Abstract
FOXP3 is a transcription factor and well-known hallmark of immune suppressive T regulatory cells (Tregs). Recent studies indicate that, in addition to its association with Treg function in the immune system, FOXP3 plays an important role in tumor development. And important tumor suppressor relay between the FOXP3 and Hippo pathways was found in human cancer. Thus, we investigated tumoral FOXP3, infiltrated Tregs count, Lats2, and YAP expression in gastric adenocarcinoma, and the relationships between expression of these three proteins and p53, Ki67, and other clinicopathological variables. We used 118 gastric adenocarcinoma tissues via immunohistochemical analysis, using a tissue microarray, in relation to survival and other clinicopathological factors. We report the several novel observations about the relationship between tumoral FOXP3 and Hippo pathway components in gastric adenocarcinoma. Positive tumoral FOXP3 expression was significantly related with smaller tumor size, tubular tumor type, lower histological grade, lower T stage, lower recurrence rate, less lymphatic invasion, and less neural invasion. Furthermore, patients with positive tumoral FOXP3 experienced significantly better disease-free and overall survival compared to patients with negative tumoral FOXP3. These findings show that tumoral FOXP3 expression is associated with favorable clinicopathological variables in gastric adenocarcinoma. And we report the novel observation of a relationship between tumoral FOXP3 and Hippo pathway components in gastric adenocarcinoma. Tumoral FOXP3 expression, infiltrated Tregs count, and Lats2 expression were all positively correlated with YAP expression. These findings suggest that the Hippo pathway in gastric adenocarcinoma might be influenced by both tumoral FOXP3 and infiltrated Tregs. In conclusion, the loss of FOXP3 expression in cancer cells is thought to contribute to tumorigenesis and progression of gastric adenocarcinoma. The expression of FOXP3 in gastric adenocarcinoma is related with Lats2 and YAP expression of the Hippo pathway.
Keywords: Gastric adenocarcinoma, FOXP3, Lats2, YAP
Introduction
Globally, gastric cancer is the fourth most common cancer and second leading cause of cancer-related deaths [1]. Gastric cancers are characterized by genetic and epigenetic changes that affect oncogenes, tumor suppressor genes, and DNA mismatch repair. Consequently, deregulation of cellular proliferation, adhesion, differentiation, and signal transduction are related to tumorigenesis and progression of gastric adenocarcinoma [2].
FOXP3 is a transcription factor and well-known hallmark of immune suppressive T regulatory cells (Tregs) [3]. Relatively well conserved in mammals [4], the FOXP3 gene is located on the short arm of the X chromosome at Xp.11.23 [5].
Recent studies indicate that, in addition to its association with Treg function in the immune system, FOXP3 plays an important role in tumor development [6,7]. FOXP3 expression in tumor cells has been reported in pancreatic cancer [6], melanoma, and other tumor cell lines [8]. There is great interest in the role of the FOXP3 gene in tumor development and the mechanisms that regulate FOXP3 expression. Merlo et al. demonstrated that FOXP3 expression level in breast carcinoma cells is associated with patient survival [9], and suggest that tumoral FOXP3 might be related to metastatic potential. Both the general mechanism by which FOXP3 expression in tumor cells affects prognosis as well as the role and function of tumoral FOXP3 in gastric adenocarcinoma remains largely unknown.
Genetic studies in Drosophila have established an important role for the Hippo pathway in the regulation of cell proliferation and apoptosis [10,11]. Li et al. revealed an important tumor suppressor relay between the FOXP3 and Hippo pathways that has been widely implicated in human cancer [12]. Lats2 (large tumor suppressor), an important enzyme of the Hippo pathway [12], is dysregulated in several cancer types [13]. This pathway largely contributes to regulation of cell cycle proliferation and apoptosis of cells by repressing expression of the oncogene YAP (Yes-associated protein) [12]. YAP is thought to regulate the balance between cell proliferation and apoptosis to maintain homeostasis [14]. FOXP3 is a direct transcriptional activator of Lats2 in epithelial cells of the prostate and breast where mutations in FOXP3 often result in decreased levels of Lats2 and an increase in YAP expression [12]. In the present study, we investigated tumoral FOXP3, Lats2, and YAP expression related to the Hippo pathway in gastric adenocarcinoma, and the relationships between expression of these three proteins and p53, Ki67, and other clinicopathological variables.
Materials and methods
Patients and tissue samples
Tissue samples were acquired from 118 cases of gastric adenocarcinoma surgically resected at Kyung Hee University Hospital at Gangdong from 2006 to 2009. For each case, two investigators (K.Y. Won and G.Y. Kim) reviewed all of the original hematoxylin and eosin-stained sections. Clinicopathological variables including age, sex, tumor type, histologic grade, tumor size, primary tumor (pT), nodal (pN) metastasis, recurrence, lymphatic invasion, vascular invasion, and neural invasion were evaluated. The mean patient follow-up duration was 30.8 months (range, 3-51 months). Among a total of 118 patients, 23 (19.5%) died of disease and 95 (80.5%) remained alive on the day when the study was initiated. Patient age ranged from 39 to 88 years (median age, 64.6 years). This study was approved by the Institutional Review Board at Kyung Hee University Hospital at Gangdong (IRB 2014-11-021-001).
Tissue microarray (TMA) construction
The H&E-stained sections of formalin-fixed paraffin embedded tumor tissue blocks were screened to identify representative, viable areas of gastric adenocarcinoma. The corresponding areas on the block were marked for tissue core punches. The TMAs were assembled using a commercially available manual tissue microarrayer (Quick-Ray; UNITMA Co., Ltd, Seoul, Korea). Briefly, three representative tumor cores with diameters of 2.0 mm were punched from each tumor tissue block, and arrayed into three recipient paraffin blocks, respectively. We arrayed three cores per case to increase the concordance rate between the immunohistochemistry results of the TMAs and the whole sections. Each of the tissue microarray blocks also contained four normal gastric tissue cores. H&E staining was performed for each block to verify tumor cell content. Cases with stromal tissue only or insufficient carcinoma tissue in the cores were excluded from analysis. Serial sectioned slides were produced, and H&E staining was performed.
Immunohistochemical staining
Immunohistochemistry was performed on 4 µm tissue sections from each TMA block using the Bond Polymer Intense Detection system (Vision BioSystems, Victoria, Australia) according to the manufacturer’s instructions with minor modifications. In brief, 4 µm sections of formalin-fixed, paraffin-embedded tissue were deparaffinized with Bond Dewax Solution (Vision BioSystems), and an antigen retrieval procedure was performed using Bond ER Solution (Vision BioSystems) for 30 minutes at 100°C. Endogenous peroxidases were quenched by incubating the tissue with hydrogen peroxide for 5 minutes. Sections were incubated for 15 minutes at ambient temperature with primary polyclonal antibodies to FOXP3 (1:100, PCH101, eBioscience, Cambridge, UK), YAP (1:100, Cell Signaling Technology, MA, USA), Lats2 (1:1000, Proteintech, Chicago, IL, USA), Ki-67 (1:200, M 7240; Dako, Glostrup, Denmark), and p53 (1:500, DO-7, Dako, Novocastra, Newcastle, UK) using a biotin-free polymeric horseradish peroxidase-linker antibody conjugate system in a Bond-max automatic slide stainer (Vision BioSystems). Nuclei were counterstained with hematoxylin. The negative control was treated in an identical manner using mouse IgG instead of primary antibody.
Evaluation of immunohistochemical staining
Tumoral FOXP3 expression was observed in the nuclei and cytoplasm of carcinoma cells. Staining of at least 20% of the cells was considered as positive FOXP3 expression [15]. FOXP3 expression in Tregs appeared as nuclear staining. The number of FOXP3-expressing Tregs in tumoral epithelium and stroma was counted in three high power fields (HPF, ×400 magnification), and the average scores were correlated with clinicopathological variables. We defined cases with ≥15 FOXP3 positive cells/HPF as positive expression [16]. YAP expression was observed in the nuclei and cytoplasm of carcinoma cells. Staining of at least 10% of the cells was considered as positive YAP expression [17]. Lats2 expression was observed in the nuclei and cytoplasm. Staining of at least 20% of the cells was considered as positive Lats2 expression. All slides were evaluated independently by two investigators (W.K.Y. and K.G.Y) without knowledge of patient identity or clinical outcome. Ki67 and p53 were considered positive if there was >10% positive average nuclear staining of strong intensity.
Statistical analysis
Pearson’s chi-square test was used to evaluate the association between tumoral FOXP3, Lats2, YAP, p53, Ki67 expression and several clinicopathological variables. The Kaplan-Meier method was used to determine the probability of disease-free and overall survival, and the data were analyzed by the log-rank test. A P-value <0.05 was considered significant. Statistical analyses were performed using the SPSS software package (version 15.0; SPSS, Inc., Chicago, IL, USA). Overall survival was defined as survival from the date of surgery to the date of death due to cancer.
Results
Relationship between tumoral FOXP3, Tregs, Lats2, and YAP expression and clinicopathological variables
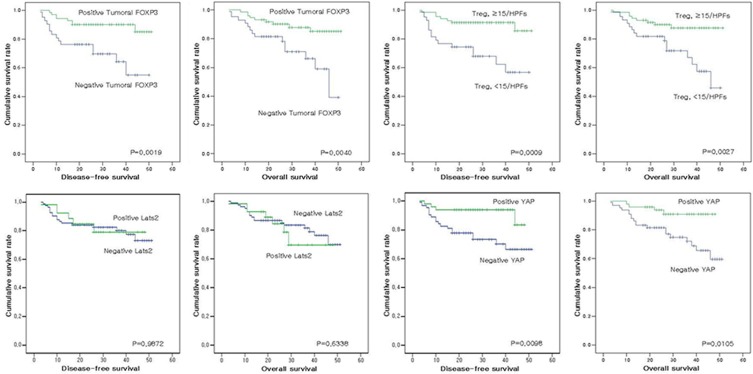
Positive tumoral FOXP3 expression was observed in 63.2% (74/117) of the gastric adenocarcinomas (Figure 1). The cases of infiltrated FOXP3-expressing Tregs (≥15 FOXP3 positive cells/HPF) were observed in 62.1% (72/116) (Figure 1B, 1D), positive Lats2 expression in 25.2% (28/111) (Figure 2A, 2B), and positive YAP expression in 43.1% (50/116) of the gastric adenocarcinomas (Figure 2C, 2D). Normal gastric mucosal epithelial cells were negative for FOXP3 expression. As shown in Tables 1 and 2, positive tumoral FOXP3 expression was significantly related with smaller tumor size, tubular tumor type, lower histological grade, lower T stage, lower recurrence rate, less lymphatic invasion, and less neural invasion. The cases of infiltrated FOXP3-expressing Tregs (≥15 FOXP3 positive cells/HPF) were significantly related with smaller tumor size, lower T stage, negative lymph node metastasis, lower recurrence rate, less lymphatic invasion, less vascular invasion, less neural invasion, and higher Ki67 expression. Positive Lats2 expression was significantly related with less neural invasion. Positive YAP expression was significantly related with smaller tumor size, tubular tumor type, lower histologic grade, lower T stage, lower recurrence rate, and higher Ki67 expression.
Figure 1.
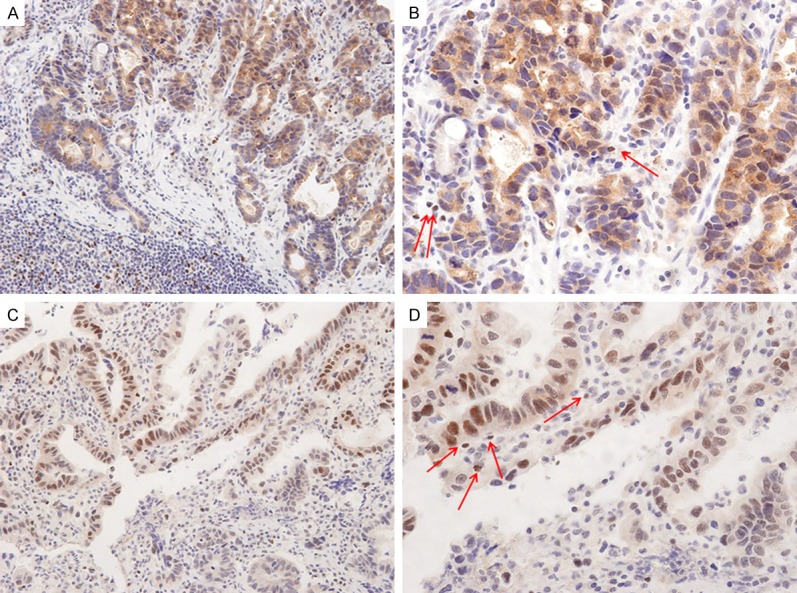
Representative photographs of FOXP3 expression in gastric adenocarcinomas. (A) Strong tumoral FOXP3 expression in nucleus and cytoplasm of gastric adenocarcinomas (original magnification, ×100). (B) Magnified view of (A) (original magnification, ×200). The carcinoma cells show granular cytoplasmic with focal nuclear tumoral FOXP3 expression. The tumor stroma also shows some FOXP3 positive Tregs (red arrows). (C) Another case of gastric adenocarcinoma shows strong tumoral FOXP3 (original magnification, ×100). (D) Magnified view of (C) (original magnification, ×200). Many carcinoma cells show strong nuclear tumoral FOXP3 expression. The tumor stroma also shows some FOXP3 positive Tregs (red arrows).
Figure 2.
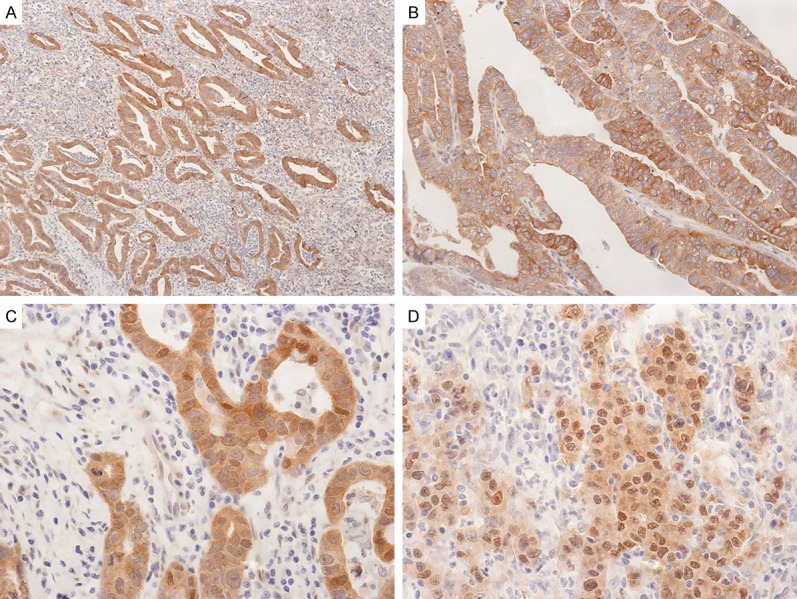
Representative photographs of Lats2 and YAP expression in gastric adenocarcinomas. A. Carcinoma cells show strong cytoplasmic and membranous Lats2 expression (original magnification, ×40). B. Another case of gastric adenocarcinoma shows cytoplasmic and membranous Lats2 expression (original magnification, ×100). C. Carcinoma cells show strong cytoplasmic and some nuclear YAP expression (original magnification, ×200). D. Carcinoma cells show cytoplasmic and many nuclear YAP expression (original magnification, ×200).
Table 1.
Correlation between Tumoral FOXP3 expression, Treg and clinicopathological variables in gastric adenocarcinoma
| Tumoral Foxp3 | Treg count | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Positive | Negative | P value | ≥15/HPFs | <15/HPFs | P value | |
| Tumor size | ||||||
| ≤3 cm | 38 (76.0) | 12 (24.0) | 0.011* | 36 (73.5) | 13 (26.5) | 0.024* |
| >3 cm | 36 (53.7) | 31 (46.3) | 36 (53.7) | 31 (46.3) | ||
| Histologic type | ||||||
| Mixed | 6 (33.3) | 12 (66.7) | 0.005* | 12 (66.7) | 6 (33.3) | 0.437 |
| Tubular | 68 (68.7) | 31 (31.3) | 60 (61.2) | 38 (38.8) | ||
| Histologic grade | ||||||
| Well/moderately | 45 (73.8) | 16 (26.2) | 0.027* | 38 (63.3) | 22 (36.7) | 0.531 |
| Poorly | 29 (54.7) | 45 (73.8) | 33 (62.3) | 20 (37.7) | ||
| Primary tumor (T) | ||||||
| I/II | 52 (71.2) | 21 (28.8) | 0.018* | 51 (70.8) | 21 (29.2) | 0.011* |
| III/IV | 22 (50.0) | 22 (50.0) | 21 (47.7) | 23 (52.3) | ||
| Lymph node metastasis (N) | ||||||
| Absent | 48 (69.6) | 21 (30.4) | 0.067 | 49 (72.1) | 19 (27.9) | 0.007* |
| Present | 26 (54.2) | 22 (45.8) | 23 (47.9) | 25 (52.1) | ||
| Recurrence | ||||||
| Absent | 66 (69.5) | 29 (30.5) | 0.004* | 65 (69.1) | 29 (30.9) | 0.001* |
| Present | 8 (36.4) | 14 (63.6) | 7 (31.8) | 15 (68.2) | ||
| Lymphatic invasion | ||||||
| Absent | 46 (70.8) | 19 (29.2) | 0.045* | 46 (71.9) | 18 (28.1) | 0.013* |
| Present | 28 (53.8) | 24 (46.2) | 26 (50.0) | 26 (50.0) | ||
| Vascular invasion | ||||||
| Absent | 72 (64.9) | 39 (35.1) | 0.131 | 71 (64.5) | 39 (35.5) | 0.029* |
| Present | 2 (33.3) | 4 (66.7) | 1 (16.7) | 5 (83.3) | ||
| Neural invasion | ||||||
| Absent | 68 (67.3) | 33 (32.7) | 0.023* | 67 (67.0) | 33 (33.0) | 0.008* |
| Present | 6 (37.5) | 10 (62.5) | 5 (31.3) | 11 (68.8) | ||
| Ki67 expression | ||||||
| Low | 8 (33.3) | 16 (66.7) | 0.001* | 10 (41.7) | 14 (58.3) | 0.018* |
| High | 65 (71.4) | 26 (28.6) | 61 (67.8) | 29 (32.2) | ||
| p53 expression | ||||||
| Low | 25 (65.8) | 13 (34.2) | 0.414 | 26 (68.4) | 12 (31.6) | 0.204 |
| High | 45 (61.6) | 28 (38.4) | 42 (58.3) | 30 (41.7) | ||
NOTE. Values are n (%).
Significantly different by the chi-squared test.
Table 2.
Correlation between Lats2, and YAP expression and clinicopathological variables in gastric adenocarcinoma
| Lats2 | YAP | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Positive | Negative | P value | Positive | Negative | P value | |
| Tumor size | ||||||
| ≤3 cm | 10 (22.7) | 34 (77.3) | 0.397 | 27 (55.1) | 22 (44.9) | 0.021* |
| >3 cm | 18 (26.9) | 49 (73.1) | 23 (34.3) | 44 (65.7) | ||
| Histologic type | ||||||
| Mixed | 5 (27.8) | 13 (72.2) | 0.496 | 4 (22.2) | 14 (77.8) | 0.043* |
| Tubular | 23 (24.7) | 70 (75.3) | 46 (46.9) | 52 (53.1) | ||
| Histologic grade | ||||||
| Well/moderately | 17 (29.8) | 40 (70.2) | 0.158 | 32 (52.5) | 29 (47.5) | 0.027* |
| Poorly | 10 (19.6) | 41 (80.4) | 17 (32.7) | 35 (67.3) | ||
| Primary tumor (T) | ||||||
| I/II | 17 (25.4) | 50 (74.6) | 0.574 | 37 (51.4) | 35 (48.6) | 0.017* |
| III/IV | 11 (25.0) | 33 (75.0) | 13 (29.5) | 31 (70.5) | ||
| Lymph node metastasis (N) | ||||||
| Absent | 15 (23.4) | 49 (76.6) | 0.386 | 34 (49.3) | 35 (50.7) | 0.075 |
| Present | 13 (27.7) | 34 (72.3) | 16 (34.0) | 31 (66.0) | ||
| Recurrence | ||||||
| Absent | 23 (25.8) | 66 (74.2) | 0.5 | 46 (48.9) | 48 (51.1) | 0.007* |
| Present | 5 (22.7) | 17 (77.3) | 4 (18.2) | 18 (81.8) | ||
| Lymphatic invasion | ||||||
| Absent | 14 (23.3) | 46 (76.7) | 0.389 | 31 (47.7) | 34 (52.3) | 0.174 |
| Present | 14 (27.5) | 37 (72.5) | 19 (37.3) | 32 (62.7) | ||
| Vascular invasion | ||||||
| Absent | 27 (25.7) | 78 (74.3) | 0.526 | 49 (44.5) | 61 (55.5) | 0.181 |
| Present | 1 (16.7) | 5 (83.3) | 1 (16.7) | 5 (83.3) | ||
| Neural invasion | ||||||
| Absent | 27 (28.4) | 68 (71.6) | 0.048* | 46 (46.0) | 54 (54.0) | 0.095 |
| Present | 1 (6.3) | 15 (93.8) | 4 (25.0) | 12 (75.0) | ||
| Ki67 expression | ||||||
| Low | 5 (21.7) | 18 (78.3) | 0.435 | 5 (21.7) | 18 (78.3) | 0.017* |
| High | 23 (26.4) | 64 (73.6) | 44 (48.4) | 47 (51.6) | ||
| p53 expression | ||||||
| Low | 9 (25.0) | 27 (75.0) | 0.427 | 17 (44.7) | 21 (55.3) | 0.401 |
| High | 15 (21.4) | 55 (78.6) | 29 (40.3) | 43 (59.7) | ||
Note: Values are n (%).
Significantly different by the chi-squared test.
Interrelationship between tumoral FOXP3, Tregs, Lats2, and YAP expression in gastric adenocarcinoma
As shown in Table 3, positive tumoral FOXP3 expression was significantly related with Treg count (P=0.005) and YAP expression (P=0.025). YAP expression was significantly related with Treg count (P=0.021) and Lats2 expression (P=0.043). Tumoral FOXP3 expression was not significantly correlated with Lats2 expression.
Table 3.
Correlation among Tumoral FOXP3, Treg, Lats2, and YAP in gastric adenocarcinoma
| Tumoral FOXP3 | Lats2 | YAP | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Positive | Negative | P value | Positive | Negative | P value | Positive | Negative | P value | |
| Treg count | |||||||||
| <15/HPFs | 21 (28.4) | 23 (54.8) | 0.005* | 8 (28.6) | 33 (40.2) | 0.191 | 13 (26.0) | 30 (46.2) | 0.021* |
| ≥15/HPFs | 53 (71.6) | 19 (45.2) | 20 (71.4) | 49 (59.8) | 37 (74.0) | 35 (53.8) | |||
| Lats2 | |||||||||
| Positive | 20 (29.0) | 8 (19.0) | 0.173 | 16 (34.8) | 12 (18.5) | 0.043* | |||
| Negative | 49 (71.0) | 34 (81.0) | 30 (65.2) | 53 (81.5) | |||||
| YAP | |||||||||
| Positive | 37 (50.7) | 13 (30.2) | 0.025* | ||||||
| Negative | 36 (49.3) | 30 (69.8) | |||||||
Note: Values are n (%).
Significantly different by the chi-squared test.
Tumoral FOXP3, Tregs, Lats2, and YAP expression and disease-free and overall survival rate
Adequate clinical follow-up information was available for all 118 patients with gastric adenocarcinomas. As shown in Table 4, univariate analyses for disease-free survival revealed an association with larger tumor size (P=0.0010), higher histologic grade (P=0.0380), higher primary tumor stage (P<0.00001), lymph node metastasis (P<0.00001), lymphatic invasion (P=0.0041), neural invasion (P=0.0367), tumoral FOXP3 expression (P=0.0019), Treg count (P=0.0009), YAP expression (P=0.0098), and Ki67 expression (P<0.00001). In univariate analyses, overall survival was related to larger tumor size (P=0.0163), higher primary tumor stage (P<0.00001), lymph node metastasis (P=0.0003), lymphatic invasion (P=0.0040), tumoral FOXP3 expression (P=0.0040), Treg count (P=0.0027), YAP expression (P=0.0105), and Ki67 expression (P=0.0013) (Figure 3).
Table 4.
Univariate analysis of clinicopathological variables for overall survival rate in gastric adenocarcinomas
| Variables | Disease-free survival (P value) | Overall survival (P value) |
|---|---|---|
| Tumor size (<3.0 cm vs. ≥3.0 cm) | 0.0010* | 0.0163* |
| Tumor type (tubular vs. mixed) | 0.2419 | 0.3431 |
| Histologic grade (well to mod vs. poor) | 0.0380* | 0.1868 |
| Primary tumor (T) (I, II vs. III, IV) | <0.00001* | <0.00001* |
| Lymph node metastasis | <0.00001* | 0.0003* |
| Recurrence | N.A | <0.00001* |
| Lymphatic invasion | 0.0041* | 0.0040* |
| Vascular invasion | 0.6434 | 0.6211 |
| Neural invasion | 0.0367* | 0.0953 |
| Tumoral FOXP3 expression | 0.0019* | 0.004* |
| Treg count | 0.0009* | 0.0027* |
| Lats2 expression | 0.9872 | 0.6338 |
| YAP expression | 0.0098* | 0.0105* |
| Ki 67 expression (<20% vs. ≥20%) | <0.00001* | 0.0013* |
| p53 expression (<10% vs. ≥10%) | 0.8686 | 0.7132 |
Statistically significant;
N.A: not applicable.
Figure 3.
Analysis of disease-free survival and overall survival according to each protein expression.
Discussion
FOXP3 is a member of the forkhead family of transcription factors and plays a key role in regulatory T cell function [18]. FOXP3 expression had been thought to be restricted to the T cell lineage [19]. Increased infiltrated FOXP3-positive Tregs in stroma of tumor have been reported as a poor prognostic factor in several carcinomas, including breast, pancreas, stomach, liver, and lung cancers [20-25]. Recently, various studies have shown that FOXP3 is expressed in tumor cells such as breast cancer cells, melanoma cells, and cell lines derived from a variety of solid tumors [6,26,27]. Some studies report that the FOXP3 gene functions as a tumor suppressor gene for breast [26], prostate [28], and non-small cell lung cancer [15]. Other studies show that FOXP3 expressed by tumors has an oncogenic feature that induces an immunosuppressive environment in stomach cancer [29] and is associated with a high risk of hepatocellular carcinoma [30]. These indicate that the functions of tumoral FOXP3 are diverse and controversial.
In present study about gastric adenocarcinoma, we observed that positive tumoral FOXP3 expression was significantly related with an increase in the number of infiltrated FOXP3-expressing Tregs. Hinz et al. showed an inhibitory influence of FOXP3-expressing pancreatic cancer cells on T cell proliferation in vitro and suggested that FOXP3-positive cancer cells acquire an immune evasion system [6]. Yoshii et al. reported that FOXP3 positive tumor cells occur at a much higher frequency in signet ring cell carcinoma than in carcinomas of other histologic types, suggesting that signet ring cell carcinoma itself might induce immune tolerance through FOXP3 gene expression [29].
Our observations led to some new findings about the role of tumoral FOXP3 in gastric adenocarcinoma. Positive tumoral FOXP3 expression was significantly related with smaller tumor size, tubular tumor type, lower histological grade, lower T stage, lower recurrence rate, less lymphatic invasion, and less neural invasion. Furthermore, patients with positive tumoral FOXP3 experienced significantly better disease-free and overall survival compared to patients with negative tumoral FOXP3. These findings show that tumoral FOXP3 expression is associated with favorable clinicopathological variables in gastric adenocarcinoma. The loss of FOXP3 expression in cancer cells is thought to contribute to tumorigenesis and progression of gastric adenocarcinoma. These results are similar to findings in breast cancer, where loss of FOXP3 expression contributes to tumorigenesis by allowing enhanced expression of the HER-2/ErbB2 oncogene, which plays a key role in breast cancer progression [31].
In present study, the cases of stromal infiltrated FOXP3-expressing Tregs (≥15 FOXP3 positive cells/HPF) were significantly related with smaller tumor size, lower T stage, negative lymph node metastasis, lower recurrence rate, less lymphatic invasion, less vascular invasion, less neural invasion, and higher Ki67 expression. Recent studies have revealed that the accumulation of Tregs is associated with advanced tumor growth and poor prognosis in several types of malignant tumors [24,32,33]. However, Tao et al. reported that tumoral FOXP3 expression attenuates the negative influence of Treg accumulation on survival, suggesting that in NSCLC (non-small cell lung cancer), tumoral FOXP3 functions as a tumor suppressor gene and exerts an inhibitory influence on the progression of Tregs. They further reported that tumoral FOXP3 expression seems to have a substantial prognostic impact on NSCLC patients when assessed in combination with Treg count, and speculated that FOXP3-expressing lung cancer cells have suppressive effects on Treg functions [15]. Interestingly, Hinz et al. has shown in human pancreatic carcinoma cells that down-regulation of FOXP3 results in up-regulation of proinflammatory cytokines IL-6 and IL-8 [6]. IL-6 plays important roles in T cell differentiation and homeostasis such as inhibiting Treg function and expansion [34]. Taken together, these data suggest that tumoral FOXP3 expression, which has an tumor suppressive role, attenuates the negative influence of Treg accumulation on survival in gastric adenocarcinoma patients, as in NSCLC patients.
Furthermore, we investigated the mechanisms of tumoral FOXP3 in connection with the Hippo pathway, which contributes to regulating cell cycle proliferation and apoptosis of cells by repressing expression of the oncogene YAP [12]. The components of the Hippo pathway include YAP and Lats2. Recently, tumoral FOXP3 was shown to relate to the Hippo pathway. FOXP3 is a direct transcriptional activator of Lats2 in epithelial cells of the prostate and breast [12]. Thus, we studied the functions of tumoral FOXP3 in relation with Lats2 and YAP expression in gastric adenocarcinomas. YAP expression in gastric adenocarcinoma correlated with smaller tumor size, tubular tumor type, lower histologic grade, lower T stage, lower recurrence rate, and higher Ki67 expression. Patients with positive YAP expression experienced significantly better disease-free and overall survival compared to patients with negative YAP expression. These observations favor that YAP expression has a tumor suppressor function in gastric adenocarcinoma. Yuan et al. reported that up-regulation of YAP correlated with better survival in a cohort of breast cancer patients [35]. YAP activity is thought to favor tumor suppression, although whether YAP is anti- or pro-tumorigenic may depend on cell context and type of stimuli [17].
Additionally, we discovered the novel observation of a relationship between tumoral FOXP3 and Hippo pathway components in gastric adenocarcinoma. Tumoral FOXP3 expression, infiltrated Tregs count, and Lats2 expression were all positively correlated with YAP expression. The Hippo pathway in gastric adenocarcinoma was influenced by both tumoral FOXP3 and infiltrated Tregs. In prostate and breast cancer, tumoral FOXP3 is a direct transcriptional activator of Lats2. Reduced expression and somatic mutations of FOXP3 correlate strongly with defective Lats2 expression in micro-dissected prostate cancer tissues [12]. They identified a tumor suppressor relay between FOXP3 and the Hippo pathway in breast and prostate cancers [12]. Although we did not find a direct connection between tumoral FOXP3 and Lats2 expression, we had the evidence to support a relationship between tumoral FOXP3 and Lats2 expression via YAP expression in gastric adenocarcinoma. Taken together, the data suggest the possibility that the tumor suppressor function of FOXP3 in gastric adenocarcinomas is related to the Hippo pathway.
FOXP3 has been studied as a therapeutic target, and vaccination to eradicate FOXP3-expressing Tregs enhances tumor immunity [36]. The finding that FOXP3 can be expressed not only by tumor-infiltrating Tregs but also by tumor cells has important implications. Our findings provide new insight that may help develop more effective therapeutic approaches.
In conclusion, tumoral FOXP3 expression is associated with favorable clinicopathological variables in gastric adenocarcinoma. The loss of FOXP3 expression in cancer cells is thought to contribute to tumorigenesis and progression of gastric adenocarcinoma. The expression of FOXP3 in gastric adenocarcinoma is related with Lats2 and YAP expression of the Hippo pathway.
Acknowledgements
This study was funded by the program of the Kyung Hee University Hospital at Gangdong in 2014 (KHU-KHNMC-201401).
Disclosure of conflict of interest
None.
References
- 1.Wang LH, Su L, Wang JT. Correlation between elevated FOXP3 expression and increased lymph node metastasis of gastric cancer. Chin Med J. 2010;123:3545–9. [PubMed] [Google Scholar]
- 2.Pinto M, Wu Y, Mensink RG, Cirnes L, Seruca R, Hofstra RM. Somatic mutations in mismatch repair genes in sporadic gastric carcinomas are not a cause but a consequence of the mutator phenotype. Cancer Genet Cytogenet. 2008;180:110–4. doi: 10.1016/j.cancergencyto.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 3.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 4.Ziegler SF. FOXP3: of mice and men. Ann Rev Immunol. 2006;24:209–26. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 5.Bennett CL, Yoshioka R, Kiyosawa H, Barker DF, Fain PR, Shigeoka AO, Chance PF. X-Linked syndrome of polyendocrinopathy, immune dysfunction, and diarrhea maps to Xp11.23-Xq13.3. Am J Human Genet. 2000;66:461–8. doi: 10.1086/302761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hinz S, Pagerols-Raluy L, Oberg HH, Ammerpohl O, Grussel S, Sipos B, Grutzmann R, Pilarsky C, Ungefroren H, Saeger HD, Kloppel G, Kabelitz D, Kalthoff H. Foxp3 expression in pancreatic carcinoma cells as a novel mechanism of immune evasion in cancer. Cancer Res. 2007;67:8344–50. doi: 10.1158/0008-5472.CAN-06-3304. [DOI] [PubMed] [Google Scholar]
- 7.Jung DJ, Jin DH, Hong SW, Kim JE, Shin JS, Kim D, Cho BJ, Hwang YI, Kang JS, Lee WJ. Foxp3 expression in p53-dependent DNA damage responses. J Biol Chem. 2010;285:7995–8002. doi: 10.1074/jbc.M109.047985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebert LM, Tan BS, Browning J, Svobodova S, Russell SE, Kirkpatrick N, Gedye C, Moss D, Ng SP, MacGregor D, Davis ID, Cebon J, Chen W. The regulatory T cell-associated transcription factor FoxP3 is expressed by tumor cells. Cancer Res. 2008;68:3001–9. doi: 10.1158/0008-5472.CAN-07-5664. [DOI] [PubMed] [Google Scholar]
- 9.Merlo A, Casalini P, Carcangiu ML, Malventano C, Triulzi T, Menard S, Tagliabue E, Balsari A. FOXP3 expression and overall survival in breast cancer. J. Clin. Oncol. 2009;27:1746–52. doi: 10.1200/JCO.2008.17.9036. [DOI] [PubMed] [Google Scholar]
- 10.Harvey K, Tapon N. The Salvador-Warts-Hippo pathway-an emerging tumour-suppressor network. Nat Rev Cancer. 2007;7:182–91. doi: 10.1038/nrc2070. [DOI] [PubMed] [Google Scholar]
- 11.O’Neill E, Kolch W. Taming the Hippo: Raf-1 controls apoptosis by suppressing MST2/Hippo. Cell Cycle. 2005;4:365–7. doi: 10.4161/cc.4.3.1531. [DOI] [PubMed] [Google Scholar]
- 12.Li W, Wang L, Katoh H, Liu R, Zheng P, Liu Y. Identification of a tumor suppressor relay between the FOXP3 and the Hippo pathways in breast and prostate cancers. Cancer Res. 2011;71:2162–71. doi: 10.1158/0008-5472.CAN-10-3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao F, Liu H, Li Z, Zhong C, Fang W. Downregulation of LATS2 in non-small cell lung cancer promoted the growth and motility of cancer cells. Tumour Biol. 2015;36:2049–57. doi: 10.1007/s13277-014-2812-1. [DOI] [PubMed] [Google Scholar]
- 14.Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, Zheng P, Ye K, Chinnaiyan A, Halder G, Lai ZC, Guan KL. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–61. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tao H, Mimura Y, Aoe K, Kobayashi S, Yamamoto H, Matsuda E, Okabe K, Matsumoto T, Sugi K, Ueoka H. Prognostic potential of FOXP3 expression in non-small cell lung cancer cells combined with tumor-infiltrating regulatory T cells. Lung Cancer. 2012;75:95–101. doi: 10.1016/j.lungcan.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Bohling SD, Allison KH. Immunosuppressive regulatory T cells are associated with aggressive breast cancer phenotypes: a potential therapeutic target. Mod Pathol. 2008;21:1527–32. doi: 10.1038/modpathol.2008.160. [DOI] [PubMed] [Google Scholar]
- 17.Kang W, Tong JH, Chan AW, Lee TL, Lung RW, Leung PP, So KK, Wu K, Fan D, Yu J, Sung JJ, To KF. Yes-associated protein 1 exhibits oncogenic property in gastric cancer and its nuclear accumulation associates with poor prognosis. Clin Cancer Res. 2011;17:2130–9. doi: 10.1158/1078-0432.CCR-10-2467. [DOI] [PubMed] [Google Scholar]
- 18.Ito T, Hanabuchi S, Wang YH, Park WR, Arima K, Bover L, Qin FX, Gilliet M, Liu YJ. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity. 2008;28:870–80. doi: 10.1016/j.immuni.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–41. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Mizukami Y, Kono K, Kawaguchi Y, Akaike H, Kamimura K, Sugai H, Fujii H. Localisation pattern of Foxp3+ regulatory T cells is associated with clinical behaviour in gastric cancer. Br J Cancer. 2008;98:148–53. doi: 10.1038/sj.bjc.6604149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J. Clin. Oncol. 2007;25:2586–93. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 22.Petersen RP, Campa MJ, Sperlazza J, Conlon D, Joshi MB, Harpole DH Jr, Patz EF Jr. Tumor infiltrating Foxp3+ regulatory T-cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer. 2006;107:2866–72. doi: 10.1002/cncr.22282. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu K, Nakata M, Hirami Y, Yukawa T, Maeda A, Tanemoto K. Tumor-infiltrating Foxp3+ regulatory T cells are correlated with cyclooxygenase-2 expression and are associated with recurrence in resected non-small cell lung cancer. J Thorac Oncol. 2010;5:585–90. doi: 10.1097/JTO.0b013e3181d60fd7. [DOI] [PubMed] [Google Scholar]
- 24.Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12:5423–34. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- 25.Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH. Quantification of regulatory T cells enables the identification of highrisk breast cancer patients and those at risk of late relapse. J. Clin. Oncol. 2006;24:5373–80. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 26.Zuo T, Liu R, Zhang H, Chang X, Liu Y, Wang L, Zheng P. FOXP3 is a novel transcriptional repressor for the breast cancer oncogene SKP2. J Clin Invest. 2007;117:3765–73. doi: 10.1172/JCI32538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Won KY, Kim HS, Sung JY, Kim GY, Lee J, Park YK, Kim YW, Suh JH, Lim SJ. Tumoral FOXP3 has potential oncogenic function in conjunction with the p53 tumor suppressor protein and infiltrated Tregs in human breast carcinomas. Pathol Res Pract. 2013;209:767–73. doi: 10.1016/j.prp.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Liu R, Li W, Chen C, Katoh H, Chen GY, McNally B, Lin L, Zhou P, Zuo T, Cooney KA, Liu Y, Zheng P. Somatic single hits inactivate the X-linked tumor suppressor FOXP3 in the prostate. Cancer Cell. 2009;16:336–46. doi: 10.1016/j.ccr.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshii M, Tanaka H, Ohira M, Muguruma K, Iwauchi T, Lee T, Sakurai K, Kubo N, Yashiro M, Sawada T, Hirakawa K. Expression of Forkhead box P3 in tumour cells Causes Immunoregulatory Function Of Signet Ring Cell Carcinoma of The Stomach. Br J Cancer. 2012;106:1668–74. doi: 10.1038/bjc.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang WH, Jiang CL, Yan W, Zhang YH, Yang JT, Zhang C, Yan B, Zhang W, Han W, Wang JZ, Zhang YQ. FOXP3 expression and clinical characteristics of hepatocellular carcinoma. World J Gastroenterol. 2010;16:5502–9. doi: 10.3748/wjg.v16.i43.5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zuo T, Wang L, Morrison C, Chang X, Zhang H, Li W, Liu Y, Wang Y, Liu X, Chan MW, Liu JQ, Love R, Liu CG, Godfrey V, Shen R, Huang TH, Yang T, Park BK, Wang CY, Zheng P, Liu Y. FOXP3 is an X-linked breast cancer suppressor gene and an important repressor of the HER-2/ErbB2 oncogene. Cell. 2007;129:1275–86. doi: 10.1016/j.cell.2007.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi N, Hiraoka N, Yamagami W, Ojima H, Kanai Y, Kosuge T, Nakajima A, Hirohashi S. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007;13:902–11. doi: 10.1158/1078-0432.CCR-06-2363. [DOI] [PubMed] [Google Scholar]
- 34.Zheng SG, Wang J, Horwitz DA. Cutting edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. J Immunol. 2008;180:7112–6. doi: 10.4049/jimmunol.180.11.7112. [DOI] [PubMed] [Google Scholar]
- 35.Yuan M, Tomlinson V, Lara R, Holliday D, Chelala C, Harada T, Gangeswaran R, Manson- Bishop C, Smith P, Danovi SA, Pardo O, Crook T, Mein CA, Lemoine NR, Jones LJ, Basu S. Yesassociated protein (YAP) functions as a tumor suppressor in breast. Cell Death Differ. 2008;15:1752–9. doi: 10.1038/cdd.2008.108. [DOI] [PubMed] [Google Scholar]
- 36.Nair S, Boczkowski D, Fassnacht M, Pisetsky D, Gilboa E. Vaccination against the forkhead family transcription factor Foxp3 enhances tumor immunity. Cancer Res. 2007;67:371–80. doi: 10.1158/0008-5472.CAN-06-2903. [DOI] [PubMed] [Google Scholar]