Abstract
Due to the increase in overweight and obesity in humans, various studies have been conducted in recent years that demonstrate the detrimental effects on tissues and organs. The aim of this study was to assess the morphoquantitative changes produced in the ileum of mice, associated with high-fat diets. Fourteen male C57BL/6 mice, 5 months old, were fed two types of diets for 14 weeks. The control group (C) was fed a standard diet (10% fat, AIN-93M) and the experimental group (E) was fed a high-fat diet (42% fat, AIN-93M-AG). The assessments included: body weight, calorie consumption, food efficiency, biochemical analysis of plasma lipids, diameter, total wall thickness, thickness of the tunica mucosa and tunica muscularis, length and width of the intestinal villi, depth of the intestinal crypts and number of goblet cells per mm-2 (NA). For the statistical analysis the Student’s t-test was used, considering a P value less than 0.05. The mice in the E group presented greater weight gain (P = 0.028), higher levels of total and LDL cholesterol (P = 0.03 and P = 0.01, respectively), and length of the intestinal villi (P = 0.000). The width of the intestinal villi and the NA of PAS-positive goblet cells presented significantly lower values (P = 0.037 and P = 0.039, respectively) than the C group. The observed changes could be related to the higher demand for fat absorption and to possible alterations in the intestinal microflora and inflammation by action of high-fat diets.
Keywords: High-fat diet, ileum, mouse
Introduction
Given the increase in overweight and obesity in humans, many studies have been conducted in recent years proving the detrimental effects of high-fat diets on different tissues and organs [1-7].
In this context, studies conducted on the digestive system have shown that the amount, origin (animal or vegetable) and type (saturated, monounsaturated and polyunsaturated) of fatty acids influence certain types of cancer. Newmark et al. [8,9] found visible lesions in the alimentary canal after administering high-fat diets for more than 12 months, observing significant differences in the appearance of hyperplastic lesions and tumors when the diet continued up to 18 and 24 months. Some studies have reported that the appearance of these lesions varies between the segments of the alimentary canal. Aslam et al. [10] did not detect any lesions in the small intestine or stomach of mice fed a high-fat diet for 15 months, but they did find the formation of polyps in the large intestine and abnormal lesions in the colonic mucosa.
It has also been shown that the composition of the foods that comprise a diet can produce morphological changes in the tissues of the small intestine. In relation to diets with different fiber content, several authors have observed significant changes in the thickness of the tunica mucosa and tunica muscularis, the length of the villi, depth of the intestinal crypts and number of goblet cells [11,12].
In relation to the diets with a high fat content, Petit et al. [13] observed an increase in the size of the jejunal villi in mice and alterations in the expression of genes involved in absorption, fatty acid transport and lipoprotein synthesis.
Additionally, de Wit et al. [14] investigated the effects of high-fat diets on molecular changes in the small intestine. These authors observed changes in the gene expression related to the lipid metabolism, cell cycle and inflammatory and immune responses. They reported that there are local effects modulated by important metabolic regulators in response to this type of diet. They also found differences in the gene expression of small proteins secreted by the small intestine related to the lipid metabolism, particularly the synthesis of chylomicrons and inflammatory response signaling molecules, such as IL18, FGF15, MIF, IGFBP3 and ANGPTL4, which can have metabolic effects on the liver, muscle and fatty tissue that underlie the development of metabolic syndrome.
Studies also indicate that the high-fat diet involves an alteration in the microflora, producing intestinal inflammation. This alteration causes an increase in luminal and plasma lipopolysaccharides (LPS), proinflammatory cytokines and changes to the occluding junctions of the epithelium [15-18].
Finally, it should be noted that the small intestine plays an important role in the digestion and absorption of consumed food, as well as the elimination of undigested food, microbes and microbial products. The functional integrity of the intestinal mucosa depends on the coordinated regulation of the different elements that comprise it, such as squamous enterocytes, exocrinocytes and the layer of mucus that these produces, the resident microflora and the individual’s immune response. According to the literature, the small intestine can undergo changes in its normal morphology by exposure to inadequate diets, which could cause functional alterations to this structure. Since the ileum is one of the segments of the intestine where most of lipids in the diet are processed and absorbed, the aim of this study was to evaluate the morphoquantitative changes produced by the consumption of high-fat diets in C57B/l6 mice.
Materials and methods
Animals
Fourteen clinically healthy male C57BL/6 mice (Mus musculus), five months old, were used. The experiments were conducted according to the guidelines in the Guide for the Care and Use of Laboratory Animals [19]. The experimental protocol was approved by the Ethics Committee of the Universidad de La Frontera (approval n° 06/2013).
Diet
The mice were divided randomly into two groups of seven animals each: the control (C) group and the experimental (E) group. The animals in the C group were fed a standard diet for rodents, composed of 10% fat in accordance with AIN-93M. The E group was fed a diet that contained 42% fat (AIN-93M-AG) (American Institute of Nutrition’s Recommendations For Laboratory Rodents) [20].
The diets were prepared by PRAGSOLUÇÕES Biociências (www.pragsolucoes.com.br, Jau, SP, Brazil) and stored at -20°C for the duration of the study. The composition of the two diets is detailed in Table 1, and the percentage of macronutrients in Table 2. The vitamin and mineral contents of the two diets were identical.
Table 1.
Composition of diets according to the American Institute of Nutrition’s Recommendations for Laboratory Rodents
| Ingredients (g/Kg) | AIN-93M | AIN-93M-AG |
|---|---|---|
| Casein | 140 | 190 |
| Cornstarch | 620 | 250 |
| Sucrose | 100 | 100 |
| Soybean oil | 40 | 40 |
| Lard | - | 320 |
| Fiber | 50 | 50 |
| Vitamin mixture* | 10 | 10 |
| Mineral mixture* | 35 | 35 |
| Cystine | 1.8 | 1.8 |
| Antioxidants | 0.008 | 0.008 |
| Choline | 2.5 | 2.5 |
| Total grams | 1.000 | 1.000 |
AIN-93M (American Institute of Nutrition, maintenance diet for adult rodents), AIN-93M-AG (American Institute of Nutrition, high-fat diet).
The mixture of vitamins and minerals complied with the recommendations for AIN-93.
Table 2.
Total percentage of macronutrients according to the American Institute of Nutrition’s Recommendations for Laboratory Rodents
| Macronutrients | AIN-93M | AIN-93M-AG |
|---|---|---|
| Sucrose (%) | 8 | 8 |
| Carbohydrates (%) | 76 | 44 |
| Proteins (%) | 14 | 14 |
| Lipids (%) | 10 | 42 |
| Total energy (Kcal/Kg) | 3.870 | 5.407 |
AIN-93M (American Institute of Nutrition, maintenance diet for adult rodents), AIN-93M-AG (American Institute of Nutrition, high-fat diet).
In order to familiarize the mice gradually to the new food and to avoid diarrhea, for two weeks prior to the experiment, approximately 2.7 g of the new diet were added per day until reaching 38 g. After the adaptation period, the animals in each group were given the corresponding diets and water ad libitum for a period of 14 weeks. Every two days the food was removed and replaced to avoid contamination and fat oxidation.
Recording weight, food consumption and efficiency
The body weight of each animal was recorded at the beginning of the study and every seven days for 14 weeks. Daily food consumption was calculated from the differences in the daily weight of the food. Once the 14 weeks were complete, the total calorie consumption in each group was determined on the basis of 3.870 Kcal/Kg in the AIN-93M diet and 5.407 Kcal/Kg in the AIN-93M-AG diet. In addition, food efficiency (FE) was calculated according to the following formula:
[(weight gained/Kcal consumed) × 100].
Euthanasia and biochemical analysis of plasma lipids
At 14 weeks, the animals were euthanized by asphyxia with carbon dioxide and blood samples were taken in a tube with EDTA by cardiac puncture of the right vestibule. For the biochemical analysis, the plasma was separated from the blood by centrifugation at room temperature for 15 min at 120 G and stored at -20 C until analysis of the lipids. The total cholesterol (CHOL-T), HDL cholesterol (HDL-C), LDL cholesterol (LDL-C) and serum triglycerides were determined in each animal using commercial kits from Labtest Diagnóstica S.A., Brazil: COLESTEROL Liquiform, COLESTEROL HDL, LDL Liquiform and TRIGLICÉRIDES Liquiform, respectively. Finally, the VLDL was calculated according to the formula of Friedewald et al. [21], validated by Fukuyama et al. [22] (VLDL = CT-HDL-C-LDL-C). Then the values obtained were compared with the reference values reported by Zhou & Hansson [23], Champy et al. [24], Wirth-Dzięciołowska et al. [25], Martins et al. [26] and Bargut et al. [27].
Acquisition of ileum samples and morphological analysis
The abdominal cavity was accessed by laparotomy and the presence and distribution of peritoneal fat were recorded. Then, the alimentary canal of each animal was removed and dissected under 10× magnification. As there is no anatomical limit defined between the jejunum and the ileum for obtaining ileum samples, 1 cm proximal to the ileocecal valve was considered. The samples were fixed in 10% buffered formalin, pH 7.4 for 48 hours and processed following the conventional protocol for embedding in Histosec® paraffin (Merck). Then, 4 µm-thick sections were made in a Microm HM325 microtome and stained with hematoxylin-eosin (H&E) and periodic acid-Schiff (PAS) for histological analysis.
For the morphometric analysis of the intestinal wall of the ileum, 15 histological slides per sample were taken, selected at random considering the complete length of the ileum. On each slide, the diameter of the ileum, total thickness of the wall and thickness of the tunica mucosa were determined. In addition, the length and width of the intestinal villi and depth of intestinal crypts were evaluated (measured from the base of the intestinal villi to the lamina muscularis mucosae) (Figure 1).
Figure 1.
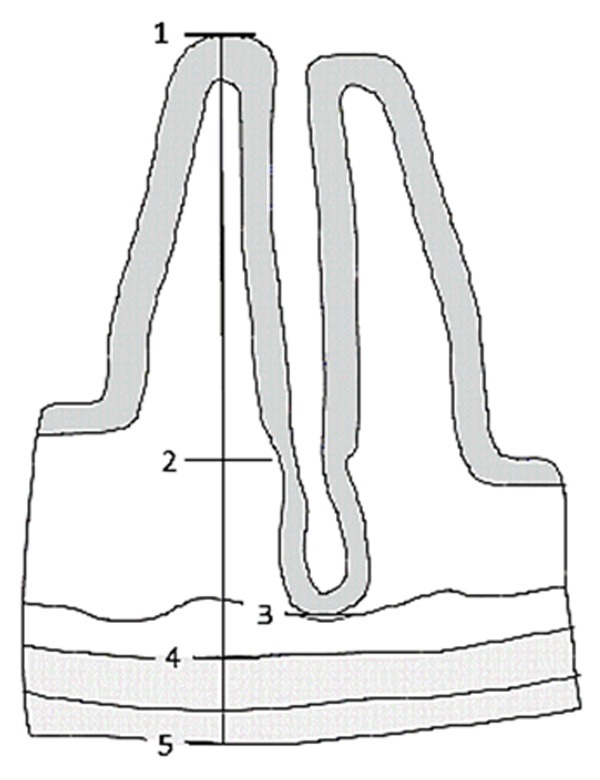
Diagram of the wall of the small intestine. Morphometric measurements of the intestinal wall, tunica mucosa, intestinal villi and crypts of the ileum. 1-5. Total wall thickness. 1-3. Thickness of the tunica mucosa. 4-5. Thickness of the tunica muscularis. 1-2. Length of the intestinal villi. 2-3. Depth of the intestinal crypt.
To do this, two villi and two intestinal crypts per slide were studied, assessing a total of 30 per sample. In each sample the number of goblet cells per mm2 was determined (NA) using the multipurpose test system M42. Five slides were selected at random, considering the complete length of the ileum, and five fields were studied on each slide. The morphometric analysis was performed from images taken with a Motic SMZ-171 microscope at 40X and 50X magnification according to sample size. In order to determine the NA of goblet cells a Leica® DM 2000 LED stereological microscope was used and the slides were photographed with a CMOS INFINITY HD digital microscopy camera. The images were projected on to a View Sonic® flat screen monitor and analyzed using Image Pro Extra 2.0 software. Calibration was done with a micrometric ruler as appropriate.
Statistical analysis
The statistical analysis was done with IBM SPSS Statistic 21© software and the assumptions were verified with the Kolmogorov-Smirnov test (data normality test) and Levene’s test (homoscedasticity analysis). The Student’s t-test (normal distribution data) was used to compare the C and E groups. The P values were considered significant when they were less than 0.05.
Results
Weight and food
When the study began, the average weight of the C group was 26.15±2.64 g and the E group was 23.54±3.02 g, finding no significant differences between these groups (P = 0.111). At the end of the 14 weeks, the final average weight of the mice in the C group was 27.68±3.36 g and in the E group it was 38.25±13.56 g, with no significant differences (P = 0.087). The weight gain in the E group (14.71±12.10 g) was significantly greater (P = 0.028) than the C group (1.53±0.81 g).
In terms of food consumption, the C group consumed on average 70.57 Kcal/day, whereas E consumed 94.78 Kcal/day. The total food consumed was 6915.53 Kcal in C and 9288.69 Kcal in E. The FE of the C group was 0.097% and for E it was 0.841%.
Biochemical analysis of plasma lipids
Only CHOL-T, LDL-C and HDL-C showed significant differences. The lipid profile of the two groups is in Table 3, and the reference values are in Table 4.
Table 3.
Descriptive statistics of the serum lipid profile of male C57BL/6 mice fed an AIN-93M diet and an AIN-93M-AG diet
| Plasmatic lipids (mmol/L) | X̅ (SD) | P | |
|---|---|---|---|
|
| |||
| C Group | E Group | ||
| CHOL-T | 2.56±0.29 | 4.42±1.07 | 0.003 |
| VLDL-C | 0.57±0.33 | 0.54±0.15 | 0.824 |
| LDL-C | 0.87±0.39 | 1.82±0.44 | 0.001 |
| HDL-C | 1.10±0.52 | 2.06±0.85 | 0.026 |
| TG | 1.08±0.25 | 1.40±0.69 | 0.260 |
Table 4.
Reference values of the serum lipid profile of C57BL/6 male mice
| References | Plasma lipids (mmol/L) | ||||
|---|---|---|---|---|---|
|
|
|||||
| Age (weeks) | X̅ (SD) | ||||
|
| |||||
| CHOL-T | LDL-C | HDL-C | TG | ||
| Zhou & Hanson [23] | 20 | 2.48±0.20 | - | 1.77±0.21 | 1.31±0.19 |
| Champy et al. [24] | 24 | 2.57±0.09 | 0.43±0.01 | 1.79±0.07 | 1.24±0.08 |
| Wirth-Dzięciołowska et al. [25] | 16 | 1.76±0.35 | - | - | 1.11±0.09 |
| Martins et al. [26] | 22 | 2.10±2.60 | - | - | 0.80±0.03 |
| Bargut et al. [27] | 20 | 2.03±0.09 | 0.56±0.05 | 1.21±0.11 | 0.73±0.01 |
Morphological analysis
The ileum of the mice in both groups presented a preserved general structure with no visible lesions. The muscular-serous layer and intestinal crypts did not show any alterations in size or shape. In the E group, the mucosal layer showed longer intestinal villi and fewer PAS-positive goblet cells. Also observed were the presence of somewhat dilated blood vessels and an increase in mononuclear infiltrates in both the lamina propria and in the submucosa (Figure 2).
Figure 2.

Ileal mucosa of C57BL/6 mice. A. The C group fed a standard diet (AIN-93M). B. E group fed a high-fat diet (AIN-93M-AG). A greater presence of mononuclear infiltrates (arrow heads) in the tunica mucosa of the ileum in E group.
No differences were observed between the groups in the morphometric variables of the diameter, wall height, height of the mucosa, thickness of the tunica muscularis and depth of intestinal crypts of the mouse ileum (Table 5).
Table 5.
Descriptive statistics of morphometric variables of the ileal wall of C57BL/6 mice in C group fed with an AIN-93M diet and of the E group fed with an AIN-93M-AG diet
| Morphometric measurements (µm) | X̅ (SD) | P | |
|---|---|---|---|
|
| |||
| C Group | E Group | ||
| Diameter | 5937±820 | 6197±1378 | 0.099 |
| Wall thickness | 355±61 | 349±45 | 0.459 |
| Thickness tunica mucosa | 272±56 | 279±37 | 0.274 |
| Thickness tunica muscularis | 63±14 | 60±11 | 0.082 |
| Depth intestinal crypts | 85±17 | 85±13 | 0.677 |
Significant differences were found in the length and width of the villi of the ileal mucosa. The intestinal villi of the mucosa of the mice in the E group (180.15±29.0 µm) were significantly longer than those in the C group (166.40±40.8 µm), P = 0.000. However, the width of the intestinal villi in the E group was significantly less (73.78±15.7 µm) than in C group (70.71±14.1), P = 0.037 (Figure 3).
Figure 3.
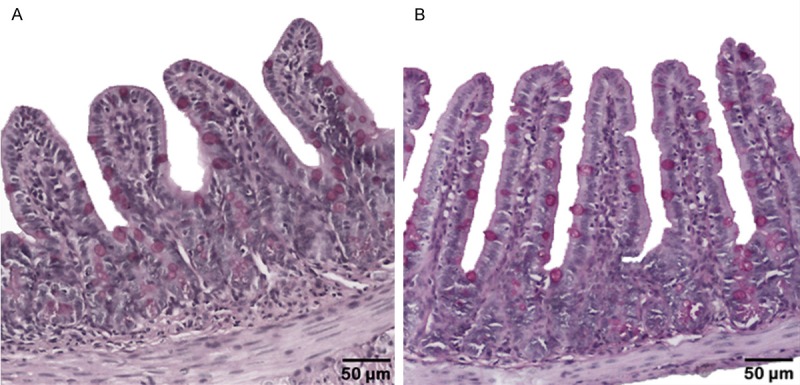
Ileal mucosa of C57BL/6 mice. A. C group fed with standard diet (AIN-93M). B. E group fed with a high-fat diet (AIN-93M-AG). Differences in length and width were observed between the two groups. PAS staining.
In terms of the area density, the PAS-positive goblet cells, significantly lower values (P = 0.039) were found in the E group (1771±423.7/mm2) than in the C group (1893.2±505.2/mm2).
Discussion
As several studies have reported, a high-fat diet produces significant weight gain [4,5,9,10,14,18,28]. In our study, the mice fed with the AIM-93M-AG diet were showing greater weight gain the C group even by the second week, and at 14 weeks they presented significant differences (P = 0.028). The weight gain was related to increased body size and visceral fat deposit, results that were also observed by Woods et al. [29] and Yang et al. [30], who reported that rats fed with hyperlipidic diets presented 52% more fat.
With respect to the plasma lipid studies on animal models fed with hyperlipidic diets, various results have been observed. Kim et al. [18] reported a significant increase in the plasma values of CHOL-T and TG, whereas other authors observed a significant increase only in TG [30] or only in CHOL-T [31]. Nevertheless, other studies reported no significant differences in the plasma lipid values compared to the C group [3,32]. Our results showed a significant increase in the plasma values of CHOL-T and LDL-C in the E group over the C group (P = 0.003, P = 0.001, respectively). In terms of the triglycerides, although there were differences, these were not significant (P = 0.260). These discrepancies could be due to such factors as variability in the model used, type and number of lipids, form and time of administration of the diet, among others [3,23-27]. Therefore, protocols or standardized models need to be created to achieve a suitable comparison of these parameters.
On the other hand, in both groups of mice, the histopathological analysis did not show any alterations in the layers of the ileal wall, which was correlated with the morphometric analysis of this structure, finding significant differences only in the length and width of the intestinal villi (P = 0.000 and P = 0.037, respectively). Newmark et al. [8,9], however, observed that a longer consumption time of high-fat diets could cause hyperplastic lesions in the mucosa.
The increase in the length of the villi observed in the E group could correspond, according to Petit et al. [13] and de Wit et al. [14], to a physiological modification in response to a greater demand for lipid absorption due to the diet. The authors indicated that these changes could be related to the increase in the Ki-67 proliferation marker in the nuclei of the enterocytes and alterations in the genetic regulation of the cell cycle processes, such as reduction of apoptosis, increase in proliferation and relative increase in intestinal mass.
It was also demonstrated in this study that the administration of AIN-93M-AG diets produced a significant reduction (P = 0.039) in the number of PAS-positive goblet cells. Although there are no studies directly linking the effect of this type of diet on goblet cells, there are studies linking the effect of this diet on the intestinal microflora [15,18,33,34] and other studies that indicate a direct connection between the intestinal microflora and goblet cells [11,34-36].
Diets with a high fat content promote the production of endotoxins by the microflora, providing a favorable means for the proliferation of Gram-negative pathogenic bacteria, such as enterobacteria, and a decrease in positive microflora, like bifidobacteria [15,18]. These changes alter the protective mucus barrier, damaging the intestinal epithelium [33].
It is known that the proliferation of Gram-negative pathogenic bacteria produces a decreased in PAS-positive goblet cells. Therefore, this reduction could be explained by two possible mechanisms: a) Mucin is secreted in defense against an infection over a prolonged period of time, causing cellular exhaustion of the goblet cells and therefore a reduction in mucin synthesis [33,34]. b) The immune system itself, in particular a subgroup of T lymphocytes, decreases the mucin secretion to reduce a possible source of nutrients, thereby inhibiting the growth of pathogenic bacteria [36].
Several studies have shown that high-fat diets produce inflammation in organs, including the kidneys, liver and pancreas [4-6,10,18]. However, the alimentary canal has not been widely explored as a potential source of inflammation associated with this type of diet [10,17]. It must also be taken into account that the intestine is the first organ exposed to the ingested nutrients, that it can be influenced by changes in the intestinal microflora, and that it also has an intrinsic resident immune system [18].
In our study, the histopathological analysis showed increased mononuclear cells in the mucosa and submucosa of the ileum in mice in the E group, demonstrating local inflammation. These results are correlated with observations made by de Wit et al. [14], who reported that a high-fat diet induces changes in the gene expression related to the lipid metabolism, cell cycle and inflammatory and immune response in the small intestine; moreover, they found changes in the expression of signaling molecules like IL18, FGF15, MIF, IGFBP3 and ANGPTL4, which that can lead to metabolic effects in the liver, muscle and fatty tissue that underlie the development of metabolic syndrome.
In addition, the change in the intestinal microflora due to the consumption of a hyperlipidic diet alters structural proteins of the occluding junctions, affecting the intestinal epithelial barrier, favoring the translocation of luminal antigens, microorganisms and their toxins [34,37]. The pathogenic bacteria produce an increase in endotoxins in the intestinal lumen, which under this condition, enter the lamina propria of the intestine, stimulating the macrophages to produce proinflammatory cytokines, which increases the local inflammation [18].
Nevertheless, studies have shown that the alterations caused by high-fat diets can decrease under certain conditions. Yang et al. [30] observed in rats that when the number of lipids in the diet is restricted, not only is body weight reduced, but also the serum glucose levels, total cholesterol and triglycerides. Additionally, Cani et al. [38] demonstrated that mice treated with probiotics showed a decrease in lipopolysaccharides, plasma cytokines and a reduction in the hepatic expression of inflammatory markers and oxidative stress. This reduction in inflammation was associated with lower intestinal permeability and improvement in the integrity of the occluding junctions. These beneficial effects could be due to commensal bacteria like bifidobacteria, which can adhere to the epithelium, preventing adhesion of pathogenic bacteria and thus the deleterious effects [34].
In this context, we can conclude that the observed changes could be related to the higher demand for fat absorption and to possible alterations in the intestinal microflora and inflammation by action of high-fat diets. Therefore, it is important to emphasize that the alterations observed can be reversed to a large extent by changing eating habits to a more balanced diet.
Disclosure of conflict of interest
None.
References
- 1.Aguila MB, Mandarim-de-Lacerda CA. Effects of chronic high fat diets on renal function and cortical structure in rats. Exp Toxicol Pathol. 2003;55:187–195. doi: 10.1078/0940-2993-00313. [DOI] [PubMed] [Google Scholar]
- 2.Aguila MB, Pinheiro AR, Parente LB, Mandarimde-Lacerda CA. Dietary effect of different highfat diet on rat liver stereology. Liver Int. 2003;23:363–370. doi: 10.1034/j.1478-3231.2003.00858.x. [DOI] [PubMed] [Google Scholar]
- 3.Jiang T, Wang Z, Proctor G, Moskowitz S, Liebman SE, Rogers T, Lucia MS, Li J, Levi M. Dietinduced obesity in C57BL/6J mice causes increased renal lipid accumulation and glomerulosclerosis via a sterol regulatory element-binding protein-1c-dependent pathway. J Biol Chem. 2005;280:32317–3225. doi: 10.1074/jbc.M500801200. [DOI] [PubMed] [Google Scholar]
- 4.Altunkaynak BZ. Effects of high fat diet induced obesity on female rat livers (a histochemical study) Eur J Gen Med. 2005;2:100–109. [Google Scholar]
- 5.Altunkaynak ME, Ozbek E, Altunkaynak BZ, Can I, Unal D, Unal B. The effects of high-fat diet on the renal structure and morphometric parametric of kidneys in rats. J Anat. 2008;212:845–852. doi: 10.1111/j.1469-7580.2008.00902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Cui Y, Fang L, Li F. Chronic high-fat diets induce oxide injuries and fibrogenesis of pancreatic cells in rats. Pancreas. 2008;37:e31–8. doi: 10.1097/MPA.0b013e3181744b50. [DOI] [PubMed] [Google Scholar]
- 7.Frantz ED, Penna-de-Carvalho A, Batista Tde M, Aguila MB, Mandarim-de-Lacerda CA. Comparative effects of the renin-angiotensin system blockers on nonalcoholic fatty liver disease and insulin resistance in C57BL/6 mice. Metab Syndr Relat Disord. 2014;12:191–201. doi: 10.1089/met.2013.0129. [DOI] [PubMed] [Google Scholar]
- 8.Newmark HL, Yang K, Lipkin M, Kopelovich L, Liu Y, Fan K, Shinozaki H. A Western-style diet induces benign and malignant neoplasms in the colon of normal C57Bl/6 mice. Carcinogenesis. 2001;22:1871–1875. doi: 10.1093/carcin/22.11.1871. [DOI] [PubMed] [Google Scholar]
- 9.Newmark HL, Yang K, Kurihara N, Fan K, Augenlicht LH, Lipkin M. Western-style diet-induced colonic tumors and their modulation by calcium and vitamin D in C57Bl/6 mice: a preclinical model for human sporadic colon cancer. Carcinogenesis. 2009;30:88–92. doi: 10.1093/carcin/bgn229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aslam MN, Paruchuri T, Bhagavathula N, Varani J. A mineral-rich red algae extract inhibits polyp formation and inflammation in the gastrointestinal tract of mice on a high-fat diet. Integr Cancer Ther. 2010;9:93–99. doi: 10.1177/1534735409360360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCullough JS, Ratcliffe B, Mandir N, Carr KE, Goodlad RA. Dietary fibre and intestinal microflora: effects on intestinal morphometry and crypt branching. Gut. 1998;42:799–806. doi: 10.1136/gut.42.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Arruda AMV, Fernandes RTV, da Silva JM, Lopes DC. Avaliação morfo-histológica da mucosa intestinal de coelhos alimentados com diferentes níveis e fontes de fibra. Rev Caatinga. 2008;21:1–11. [Google Scholar]
- 13.Petit V, Arnould L, Martin P, Monnot MC, Pineau T, Besnard P, Niot I. Chronic high-fat diet affects intestinal fat absorption and postprandial triglyceride levels in the mouse. J Lipid Res. 2007;48:278–287. doi: 10.1194/jlr.M600283-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.de Wit NJ, Bosch-Vermeulen H, de Groot PJ, Hooiveld GJ, Bromhaar MM, Jansen J, Müller M, van der Meer R. The role of the small intestine in the development of dietary fat-induced obesity and insulin resistance in C57BL/6J mice. BMC Med Genomics. 2008;1:14. doi: 10.1186/1755-8794-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen YY, Knight R, Ahima RS, Bushman F, Wu GD. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137:1716–1724. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to highfat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299:G440–G448. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding S, Chi MM, Scull BP, Rigby R, Schwerbrock NM, Magness S, Jobin C, Lund PK. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS One. 2010;5:e12191. doi: 10.1371/journal.pone.0012191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim KA, Gu W, Lee IA, Joh EH, Kim DH. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One. 2012;7:e47713. doi: 10.1371/journal.pone.0047713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research (ILAR), Division on Earth and Life Studies (DELS), National Research Council. Guide for the Care and Use of Laboratory animals. 8th edition. Washington (DC): The National Academies Press; 2011. [Google Scholar]
- 20.Reeves PG, Nielsen FH, Fahey GC. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 21.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 22.Fukuyama N, Homma K, Wakana N, Kudo K, Suyama A, Ohazama H, Tsuji C, Ishiwata K, Eguchi Y, Nakazawa H, Tanaka E. Validation of the Friedewald equation for evaluation of plasma LDL-cholesterol. J Clin Biochem Nutr. 2008;43:1–5. doi: 10.3164/jcbn.2008036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou X, Hansson GK. Effect of sex and age on serum biochemical reference ranges in C57BL/6J mice. Comp Med. 2004;54:176–178. [PubMed] [Google Scholar]
- 24.Champy MF, Selloum M, Zeitler V, Caradec C, Jung B, Rousseau S, Pouilly L, Sorg T, Auwerx J. Genetic background determines metabolic phenotypes in the mouse. Mamm Genome. 2008;19:318–331. doi: 10.1007/s00335-008-9107-z. [DOI] [PubMed] [Google Scholar]
- 25.Wirth-Dzięciołowska E, Karaszewska TS, Pyśniak MG. Selected blood serum biochemical indicators in twelve inbred strains of laboratory mice. Anim Sci Pap Rep. 2009;27:159–167. [Google Scholar]
- 26.Martins MA, Catta-Preta M, Mandarim-de-Lacerda CA, Aguila MB, Brunini TCM, Mendes-Ribeiro AC. High fat diets modulate nitric oxide biosynthesis and antioxidant defence in red blood cells from C57BL/6 mice. Arch Biochem Biophys. 2010;499:56–61. doi: 10.1016/j.abb.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 27.Bargut TCL, Frantz ED, Mandarim-de-Lacerda CA, Aguila MB. Effects of a diet rich in n-3 polyunsaturated fatty acids on hepatic lipogenesis and beta-oxidation in mice. Lipids. 2014;49:431–444. doi: 10.1007/s11745-014-3892-9. [DOI] [PubMed] [Google Scholar]
- 28.Steinbach G, Kumar SP, Reddy BS, Lipkin M, Holt PR. Effects of caloric restriction and dietary fat on epithelial cell proliferation in rat colon. Cancer Res. 1993;53:2745–2749. [PubMed] [Google Scholar]
- 29.Woods SC, Seeley RJ, Rushing PA, D’Alessio D, Tso P. A controlled high-fat diet induces an obese syndrome in rats. J Nutr. 2003;133:1081–1087. doi: 10.1093/jn/133.4.1081. [DOI] [PubMed] [Google Scholar]
- 30.Yang N, Ying C, Xu M, Zuo X, Ye X, Liu L, Nara Y, Sun X. High-fat diet up-regulates caveolin-1 expression in aorta of diet-induced obese but not in diet-resistant rats. Cardiovasc Res. 2007;76:167–174. doi: 10.1016/j.cardiores.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 31.Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EH, Piersma AH, Ozanne SE, Twinn DF, Remacle C, Rowlerson A, Poston L, Taylor PD. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension. 2008;51:383–392. doi: 10.1161/HYPERTENSIONAHA.107.101477. [DOI] [PubMed] [Google Scholar]
- 32.Ble-Castillo JL, Aparicio-Trapala MA, Juárez-Rojop IE, Torres-Lopez JE, Mendez JD, Aguilar-Mariscal H, Olvera-Hernández V, Palma-Cordova LC, Diaz-Zagoya JC. Differential effects of high-carbohydrate and high-fat diet composition on metabolic control and insulin resistance in normal rats. Int J Environ Res Public Health. 2012;9:1663–1676. doi: 10.3390/ijerph9051663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep. 2010;12:319–330. doi: 10.1007/s11894-010-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cinova J, De Palma G, Stepankova R, Kofronova O, Kverka M, Sanz Y, Tuckova L. Role of intestinal bacteria in gliadin-induced changes in intestinal mucosa: study in germ-free rats. PLoS One. 2011;6:e16169. doi: 10.1371/journal.pone.0016169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kandori H, Hirayama K, Takeda M, Doi K. Histochemical, lectin-histochemical and morphometrical characteristics of intestinal goblet cells of germfree and conventional mice. Exp Anim. 1996;45:155–160. doi: 10.1538/expanim.45.155. [DOI] [PubMed] [Google Scholar]
- 36.Bergstrom KS, Guttman JA, Rumi M, Ma C, Bouzari S, Khan MA, Gibson DL, Vogl AW, Vallance BA. Modulation of intestinal goblet cell function during infection by an attaching and effacing bacterial pathogen. Infect Immun. 2008;76:796–811. doi: 10.1128/IAI.00093-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boyle EC, Brown NF, Finlay BB. Salmonella enterica serovar Typhimurium effectors SopB, SopE, SopE2 and SipA disrupt tight junction structure and function. Cell Microbiol. 2006;8:1946–1957. doi: 10.1111/j.1462-5822.2006.00762.x. [DOI] [PubMed] [Google Scholar]
- 38.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin JF, Ferrières J, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]


