP, TRAF6 and CTSK were significantly up-regulated upon co-culture with PDLSCs (Figure 3C, 3D).
PDLSCs increased the protein expressions of TRAP & TRAF6 in RAW264.7 cells
Western blotting was used to detect the protein expressions of osteoclastic markers in RAW264.7 cells with or without PDLSCs co-culture. Results demonstrated that the protein expressions of TRAP and TRAF6 were increased on day 3 and day 7 when compared with the control group (Figure 3E-G).
Discussion
Tissue engineering is a contemporary area of science that is based on the principles of cell biology, bioengineering, biomaterials, biochemistry and biophysics, and its ultimate goal is to solve clinical problems related to tissue loss and organ failure [20,21]. Specifically, stem cells-based therapy has been considered as one of potential treatment strategies for a variety of chronic, debilitating diseases. This holds true for periodontal regeneration in inflammatory periodontal diseases [22]. Indeed, previous data indicate that application of ex vivo-expanded stem cells in diverse animal models of periodontal defects can partly restore diseased/destroyed tissues, and PDLSCs have long been regarded as a promising type of stem cells for this goal [23,24]. It is speculated that transplanted PDLSCs not only participate directly in bone repair but also recruit host bone progenitor cells through secretion of trophic factors [14,25], which are conceived as their main mechanisms to promote bone regeneration. However, nowadays prevailing studies on the paracrine secretion of MSCs are mainly concentrated on their recruiting host cells, anti-inflammatory and immunoregulatory potentials. Few reports have been documented about the paracrine effects of MSCs on osteoclastic and osteoblastic differentiations during periodontal and bone tissue regeneration. In this study, a transwell system was used to explore the effects of PDLSCs on mature differentiation of osteoblast-like cells and osteoclast precursors, which permitted diffusion of soluble molecules between the separated compartments while blocked cell-cell contact. Thereby, it can distinguish the paracrine effects from the direct cell-cell interactions and eliminate the cell contamination from PDLSCs in the subsequent analyses. Results showed that PDLSCs enhanced ALP activity, expression of ALP, BSP, OPN mRNA and BSP, OPN protein and mineralization matrix deposition in MC3T3-E1 preosteoblasts. Meanwhile, they improved maturation and expression of TRAP, CSTK, TRAF6 mRNA and TRAP, TRAF6 protein in osteoclast precursor RAW264.7 cells. PDLSCs regulate both osteoblastic and osteoclastic differentiation, at least partially, in a paracrine fasion.
Physiologically, bone mass is maintained by the balance of bone resorption by osteoclasts and bone matrix formation by osteoblasts. These activities always exert the influence on the same bone surface, a process called bone remodeling. The cell population associated with bone remodeling is designated as the basic multi-cellular unit (BMU) [26]. Therefore, the original concept of BMU contained only osteoclasts and osteoblasts. But recently, the immune cells and precursor populations of osteoblasts and osteoclasts are found to contribute to the cell class of BMU [27]. Until now, there has been a deep insight into the functions and intercellular regulation and communication of osteoblasts and osteoclasts. However, little information about the effects of MSCs as precursor populations of osteoblasts on osteoblastic and osteoclastic differentiation has been known.
Generally, bone remodeling begins with the mature differentiation of osteoclast, bone resorption caused by osteoclast, bone matrix formation mediated by osteoblasts and matrix mineralization. Osteoblasts are related to the formation, deposition and mineralization of bone tissue by synthesizing and depositing calcium phosphate crystals, and extracellular matrix [28]. MSCs are considered as main precursor cells of osteoblasts [28]. Therefore, osteoblasts or MSCs potential to differentiate into osteoblasts have been introduced into bone tissue engineering. Indeed, osteoblasts or MSCs including PDLSCs as the seeding cells in tissue engineering have been proved to improve bone regeneration [29,30]. The mechanisms of enhancing bone regeneration by MSCs are commonly considered to be associated with direct osteoblastic differentiation [29], immunoregulation [30-32] and blood vessel regeneration and vascularization [33-35]. It has also been speculated that MSCs can exert regulation effect on osteoblastic differentiation. The experimental results conducted by Li et al. [36] showed that MSCs maintained in osteogenic medium secreted factors at specific time points that induced ALP activity in exogenous MSCs as well as their migration. Osugi et al. [37] found that the conditioned media from human bone marrow-derived mesenchymal stem cells (MSC-CM) promoted the migration, proliferation, and osteoblastic differentiation of rat MSCs (rMSCs) in vitro and strengthened bone regeneration by mobilization of endogenous stem cells in vivo. In our study, for the first time, we demonstrated by indirect co-culture system that PDLSCs enhanced the osteogenic differentiation of MC3T3-E1 cells in vitro.
Bone resorption caused by osteoclasts is the first step of physiological bone remodeling, and mature differentiation and function of osteoclasts play important roles in maintaining bone mass integrity through osteoblast-osteoclast coupling. The investigation on osteoclast deficient mice showed that osteoclast deficiency leaded to matrix disorganization, poor mineralization and decreased number and function of osteoblasts [38,39]. Dai et al. [38] found that transplantation of bone buds derived from osteoclast deficient fetal mice into normal wild type mice recovered normal development of these bone buds. Similarly, osteoclasts also play an important role in bone tissue engineering. Walker et al. [40] demonstrated that implantation of hematopoietic cells, osteoclast precursors, could restore bone resorption in mice with osteoporosis. Moreover, Sinclair et al. [41] demonstrated that when cocultured with osteoclasts on the 3-D scaffold substrates, the proliferation and osteoblastic differentiation of human mesenchymal stem cells (hMSCs) were improved. The effects of osteoclast in bone remodeling and in bone tissue engineering are from its potentials to control the proliferation and differentiation of osteoblasts. Paracrine cytokines such as hepatocyte growth factor (HGF), sclerostin, myeloid protein-1 (Mim-1) are suggested to be responsible for this action of osteoclasts [42]. Additionally, osteoclasts express the nuclear factor of activated T cells c1 (NFATc1) target gene Efnb2 (encoding ephrinB2), while osteoblasts express the receptor Ephrin type-B receptor 4 (EphB4) [43]. The forward signaling conducted through EphB4 into osteoblasts enhances osteoblastic differentiation. Therefore, Han and Zhang [43] pointed out that the absence of osteoclasts might be the main cause of the osteoblast abnormalities in bone tissue reconstruction in vitro, and osteoclast introduction would become a novel strategy in bone tissue engineering. Besides, regulation of mature differentiation and function of osteoclasts will also be a reasonable option for bone tissue engineering. The recent studies indicated that RANKL and osteoprotegerin (OPG) are both highly expressed in the MSCs, and through regulating the ratio of RANKL/OPG, MSCs actively participate in the regulation of osteoclastogenesis [44]. EphB4 and ephrinB2 are also highly expressed in MSCs, and dysregulated expressions of EphrinB2 and EphB4 in MSCs contribute, at least in part, to the development of myelomatous bone lesions [45]. Varin et al. [46] demonstrated that soluble CD200 expressed on MSCs inhibited the differentiation and maturation of osteoclast precursors in vitro. Moreover, CD200 positive MSCs inhibited RANKL-dependent osteoclast formation. However, our results using indirect coculture system showed that PDLSCs enhanced RANKL-induced terminal differentiation and the expressions of TRAP, TRAF6 and CTSK genes and proteins in RAW264.7 cells. These contradictory results are difficult to explain now, but may be related to types and differentiation status of both MSCs and osteoclast precursors. Considering that the degradation of hydroxyapatite and organic matrix by osteoclasts plays a key role in bone remodeling, it is reasonable to presume that osteoclasts may contribute to the biological degradation of supporting materials in bone tissue engineering. Therefore, further investigation on regulation of MSCs including PDLSCs on mature differentiation of osteoclast precursors and function of osteoclasts will help in more roundly understanding the mechanisms of MSCs in bone tissue engineering.
Conclusion
It is well known that MSCs can exert a therapeutic role by paracrine secretion of a variety of molecules. However, the present prevailing studies on the paracrine secretion of MSCs are mainly concentrated on their recruiting host cells and anti-inflammatory and immunoregulatory potentials. Few reports have been documented about the effects of MSCs on osteoclastic and osteoblastic differentiations. Our study using a indirect coculture system showed that PDLSCs enhanced ALP activity, expressions of ALP, BSP, OPN mRNA and BSP, OPN proteins and mineralization matrix deposition in preosteoblast MC3T3-E1 cells. Meanwhile, they improved maturation of osteoclasts and expressions of TRAP, CSTK, TRAF6 mRNA and TRAP, TRAF6 proteins in osteoclast precursor RAW264.7 cells. These data suggest that PDLSCs regulate both osteoblastic and osteoclastic differentiation, at least partially, in a paracrine fasion. Further investigation on regulation mechanisms of MSCs on mature differentiation and functions of osteoblast and osteoclast precursors will help in more roundly understanding the mechanisms of MSCs in bone tissue engineering.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81271141) and the Natural Science Foundation of Shandong Province (No. ZR2010HQ015).
Disclosure of conflict of interest
None.
References
- 1.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–20. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 2.Hynes K, Menicanin D, Gronthos S, Bartold PM. Clinical utility of stem cells for periodontal regeneration. Periodontol 2000. 2012;59:203–27. doi: 10.1111/j.1600-0757.2012.00443.x. [DOI] [PubMed] [Google Scholar]
- 3.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 4.Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–42. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 5.Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381–90. [PubMed] [Google Scholar]
- 6.Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5:362–9. doi: 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- 7.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–30. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100:5807–12. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morsczeck C, Gotz W, Schierholz J, Zeilhofer F, Kuhn U, Mohl C, Sippel C, Hoffmann KH. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005;24:155–65. doi: 10.1016/j.matbio.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–55. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 11.Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, Liu H, Gronthos S, Wang CY, Wang S, Shi S. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006;1:e79. doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu X, Ge S, Chen S, Xu Q, Zhang J, Guo H, Yang P. Human gingiva-derived mesenchymal stromal cells contribute to periodontal regeneration in beagle dogs. Cells Tissues Organs. 2013;198:428–37. doi: 10.1159/000360276. [DOI] [PubMed] [Google Scholar]
- 13.Iwata T, Yamato M, Zhang Z, Mukobata S, Washio K, Ando T, Feijen J, Okano T, Ishikawa I. Validation of human periodontal ligament-derived cells as a reliable source for cytotherapeutic use. J Clin Periodontol. 2010;37:1088–99. doi: 10.1111/j.1600-051X.2010.01597.x. [DOI] [PubMed] [Google Scholar]
- 14.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–84. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 15.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Aktmodified mesenchymal stem cells. Nat Med. 2005;11:367–8. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 16.Wada N, Maeda H, Tanabe K, Tsuda E, Yano K, Nakamuta H, Akamine A. Periodontal ligament cells secrete the factor that inhibits osteoclastic differentiation and function: the factor is osteoprotegerin/osteoclastogenesis inhibitory factor. J Periodontal Res. 2001;36:56–63. doi: 10.1034/j.1600-0765.2001.00604.x. [DOI] [PubMed] [Google Scholar]
- 17.Digirolamo CM, Stokes D, Colter D, Phinney DG, Class R, Prockop DJ. Propagation and senescence of human marrow stromal cells in culture: a simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br J Haematol. 1999;107:275–81. doi: 10.1046/j.1365-2141.1999.01715.x. [DOI] [PubMed] [Google Scholar]
- 18.Huh JB, Kim SE, Song SK, Yun MJ, Shim JS, Lee JY, Shin SW. The effect of immobilization of heparin and bone morphogenic protein-2 to bovine bone substitute on osteoblast-like cell’s function. J Adv Prosthodont. 2011;3:145–51. doi: 10.4047/jap.2011.3.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiou WF, Lee CH, Liao JF, Chen CC. 8-Prenylkaempferol accelerates osteoblast maturation through bone morphogenetic protein-2/p38 pathway to activate Runx2 transcription. Life Sci. 2011;88:335–42. doi: 10.1016/j.lfs.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Mitrano TI, Grob MS, Carrion F, Nova-Lamperti E, Luz PA, Fierro FS, Quintero A, Chaparro A, Sanz A. Culture and characterization of mesenchymal stem cells from human gingival tissue. J Periodontol. 2010;81:917–25. doi: 10.1902/jop.2010.090566. [DOI] [PubMed] [Google Scholar]
- 21.Yang H, Gao LN, An Y, Hu CH, Jin F, Zhou J, Jin Y, Chen FM. Comparison of mesenchymal stem cells derived from gingival tissue and periodontal ligament in different incubation conditions. Biomaterials. 2013;34:7033–47. doi: 10.1016/j.biomaterials.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 22.Lu H, Xie C, Zhao YM, Chen FM. Translational research and therapeutic applications of stem cell transplantation in periodontal regenerative medicine. Cell Transplant. 2013;22:205–29. doi: 10.3727/096368912X656171. [DOI] [PubMed] [Google Scholar]
- 23.Park JY, Jeon SH, Choung PH. Efficacy of periodontal stem cell transplantation in the treatment of advanced periodontitis. Cell Transplant. 2011;20:271–85. doi: 10.3727/096368910X519292. [DOI] [PubMed] [Google Scholar]
- 24.Tsumanuma Y, Iwata T, Washio K, Yoshida T, Yamada A, Takagi R, Ohno T, Lin K, Yamato M, Ishikawa I, Okano T, Izumi Y. Comparison of different tissue-derived stem cell sheets for periodontal regeneration in a canine 1-wall defect model. Biomaterials. 2011;32:5819–25. doi: 10.1016/j.biomaterials.2011.04.071. [DOI] [PubMed] [Google Scholar]
- 25.Meirelles Lda S, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419–27. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Frost HM. Dynamics of bone remodeling. Bone Biodynamics. 1964:315. [Google Scholar]
- 27.Sims NA, Martin TJ. Coupling Signals between the Osteoclast and Osteoblast: How are Messages Transmitted between These Temporary Visitors to the Bone Surface? Front Endocrinol (Lausanne) 2015;6:41. doi: 10.3389/fendo.2015.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phan TC, Xu J, Zheng MH. Interaction between osteoblast and osteoclast: impact in bone disease. Histol Histopathol. 2004;19:1325–44. doi: 10.14670/HH-19.1325. [DOI] [PubMed] [Google Scholar]
- 29.Li S, Tu Q, Zhang J, Stein G, Lian J, Yang PS, Chen J. Systemically transplanted bone marrow stromal cells contributing to bone tissue regeneration. J Cell Physiol. 2008;215:204–9. doi: 10.1002/jcp.21302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Yang R, Shi S. Systemic infusion of mesenchymal stem cells improves cell-based bone regeneration via upregulation of regulatory T cells. Tissue Eng Part A. 2015;21:498–509. doi: 10.1089/ten.tea.2013.0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li C, Wang X, Tan J, Wang T, Wang Q. The immunomodulatory properties of periodontal ligament stem cells isolated from inflamed periodontal granulation. Cells Tissues Organs. 2014;199:256–65. doi: 10.1159/000367986. [DOI] [PubMed] [Google Scholar]
- 32.Wada N, Menicanin D, Shi S, Bartold PM, Gronthos S. Immunomodulatory properties of human periodontal ligament stem cells. J Cell Physiol. 2009;219:667–76. doi: 10.1002/jcp.21710. [DOI] [PubMed] [Google Scholar]
- 33.Boomsma RA, Geenen DL. Mesenchymal stem cells secrete multiple cytokines that promote angiogenesis and have contrasting effects on chemotaxis and apoptosis. PLoS One. 2012;7:e35685. doi: 10.1371/journal.pone.0035685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–85. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 35.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–8. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 36.Li F, Whyte N, Niyibizi C. Differentiating multipotent mesenchymal stromal cells generate factors that exert paracrine activities on exogenous MSCs: Implications for paracrine activities in bone regeneration. Biochem Biophys Res Commun. 2012;426:475–9. doi: 10.1016/j.bbrc.2012.08.095. [DOI] [PubMed] [Google Scholar]
- 37.Osugi M, Katagiri W, Yoshimi R, Inukai T, Hibi H, Ueda M. Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng Part A. 2012;18:1479–89. doi: 10.1089/ten.tea.2011.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dai XM, Zong XH, Akhter MP, Stanley ER. Osteoclast deficiency results in disorganized matrix, reduced mineralization, and abnormal osteoblast behavior in developing bone. J Bone Miner Res. 2004;19:1441–51. doi: 10.1359/JBMR.040514. [DOI] [PubMed] [Google Scholar]
- 39.Sakagami N, Amizuka N, Li M, Takeuchi K, Hoshino M, Nakamura M, Nozawa-Inoue K, Udagawa N, Maeda T. Reduced osteoblastic population and defective mineralization in osteopetrotic (op/op) mice. Micron. 2005;36:688–95. doi: 10.1016/j.micron.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 40.Walker DG. Bone resorption restored in osteopetrotic mice by transplants of normal bone marrow and spleen cells. Science. 1975;190:784–5. doi: 10.1126/science.1105786. [DOI] [PubMed] [Google Scholar]
- 41.Sinclair SS, Burg KJ. Effect of osteoclast co-culture on the differentiation of human mesenchymal stem cells grown on bone graft granules. J Biomater Sci Polym Ed. 2011;22:789–808. doi: 10.1163/092050610X496260. [DOI] [PubMed] [Google Scholar]
- 42.Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T, Suda T, Matsuo K. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006;4:111–21. doi: 10.1016/j.cmet.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 43.Han D, Zhang Q. An essential requirement for osteoclasts in refined bone-like tissue reconstruction in vitro. Med Hypotheses. 2006;67:75–8. doi: 10.1016/j.mehy.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 44.Takeshita S, Fumoto T, Naoe Y, Ikeda K. Age-related marrow adipogenesis is linked to increased expression of RANKL. J Biol Chem. 2014;289:16699–710. doi: 10.1074/jbc.M114.547919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pennisi A, Ling W, Li X, Khan S, Shaughnessy JD Jr, Barlogie B, Yaccoby S. The ephrinB2/EphB4 axis is dysregulated in osteoprogenitors from myeloma patients and its activation affects myeloma bone disease and tumor growth. Blood. 2009;114:1803–12. doi: 10.1182/blood-2009-01-201954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varin A, Pontikoglou C, Labat E, Deschaseaux F, Sensebe L. CD200R/CD200 inhibits osteoclastogenesis: new mechanism of osteoclast control by mesenchymal stem cells in human. PLoS One. 2013;8:e72831. doi: 10.1371/journal.pone.0072831. [DOI] [PMC free article] [PubMed] [Google Scholar]


