Abstract
Background: To evaluate the activity of natural killer cells through their inhibitory and activating receptors and quantity in peripheral blood mononuclear cells extracted from patients with acute myocardial infarction, stable angina pectoris and the controls. Methods: 100 patients with myocardial infarction, 100 with stable angina, and 20 healthy volunteers were recruited into the study. 20 randomly chosen people per group were examined for the whole human genome microarray analysis to detect the gene expressions of all 40 inhibitory and activating natural killer cell receptors. Flow cytometry analysis was applied to all 200 patients to measure the quantity of natural killer cells. Results: In myocardial infarction group, the mRNA expressions of six inhibitory receptors KIR2DL2, KIR3DL3, CD94, NKG2A, KLRB1, KLRG1, and eight activating receptors KIR2DS3, KIR2DS5, NKp30, NTB-A, CRACC, CD2, CD7 and CD96 were significantly down-regulated (P<0.05) compared with both angina patients and the controls. There was no statistical difference in receptor expressions between angina patients and control group. The quantity of natural killer cells was significantly decreased in both infarction and angina patients compared with normal range (P<0.001). Conclusions: The significant mRNAs down-regulation of several receptors in myocardial infarction group and reduction in the quantity of natural killer cells in both myocardial infarction and angina patients showed a quantitative loss and dysfunction of natural killer cells in myocardial infarction patients.
Keywords: Myocardial infarction, stable angina pectoris, natural killer cells, natural killer cell inhibitory receptors, natural killer cell activating receptors, gene expression
Introduction
Cardiovascular diseases, with high morbidity and mortality worldwide, are caused mainly by atherosclerosis, a multifactorial and highly complex disease in which innate and adaptive immunity operates together in the progression of lesion [1]. Natural killer (NK) cells are a key cellular component of innate immune response characterized by strong cytolytic activity against susceptible target cells and the ability to release several cytokines. NK cells provide the first-line defense against infecting microbes, tumors and autoimmune diseases [2]. Although NK cells do not express classical antigen receptors of the immunoglobulin-gene family, they use an array of innate receptors to sense the environment and respond to alterations caused by infections, cellular stress, and transformation. They recognize the absence of self major histocompatibility complex class I (MHC-I) as a way to discriminate normal cells from cells in distress. In humans, this “missing-self” recognition is ensured by inhibitory receptors, which dampen NK cell activation upon interaction with their MHC-I ligands [3,4]. The array of activating receptors expressed by NK cells can trigger cytolytic process, as well as cytokine or chemokine secretion [5,6]. Synergistic signals from combinations of inhibitory and activating receptors are integrated to activate NK cells [7].
Results from numerous experimental studies indicated the important role of NK cells in atherosclerosis [8]. It is wildly accepted that the loss of NK cell functions occurred in patients with coronary atherosclerosis diseases (CAD) [9-12]. Jonasson et al. suggested that the impaired function of NK cells in CAD patients might be a mainly quantitative defect [9]. Backteman et al. described the sustained reduction of NK cells was associated with low-grade inflammation [10]. Li et al. found that the rate of spontaneous NK cell apoptosis was increased in CAD patients [11]. However, the mechanisms of controlling the suppression of NK cells in CAD patients have not been fully investigated. Only a couple of studies were performed towards all the NK cell receptors in different stage of CAD patients. We designed this in vitro study to investigate the activity of NK cells through the expression of their receptors and quantity in patients with AMI and SA. Human microarray analysis was used to systematically examine the mRNA expressions of both inhibitory and activating NK cell receptors in peripheral blood mononuclear cells (PBMCs) extracted from AMI patients, SA patients and the control group. Flow cytometry analysis was applied to test the difference in NK cell proportion in PBMCs between AMI and SA patients.
Materials and methods
Patient information
The study recruited 100 patients with myocardial infarction, 100 with stable angina, and 20 healthy volunteers. Human microarray analysis was performed for 20 randomly selected AMI patients, 20 randomly selected SA patients, and 20 healthy volunteers. The sample sizes, the number of subjects per group, were based on an assumed within-group variance of 0.50 and the targeted nominal power of 0.95 [13]. Table 1 showed the baseline demographic data. The AMI patients were admitted no more than 12 hours from the onset of symptoms to our Coronary Care Unit between January and June 2013, included 18 male and two female, with an age of 58±12 (mean ± s.d.) years. The SA group has 20 patients (18 male, two female, age 64±10). 20 volunteers (17 male, three female, age 29±3) were enrolled as the control group during the same period with similar male/female ratio. Histories, physical examination, ECG, chest radiography and routine chemical analyses showed the controls had no evidence of coronary heart diseases.
Table 1.
Baseline demographic data in three groups (x̅±s.d.)
| Index | AMI (a) (N=20) | SA (b) (N=20) | Con (c) (N=20) | P (all) | P (a v b) |
|---|---|---|---|---|---|
| Age | 57.8±11.9 | 63.6±9.9 | 28.8±3.3 | 0.000 | 0.251 |
| Sex (M/F) | 18/2 | 18/2 | 17/3 | 0.853 | 1.0 |
| BMI (kg/m2) | 23.6±2.6 | 22.8±2.7 | 21.3±1.8 | 0.102 | 0.56 |
| Ethnicity, Han | 20 | 20 | 20 | 1 | 1 |
| Smoking history (num/d) | 13.6±12.2 | 9.8±10.3 | 0 | 0.00 | 0.648 |
| SBP (mmHg) | 128.6±15.3 | 123.0±12.1 | 120.8±7.2 | 0.115 | 0.501 |
| DBP (mmHg) | 67.0±8.0 | 73.0±8.0 | 71.6±3.2 | 0.017 | 0.064 |
| LDL-C (mmol/L) | 2.5±1.0 | 2.1±0.8 | 2.9±0.5 | 0.327 | 0.548 |
| Triglycerides (mmol/L) | 1.6±1.1 | 1.5±1.4 | 1.2±0.4 | 0.73 | 0.762 |
| HDL-C (mmol/L) | 0.8±0.7 | 0.9±0.2 | 1.3±0.2 | 0.000 | 0.803 |
| FBG (mmol/L) | 5.4±0.9 | 5.0±0.8 | 4.9±0.5 | 0.61 | 0.082 |
Footnotes: LDL-C = low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; FBG: Fasting Plasma Glucose.
For the flow cytometry analysis, 100 AMI (88 male, 12 female, age 59±13) and 100 SA patients (82 male, 18 female, age 63±10) from Coronary Care Unit and Department of Cardiology were recruited between January and December 2013 (Table 2).
Table 2.
Baseline demographic data for flow cytometric analysis (x̅±s.d.)
| Index | AMI (a) (N=100) | SA (b) (N=100) | P |
|---|---|---|---|
| Age | 58.8±12.9 | 63.6±9.9 | 0.93 |
| Sex (M/F) | 88/12 | 82/18 | 0.2348 |
| BMI (kg/m2) | 23.8±3.2 | 22.6±3.0 | 0.44 |
| Ethnicity, Han | 20 | 20 | 1 |
| Smoking history (num/d) | 14.1±11.4 | 11.6±9.8 | 0.132 |
| SBP (mmHg) | 125.±12.1 | 122.8±11.6 | 0.32 |
| DBP (mmHg) | 65.9±9.1 | 74.3±8.7 | 0.067 |
| LDL-C (mmol/L) | 3.01±1.1 | 2.88±0.8 | 0.094 |
| Triglycerides (mmol/L) | 1.5±0.8 | 1.4±0.7 | 0.762 |
| HDL-C (mmol/L) | 0.9±0.2 | 1.0±0.3 | 0.461 |
| FBG (mmol/L) | 5.4±1.2 | 5.3±0.9 | 0.43 |
Footnotes: LDL-C = low-density lipoprotein cholesterol; HDL-C: highdensity lipoprotein cholesterol; FBG: Fasting Plasma Glucose.
All AMI patients were diagnosed on the basis of following criteria [14]: detection of a rise of cardiac biomarker values [preferably cardiac troponin (cTn)] with at least one value above the 99th percentile upper reference limit (URL) and with at least one of the following: 1) Symptoms of ischemia. 2) New or presumed new significant ST-segment-T wave (ST-T) changes or new left bundle branch block (LBBB). 3) Development of pathological Q waves in the ECG. 4) Imaging evidence of new loss of viable myocardium or new regional wall motion abnormality. 5) Identification of an intracoronary thrombus by angiography.
All SA patients had exclusively effort-related angina with a positive exercise stress test and at least one coronary stenosis detected at angiography (>70% reduction of lumen diameter).
There were no significant differences between AMI and SA patients in age, sex, smoking status, BMI, systolic blood pressure, diastolic blood pressure, LDL-C, HDL-C, triglycerides and fasting plasma glucose(FBG) (Tables 1 and 2).
The exclusion criteria for three groups were as follows: venous thrombosis, history of severe renal or hepatic diseases, haematological disorders, acute or chronic inflammatory diseases and malignancy.
The study protocol was approved by the ethics committee of Tongji University and informed consent form was obtained.
Gene expression chips
Agilent G4112F Whole Human Genome Oligo Microarrays purchased from Agilent (USA) were used in the chip analysis. A microarray is composed of more than 41,000 genes or transcripts, including targeted 19,596 entrez gene RNAs. Sequence information used in the microarrays was derived from the latest databases of RefSeq, Goldenpath, Ensembl and Unigene [15]. The functions of more than 70% of the genes in the microarray are already known. All patients were subjected to the chip analysis.
Total RNA isolation
Five milliliter of peripheral blood samples from median cubital vein were drawn from AMI and SA patients with PAXgene tube immediately after admission. Leucocytes were obtained through density gradient centrifugation with Ficoll solution and the remaining red blood cells were destroyed by erythrocyte lysis buffer (Qiagen, Hilden, Germany). Total RNA was extracted and purified using PAXgeneTM Blood RNA kit (Cat#762174, QIAGEN, GmBH, Germany) following the manufacturer’s instructions. It was further checked for a RIN number to inspect RNA integration by an Agilent Bioanalyzer 2100 (Agilent technologies, Santa Clara, CA, US). The sample was considered qualified when both 2100 RIN and 28S/18S are no less than 0.7.
RNA amplification and labeling
Total RNA was amplified and labeled by Low Input Quick Amp Labeling Kit, One-Color (Cat#5190-2305, Agilent technologies, Santa Clara, CA, US), following the manufacturer’s instructions. Labeled cRNA was purified by RNeasy mini kit (Cat#74106, QIAGEN, GmBH, Germany).
Microarray hybridization
Each slide was hybridized with 1.65 μg Cy3-labeled cRNA using Gene Expression Hybridization Kit (Cat#5188-5242, Agilent technologies, Santa Clara, CA, US) in Hybridization Oven (Cat#G2545A, Agilent technologies, Santa Clara, CA, US), following the manufacturer’s instructions. After 17 hours of hybridization, slides were washed in staining dishes (Cat#121, Thermo Shandon, Waltham, MA, US) with Gene Expression Wash Buffer Kit (Cat#5188-5327, Agilent technologies, Santa Clara, CA, US), according to the manufacturer’s operation manual.
Chip scan and data acquisition
Slides were scanned using Agilent Microarray Scanner (Cat#G2565CA, Agilent technologies, Santa Clara, CA, US) with default settings, Dye channel: Green, Scan resolution =3 μm, 20 bit. Data were extracted with Feature Extraction software 10.7 (Agilent technologies, Santa Clara, CA, US). Raw data were normalized using Quantile algorithm, Gene Spring Software 11.0 (Agilent technologies, Santa Clara, CA, US).
RT-PCR
The spots in the microarray were randomly selected and their expressions were confirmed by RT-PCR. Among all the genes with differential expressions, three genes were randomly selected and subjected to RT-PCR, along with the house keeping genes (GAPDH). The relative expressions were indicated as the expression of the target genes normalized to the expression of GAPDH (2-ΔΔCt). The melting curve and the 2-ΔΔCt-method were used to detect the differences in the expressions among the three groups. The results from RT-PCR were consistent with the microarray analysis.
Flow cytometry
Five milliliter peripheral bloods from median cubital vein were drawn immediately from 100 AMI and 100 SA after admission to Coronary Care Unit and department of Cardiology. The sample was incubated for 15 min in the dark with phycoerythrin-conjugated monoclonal antibody against human CD16 (Becton-Dickinson, FranklinLakes, NJ, USA) and rabbit ploycolonal antibody against human CD56 (Neomarkers, Fremont, CA). Fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulin G (SouthernBiothech, Birmingham, UK) was used to detect CD56 antibody. Then red blood cells were lysed, washed with phosphate-buffered saline, and fixed in 4% paraformaldehyde before analysis of 60,000 events after exclusion of debris and platelets. The quantity of circulating NK cells was measured by the proportion of the CD16/CD56 double positive cells per 100 peripheral blood mononuclear cells. Isotype-identical antibodies served as controls. Double color flow cytometry analyses were performed using aEBeckman Coulter (Coulter, USA) flow cytometer and performed in duplicate.
Statistical analysis
Descriptive statistics were expressed as mean ± s.d. Differences between groups were examined by one-way analysis of variance (ANOVA). After ANOVA the test of all pairwise group mean comparison was performed using the Tukey’s method. Density curve for NK cells was delineated using R software. Data were analyzed using SPSS 17.0, and P-values <0.05 were considered statistically significant.
Results
Gene expression of inhibitory NK cell receptors
The results showed mRNA expressions of 15 inhibitory NK cell receptors. The inhibitory receptors are generally divided into MHC-I specific (Figure 1A) and non-MHC specific (Figure 1B). MHC-I specific receptors include killer cell immunoglobulin-like receptors (KIRs), killer lectin-like receptors (KLRs) and LIR-1. Non-MHC specific receptors contain KLRB1, KLRG1, Siglec-7 (sialic acid binding Ig-like lectin 7), CEACAM1 (carcinoembryonic antigen-related cell adhesion molecule 1, Siglec-9) and IRp60. The KIRs consist of KIR2DL1, KIR2DL2, KIR2DL3, KIR2DL5, KIR3DL1, KIR3DL2 and KIR3DL3. The KLRs comprise CD94 (KLRD1), NKG2A, KLRG1, and KLRB1. The last two belong to non-MHC receptors. In sum the gene expressions of 15 NK cell inhibitory receptors in PBMCs from three groups of patients were detected. In PBMCs from the three groups, expressions of the genes encoding KIR2DL2, KIR3DL3, CD94, NKG2A, KLRB1 and KLRG1 were significantly different (P<0.05). In AMI group, all the 11 gene expressions of MHC specific receptors were the lowest among three groups, and mRNA expressions of KIR2DL2, KIR3DL3, CD94 and NKG2A were significantly lower (P<0.05) than in SA patients and the controls. The mRNA expression of non-MHC receptors, KLRB1 and KLRG1 in AMI patients was significantly down-regulated (P<0.05) in comparison with the SA patients and the controls. There was no statistical difference in NK cell inhibitory receptor mRNA expressions between SA and the controls.
Figure 1.
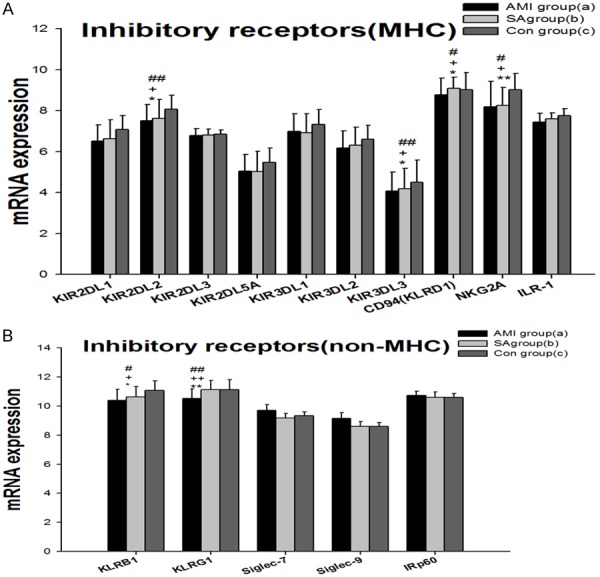
mRNA expression of specific MHC inhibitory NK cell receptors (A) and non-MHC inhibitory NK cell receptors (B) in PBMCs from three groups. Three groups *: P<0.05, **: P<0.01. a v c #: P<0.05, ##: P<0.01; b v c &: P<0.05, &&: P<0.01; a v b +: P<0.05, ++: P<0.01.
Gene expression of activating NK cell receptors
mRNA expressions of 25 activating NK cell receptors in PBMCs from three groups were examined, which included KIRs (Figure 2A), natural cytotoxicity receptors (NCR), the SLAM-related receptors (SRR) and other NK cell activating receptors containing DNAX accessory molecule-1 (DNAM-1), CD2, CD7, CD16, CD44, CD96 (Tactile) and CD160 (Figure 2B). The KIRs comprise KIR2DS1-6, KIR2DL4, KIR3DS1, KIR2DP1, NKG2C and NKG2D. NCRs contain NKp30, NKp44, NKp46 and NKP80. 2B4 (CD244), NTB-A (CD352) and CRACC (CD319) are members of the recently defined family of SRR. In PBMCs from the three groups, expressions of the gene encoding KIR2DS2, NKG2D, NKp30, NTB-A, CRACC, CD2, CD7 and CD96 were significantly different (P<0.01). In AMI group 23 gene expressions of activating receptors were lowest among the three groups. KIR2DS2, NKG2D, NKp30, NTB-A, CRACC, CD2, CD7 and CD96 mRNAs in AMI patients were significantly down-regulated when compared with in SA patients and controls (P<0.05). Between the SA and controls there is no statistically significant difference in NK cell activating receptors.
Figure 2.
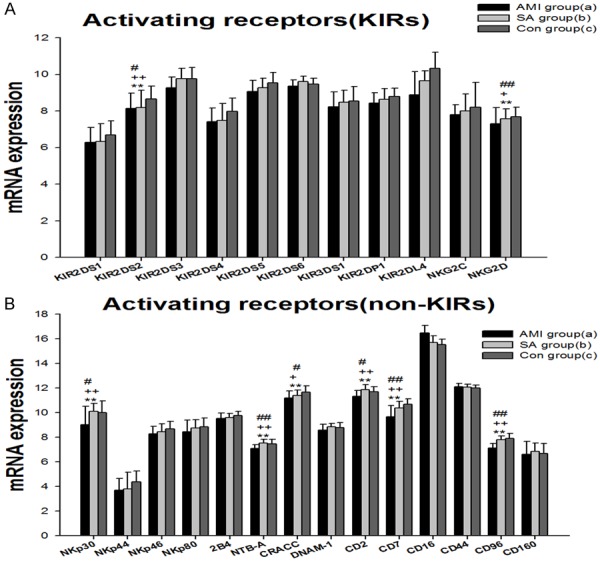
mRNA expression of activating NK cell receptors in PBMCs from three groups: KIRs NK cell activating receptors (A) and other NK cell activating receptors (B). Three groups *: P<0.05, **: P<0.01. a v c #: P<0.05, ##: P<0.01; b v c &: P<0.05, &&: P<0.01; a v b +: P<0.05, ++: P<0.01.
NK cells counting
Density curves for three groups were delineated using R software and the normal range for NK cells is from 8.6-21.1% (Figure 3) [16]. The two density curves for AMI and SA patients were substantially left shift. The number of NK cells was significantly decreased in both AMI and SA patients in PBMCs when compared with the normal range (all P<0.01). However, there was no significant difference between AMI and SA patients in the quantity of NK cells.
Figure 3.
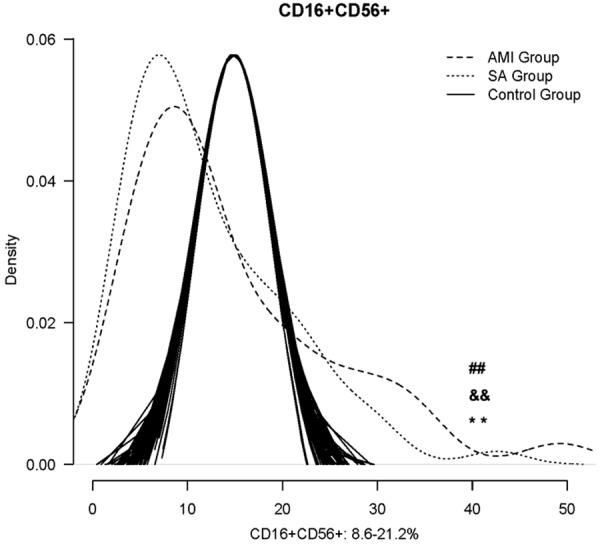
The comparison of NK cells counting among the AMI, SA patients and the normal range. *: P<0.05, **: P<0.01. a v c #: P<0.05, ##: P<0.01; b v c &&: P<0.01; +: P<0.05, ++: P<0.01; a v b &: P<0.05.
Discussion
The NK cells express an array of inhibitory and activating receptors, and the inhibitory receptors are responsible for self-tolerance and adjusting NK-cell activity based on the expression level of self-MHC I [17,18]. Considerable evidences confirmed that the signal transmitted by NK inhibitory receptors was dominant when they are present together with the activating receptors for the normal cells protection [7,19]. KIRs are the most important NK cell receptors, which recognize classical MHC class I. KIRs with short cytoplasmic domains are activating receptors, whereas ligation of the KIR with long cytoplasmic domains to their cognate HLA class I ligands transmits a cascade of inhibitory signals that mediates NK cell tolerance to self cells [20,21]. In our present study, the gene expressions of KIR2DL2, KIR3DL3, CD94 and NKG2A were significantly lower than in SA patients and the controls, suggesting the impaired ability to protect the normal cells. Recent work revealed that there was another system of NK-cell inhibition independent of MHC-I molecules. The receptors, such as KLRG1 and KLRB1, showed that MHC class I-independent inhibitory receptors also played crucial roles in inducing peripheral tolerance and these newly discovered NK-cell inhibitory receptors broadened the definition of self as seen by NK cells [22,23]. The mechanism of signals from these receptors has not been fully investigated. However, clinical studies and animal experiments showed these receptors may be related to the prevention of autoimmune diseases [24,25]. In our study the significant down-regulation of KLRG1 and KLRB1 mRNA expressions in AMI group demonstrated that the NK cells were insufficient to prevent autoimmune disease and were hyporesponsive in AMI patients. As to SA patients, the gene expressions of inhibitory NK receptors were not statistically different from those in the controls, indicating that no initiation of any inhibitory NK receptors was activated in SA group.
The activating receptors are widely expressed on the surface of NK cells. NKG2D is a central activating NK cell receptor, which can be bound to many ligands that are induced on cells under stress due to infection, transformation, or DNA damage. Therefore, NKG2D played an important role in targeting NK cell responses toward abnormal cells and eventually the lysis [26,27]. NCRs including NKp30, NKp44, NKp46 and NKp80 are one of the most important activating receptors that mediate the NK-cell cytotoxicity and cytokine production [7]. NCRs have been shown to recognize a broad spectrum of ligands, ranging from viral-, parasite- and bacterial-derived ligands to cellular ligands, and play a central role in eliminating infected or transformed cells, particularly malignant cells [28-30]. The 2B4, NTB-A and CRACC receptors belong to the family of SRR, and transmit activating signals through the SLAM-associated protein (SAP) [31]. Like NKG2D ligands, DNAM-1 ligands are frequently expressed on stressed cells, and DNAM-1 receptor participates in a cytotoxic immune response, when bound to the ligands [32-34]. Many other receptors, including CD2, CD7, CD16, CD44, CD96 and CD160 contribute to NK cell activation, but much work remains to be done in determining how and when the various NK cell receptors deliver signals for activation [7,35]. Among the activating receptors, the most dominant NKG2D and NCRs receptors, however, do not activate the NK cells by their own. Activation of NK cells by any of the tested receptors requires complementation with another activating receptor, such as 2B4, NTB-A, CRACC or DNMA-1 to obtain synergistic activation signals, and then releases the perforin and granzyme, leading to the target cells lysis directly [36]. In the meanwhile the requirement for combination may serve as a safeguard to prevent unrestrained activation of NK cells [37]. In our study, 23 gene expressions of activating receptors in AMI patients were lowest among the three groups, and KIR2DS2, NKG2D, NKp30, NTB-A, CRACC, CD2, CD7 and CD96 mRNAs were significantly decreased in comparison with SA patients and controls respectively. The result showed the transduction of activating signal was inhibited in patients with AMI. As a result, the NK cells immune activity to the targeted cells was inhibited. Just as the NK cell inhibitory receptors, there was no statistical difference in mRNA expressions between the SA patients and controls in activating receptors, indicating the NK receptors in SA patients was in a nearly inactive state.
In the present study the significant down-regulation of NK cell inhibitory receptors in AMI patients suggested the decreased protection of normal cells and the hyporesponsive level of NK cells. Meanwhile, the significant down-regulated activating NK receptors in AMI group presented the declined cytotoxicity and cytokine production activity of NK cells. However, there was no statistical difference in gene expressions of inhibitory and activating receptors between the SA patients and controls. Previous studies found the reduced proportions of NK cells in peripheral blood of CAD [5-7], but the reason is still controversial [9-12]. In line with this, the similar loss of NK cell numbers in both AMI and SA patients were also demonstrated in our study (Figure 3). We may conclude that the impaired NK cell function in different stages of CAD patients is not just a quantitative defect. In AMI patients both numbers and receptor activity decreased, while only reduction of quantity was found in SA patients. As a result, we speculated that the pathogenesis of AMI may be associated with NK cell receptor deficient or the progress of AMI restrained the NK cell innate immunity. Both sides showed the close relationship between AMI pathogenesis and the NK cell receptor activity.
In conclusion, the loss of NK cell activity in AMI patients is quantitative and involves the dysfunction of NK cells receptor activity. Consequently, improving NK cell immunity may be considered as a potential target for medical interventions in the patients with AMI.
Limitation
A limitation of our study is the statistically significant difference in age between AMI and SA patients and the controls as aging may affect the immune status. However, our study showed no statistically significant difference in the activity of NK cell receptors between the older SA patients and the younger controls. This may indicate that the aging effect is only marginal for the activity of NK cell receptors.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (No. 30570809).
Disclosure of conflict of interest
None.
References
- 1.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–12. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 2.Martín-Fontecha A, Carbone E. The social life of NK cells. Arch Immunol Ther Exp (Warsz) 2001;49(Suppl 1):S33–9. [PubMed] [Google Scholar]
- 3.Anfossi N, André P, Guia S, Falk CS, Roetynck S, Stewart CA, Breso V, Frassati C, Reviron D, Middleton D, Romagné F, Ugolini S, Vivier E. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–42. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Joncker NT, Fernandez NC, Treiner E, Vivier E, Raulet DH. NK cell responsiveness is tuned commensurate with the number of inhibitory receptors for self-MHC class I: the rheostat model. J Immunol. 2009;182:4572–80. doi: 10.4049/jimmunol.0803900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vivier E, Nunès JA, Vély F. Natural killer cell signaling pathways. Science. 2004;306:1517–9. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- 6.André P, Castriconi R, Espéli M, Anfossi N, Juarez T, Hue S, Conway H, Romagné F, Dondero A, Nanni M, Caillat-Zucman S, Raulet DH, Bottino C, Vivier E, Moretta A, Paul P. Comparative analysis of human NK cell activation induced by NKG2D and natural cytotoxicity receptors. Eur J Immunol. 2004;34:961–71. doi: 10.1002/eji.200324705. [DOI] [PubMed] [Google Scholar]
- 7.Whitman SC, Rateri DL, Szilvassy SJ, Yokoyama W, Daugherty A. Depletion of natural killer cell function decreases atherosclerosis in lowdensity lipoprotein receptor null mice. Arterioscler Thromb Vasc Biol. 2004;24:1049–54. doi: 10.1161/01.ATV.0000124923.95545.2c. [DOI] [PubMed] [Google Scholar]
- 8.Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol. 2013;31:227–58. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jonasson L, Backteman K, Ernerudh J. Loss of natural killer cell activity in patients with coronary artery disease. Atherosclerosis. 2005;183:316–21. doi: 10.1016/j.atherosclerosis.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Backteman K, Ernerudh J, Jonasson L. Natural killer (NK) cell deficit in coronary artery disease: no aberrations in phenotype but sustained reduction of NK cells is associated with low-grade inflammation. Clin Exp Immunol. 2014;175:104–12. doi: 10.1111/cei.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li W, Lidebjer C, Yuan XM, Szymanowski A, Backteman K, Ernerudh J, Leanderson P, Nilsson L, Swahn E, Jonasson L. NK cell apoptosis in coronary artery disease: relation to oxidative stress. Atherosclerosis. 2008;199:65–72. doi: 10.1016/j.atherosclerosis.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 12.Hak Ł, Myśliwska J, Wieckiewicz J, Szyndler K, Trzonkowski P, Siebert J, Myśliwski A. NK cell compartment in patients with coronary heart disease. Immun Ageing. 2007;4:3. doi: 10.1186/1742-4933-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobbin K, Simon R. Sample size determination in microarray experiments for class comparison and prognostic classification. Biostatistics. 2005;6:27–38. doi: 10.1093/biostatistics/kxh015. [DOI] [PubMed] [Google Scholar]
- 14.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD Joint ESC/ACCF/AHA/WHF Task Force for Universal Definition of Myocardial Infarction; Authors/Task Force Members Chairpersons; Thygesen K, Alpert JS, White HD Biomarker Subcommittee; Jaffe AS, Katus HA, Apple FS, Lindahl B, Morrow DA ECG Subcommittee; Chaitman BR, Clemmensen PM, Johanson P, Hod H Imaging Subcommittee; Underwood R, Bax JJ, Bonow JJ, Pinto F, Gibbons RJ Classification Subcommittee; Fox KA, Atar D, Newby LK, Galvani M, Hamm CW Intervention Subcommittee; Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J Trials & Registries Subcommittee; Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML Trials & Registries Subcommittee; Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G Trials & Registries Subcommittee; Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D Trials & Registries Subcommittee; Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S ESC Committee for Practice Guidelines (CPG); Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S Document Reviewers. Morais J, Aguiar C, Almahmeed W, Arnar DO, Barili F, Bloch KD, Bolger AF, Botker HE, Bozkurt B, Bugiardini R, Cannon C, de Lemos J, Eberli FR, Escobar E, Hlatky M, James S, Kern KB, Moliterno DJ, Mueller C, Neskovic AN, Pieske BM, Schulman SP, Storey RF, Taubert KA, Vranckx P, Wagner DR. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–98. [Google Scholar]
- 15.Wiltgen M, Tilz GP. DNA microarray analysis: principles and clinical impact. Hematology. 2007;12:271–287. doi: 10.1080/10245330701283967. [DOI] [PubMed] [Google Scholar]
- 16.Cooper MA, Colonna M, Yokoyama WM. Hidden talents of natural killers: NK cells in innate and adaptive immunity. EMBO Rep. 2009;10:1103–10. doi: 10.1038/embor.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Backström E, Kristensson K, Ljunggren HG. Activation of natural killer cells: underlying molecular mechanisms revealed. Scand J Immunol. 2004;60:14–22. doi: 10.1111/j.0300-9475.2004.01475.x. [DOI] [PubMed] [Google Scholar]
- 18.Middleton D, Curran M, Maxwell L. Natural killer cells and their receptors. Transpl Immunol. 2002;10:147–64. doi: 10.1016/s0966-3274(02)00062-x. [DOI] [PubMed] [Google Scholar]
- 19.Moretta L, Bottino C, Pende D, Vitale M, Mingari MC, Moretta A. Different checkpoints in human NK-cell activation. Trends Immunol. 2004;25:670–6. doi: 10.1016/j.it.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Campbell KS, Purdy AK. Structure/function of human killer cell immunoglobulin-like receptors: lessons from polymorphisms, evolution, crystal structures and mutations. Immunology. 2011;132:315–25. doi: 10.1111/j.1365-2567.2010.03398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gras Navarro A, Kmiecik J, Leiss L, Zelkowski M, Engelsen A, Bruserud Ø, Zimmer J, Enger PØ, Chekenya M. NK cells with KIR2DS2 immunogenotype have a functional activation advantage to efficiently kill glioblastoma and prolong animal survival. J Immunol. 2014;193:6192–206. doi: 10.4049/jimmunol.1400859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar V, McNerney ME. A new self: MHCclass-I-independent natural-killer-cell self-tolerance. Nat Rev Immunol. 2005;5:363–74. doi: 10.1038/nri1603. [DOI] [PubMed] [Google Scholar]
- 23.Lebbink RJ, Meyaard L. Non-MHC ligands for inhibitory immune receptors: novel insights and implications for immune regulation. Mol Immunol. 2007;44:2153–64. doi: 10.1016/j.molimm.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Ito M, Maruyama T, Saito N, Koganei S, Yamamoto K, Matsumoto N. Killer cell lectin-like receptor G1 binds three members of the classical cadherin family to inhibit NK cell cytotoxicity. J Exp Med. 2006;203:289–95. doi: 10.1084/jem.20051986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Hofmann M, Wang Q, Teng L, Chlewicki LK, Pircher H, Mariuzza RA. Structure of natural killer cell receptor KLRG1 bound to E-cadherin reveals basis for MHC-independent missing self recognition. Immunity. 2009;31:35–46. doi: 10.1016/j.immuni.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raulet DH, Gasser S, Gowen BG, Deng W, Jung H. Regulation of ligands for the NKG2D activating receptor. Annu Rev Immunol. 2013;31:413–41. doi: 10.1146/annurev-immunol-032712-095951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zafirova B, Wensveen FM, Gulin M, Polić B. Regulation of immune cell function and differentiation by the NKG2D receptor. Cell Mol Life Sci. 2011;68:3519–29. doi: 10.1007/s00018-011-0797-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kruse PH, Matta J, Ugolini S, Vivier E. Natural cytotoxicity receptors and their ligands. Immunol Cell Biol. 2014;92:221–9. doi: 10.1038/icb.2013.98. [DOI] [PubMed] [Google Scholar]
- 29.Koch J, Steinle A, Watzl C, Mandelboim O. Activating natural cytotoxicity receptors of natural killer cells in cancer and infection. Trends Immunol. 2013;34:182–91. doi: 10.1016/j.it.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Bhat R, Rommelaere J. NK-cell-dependent killing of colon carcinoma cells is mediated by natural cytotoxicity receptors (NCRs) and stimulated by parvovirus infection of target cells. BMC Cancer. 2013;13:367. doi: 10.1186/1471-2407-13-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Claus M, Meinke S, Bhat R, Watzl C. Regulation of NK cell activity by 2B4, NTB-A and CRACC. Front Biosci. 2008;13:956–65. doi: 10.2741/2735. [DOI] [PubMed] [Google Scholar]
- 32.de Andrade LF, Smyth MJ, Martinet L. DNAM-1 control of natural killer cells functions through nectin and nectin-like proteins. Immunol Cell Biol. 2014;92:237–44. doi: 10.1038/icb.2013.95. [DOI] [PubMed] [Google Scholar]
- 33.Tahara-Hanaoka S, Miyamoto A, Hara A, Honda S, Shibuya K, Shibuya A. Identification and characterization of murine DNAM-1 (CD226) and its poliovirus receptor family ligands. Biochem Biophys Res Commun. 2005;329:996–1000. doi: 10.1016/j.bbrc.2005.02.067. [DOI] [PubMed] [Google Scholar]
- 34.El-Sherbiny YM, Meade JL, Holmes TD, McGonagle D, Mackie SL, Morgan AW, Cook G, Feyler S, Richards SJ, Davies FE, Morgan GJ, Cook GP. The requirement for DNAM-1, NKG2D, and NKp46 in the natural killer cell-mediated killing of myeloma cells. Cancer Res. 2007;67:8444–9. doi: 10.1158/0008-5472.CAN-06-4230. [DOI] [PubMed] [Google Scholar]
- 35.Chan CJ, Smyth MJ, Martinet L. Molecular mechanisms of natural killer cell activation in response to cellular stress. Cell Death Differ. 2014;21:5–14. doi: 10.1038/cdd.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bryceson YT, March ME, Ljunggren HG, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006;214:73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lam RA, Chwee JY, Le Bert N, Sauer M, Pogge von Strandmann E, Gasser S. Regulation of self-ligands for activating natural killer cell receptors. Ann Med. 2013;45:384–94. doi: 10.3109/07853890.2013.792495. [DOI] [PubMed] [Google Scholar]


