Abstract
Distant metastasis continues to be a fatal threat to quality of life in patients with small cell lung caner (SCLC). The purpose of this work is to analyze the expressions of chemokine receptor four (CXCR4), matrix metalloproteinase-9 (MMP-9), transforming growth factor-b1 (TGF-β1), N-cadherin and vascular endothelial growth factor (VEGF) in small cell lung caner (SCLC), and to explore their correlations with the prognosis and metastasis. Sixty-five consecutive patients with stage I-III SCLC who received operation in our hospital from Jan 2003 to Oct 2009 were retrospectively analyzed. The expression of CXCR4 was found significantly correlated with bone metastasis (P = 0.004), and were marginally correlated with brain metastasis (P = 0.068) and lymph node metastasis (P = 0.085). The expression of MMP-9 was significantly associated with pathological staging (P = 0.048). Univariate analysis suggested surgical approach, clinical stage, lymph node metastasis were significantly associated with OS and PFS (P < 0.05), high expression of CXCR4 was significantly correlated with worse OS (P = 0.004) and PFS (P = 0.005). Multivariate analysis suggested surgical approach, TGF-β1, CXCR4 and lymph node metastasis were independent prognostic factor for PFS. In conclusion, High expression of CXCR4, MMP-9, TGF-β1 and VEGF were found in SCLC. High expression of MMP-9 was significantly associated with pathological staging, and high expression of CXCR4 was correlated with bone metastasis and also might correlate with brain metastasis. CXCR4 were independent prognostic factor for survival in SCLC and expanded samples should be further explored in the future.
Keywords: Small cell lung cancer, molecular factors, immunohistochemistry, metastasis, prognosis
Introduction
Lung cancer is one of the most common malignant tumors in the world, with its highest incidence, mortality and 5-year survival rate lower than 15%. Small cell lung caner (SCLC) accounts for 15%-20% of all lung cancer and tends to have wide-spread metastasis at early stage and the ability to develop resistance against chemotherapeutic drugs which are responsible for the highly malignant phenotype [1]. Chemokine receptor four (CXCR4), matrix metalloproteinase-9 (MMP-9) and transforming growth factor-b1 (TGF-β1), N-cadherin and vascular endothelial growth factor (VEGF) have been confirmed to play an important regulatory role during the process of tumor invasion and distant metastasis [2-7]. In our study, we detected and analyzed the expressions of CXCR4, MMP-9, TGF-β1, N-cadherin and VEGF in SCLC, explored their correlations with brain metastasis and prognostic significance by immunohistochemical method and further provided theoretical basis for subsequent targeted therapy of clinical research.
Materials and methods
Patients and specimens
The study was approved by the Tianjin Medical University Cancer Hospital and Institution. Sixty five SCLC patients between Jan 2003 and Oct 2009 who had undergone a lobectomy or pneumonectomy and mediastinal lymph node dissection were included in this retrospective study. Most of the patients received operation therapy due to the non-small cell lung cancer diagnosis pre-operation. The selection criteria were as follows: patients had pathologically proven SCLC, had undergone surgery, had negative pretreatment results of computed tomography or magnetic resonance imaging of the head at the time of initial diagnosis, and had no history of any other cancer. None of the patients had received PCI. TNM staging was defined according to the current American Joint Committee on Cancer (AJCC) criteria, 7th edition.
Immunohistochemistry
All the specimens used for immunohistochemistry (IHC) were fixed in 10% neutral buffered formalin and processed routinely for CXCR4, MMP9, TGF-β1, N-cadherin and VEGF according to immunohistochemistry of streptavidin-perioxidas (SP) kit. The working concentration for primary antibodies follows: CXCR4 (1:50, Abcam company, England); VEGF (1:100, Abcam, England); MMP9 (1:100, Zhongshan Golden Bridge Biological Technology, Beijing, China), TGF-β1 (1:100, Abcam, England) and N-cadherin (1:50, Abcam, England). The secondary antibodies were added to the samples for fluorescence signals (1:1000, Zhongshan Golden Bridge Biological Technology, Beijing, China). The immunocomplexes were visualized using diaminobenzidine (DAB) as a chromogenic substrate (Zhongshan Golden Bridge Biological Technology, Beijing, China). Phosphate buffered saline (PBS) was used in place of primary antibodies as negative control and positive results were used as a positive control.
Judgment of results
Two independent observers determined the percentage of cells being stained and interpreted the results in a blinded fashion. Both the intensity and percentage of positive cells were measured. The product of the staining intensity and positive cell scores determined the final results for each section. The staining intensity was determined for the following four classes: 0 = undetectable; 1 = faint buff; 2 = moderate buff; and 3 = high buff or sepia. Stained cell sections in each case were randomly selected and five high-power fields were counted under the microscope (× 400). We counted 100 cells in each region for a total number of 500 cells, calculated the percentage of positive cells as well as the score. IHC staining was scored according to the following criterion: 0, 0%-5%; 1, 6%-25%; 2, 26%-50%; 3, 51%-75% and 4, 76%-100%. Using the percentage of stained cells × staining intensity ( from 0 to 12), the integrated scoring was assessed [8]. Cell expression was stratified as follows according to our staining: negative: less than 3, moderate positive, less than 6 and strong positive for more than 6 to CXCR4, MMP9; negative: less than 3, positive for more than 3 to TGF-β1 and N-cadherin; negative: less than 4, positive for more than 4 to VEGF.
Statistical analysis
All statistical analyses were carried out using SPSS 17.0 software. Enumeration data were analyzed by X2 test and Kaplan-Meier method was conducted for survival curves, which were compared with Log-rank test, and multivariate analyses were carried out by the Cox proportional hazards regression model. P < 0.05 was considered statistically significant.
Results
The characteristics of patients
There were 44 male cases and 21 female cases, aging from 35 to 78 years with median age of 59 years. Eighteen cases received pneumonectomy and 47 cases received lobectomy; 20 cases had stage I, 15 had stage II and 30 had stage III disease according to UICC 2009; Twenty-four cases received 1-2 cycles of induction chemotherapy, 57 cases received 1-6 cycles of adjuvant chemotherapy and 17 cases received radiotherapy to the surgical stump and mediastinum with a total dose of 40-50 Gy. Thirty two cases suffered distant metastasis (including 15 bone metastasis, 11 brain metastasis and 9 with other distant metastasis as liver or adrenal gland) and 17 with local recurrence (LR) when followed up on January 1, 2015. The median overall survival (OS) and progression free survival (PFS) were 30 months and 17 months, respectively.
The expression of CXCR4, MMP-9, TGF-β1, N-cadherin and VEGF protein
IHC staining results of tumor samples demonstrated that the positive expression rates of CXCR4, MMP-9, TGF-β1, N-cadherin and VEGF were 93.8% (61/65), 70.8% (46/65) and 43.1% (28/65), 35.4% (23/65) and 50.8% (33/65) respectively. CXCR4, MMP-9 (Figure 1), TGF-β1, N-cadherin and VEGF (Figure 2) positive cells were stained with brown-yellow granules or masses, specifically in the cytoplasm. The moderate positive rate of CXCR4 was 18.0% (11/61) and that of strong positive was 82.0% (50/61). The moderate positive rate of MMP-9 was 26.1% (12/46) and that of strong positive was 73.9% (34/46).
Figure 1.
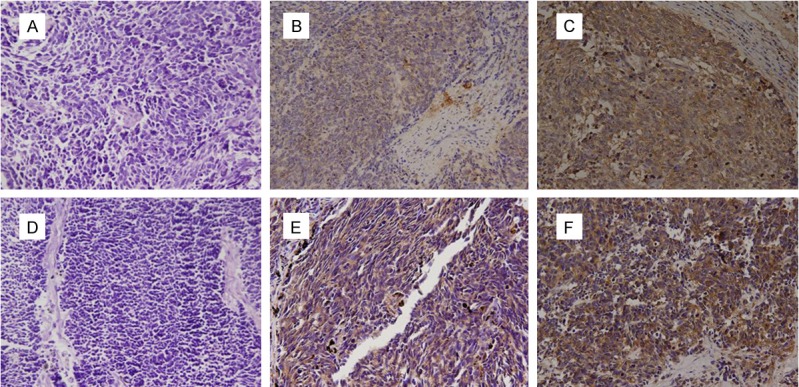
The expression of CXCR4 and MMP9 (× 200). A. Negative expression of CXCR4 protein. B. Moderate positive expression of CXCR4 protein. C. Strong positive expression of CXCR4 protein. D. Negative expression of MMP9 protein. E. Moderate positive expression of MMP9 protein. F. Strong positive expression of MMP9 protein.
Figure 2.
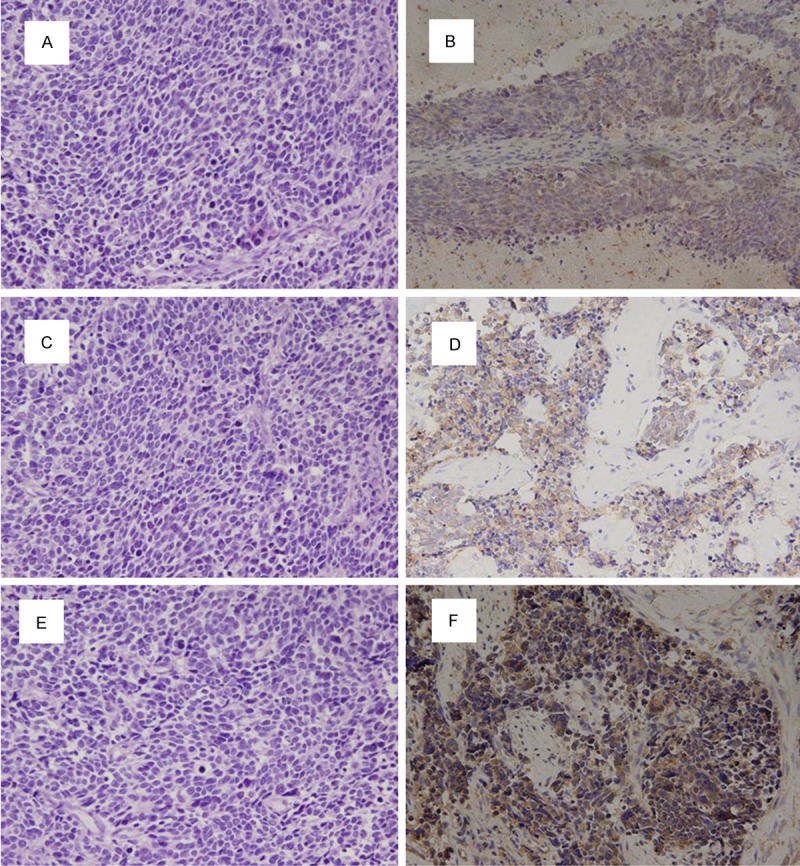
The expression of TGF-β1, N-cadherin and VEGF (× 200). A. Negative expression of TGF-β1 protein. B. Positive expression of TGF-β1 protein. C. Negative expression of N-cadherin protein. D. Positive expression of N-cadherin protein. E. Negative expression of VEGF protein. F. Positive expression of VEGF protein.
The relationship among the expression of CXCR4, MMP-9, TGF-β1, N-cadherin and VEGF to survival
The PFS and OS at 1 year, 3 years and 5 years for the whole group was 53.8%, 34.9%, 31.7% and 81.5%, 40.9%, 33.0%, respectively. Univariate analysis suggested that surgical approach, clinical stage, lymphatic metastasis were significantly associated with prognosis (P < 0.05, high expression of CXCR4 was significantly correlated with worse OS and PFS (P = 0.004 and P = 0.005, respectively) (Figure 3), while no differences were found between MMP-9 or TGF-β1 with OS (P > 0.05) (Table 1). Multivariate analysis suggested that surgical approach, TGF-β1, CXCR4 and lymph node metastasis were independent prognostic factors for PFS (Table 2), While no correlation were found between PFS and the expression of N-cadherin and VEGF (P > 0.05).
Figure 3.
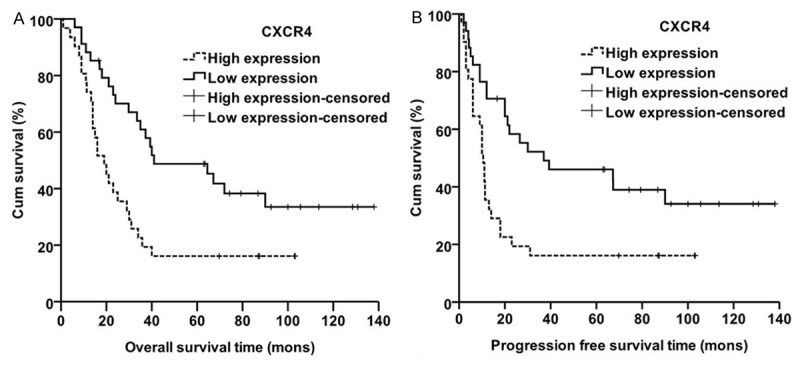
Kaplan-Meier curves for patients with the expression of CXCR4. A. Overall survival differences in CXCR4, P = 0.004. B. Progression-free survival differences in CXCR4, P = 0.005.
Table 1.
Statistical analysis of factors predicting patients’ overall survival (OS)
| (Number of patients) | OS (months) | Univariate* | Multivariate** | |
|---|---|---|---|---|
|
|
||||
| Median | P Value | P Value | 95% CI | |
| Sex | ||||
| Male (44) | 23.0 | 0.035 | ||
| Female (21) | 41.0 | |||
| Age | ||||
| < 65 years (44) | 30.0 | 0.207 | ||
| ≥ 65 years (21) | 25.0 | |||
| Smoking Index | ||||
| < 400 (36) | 25.0 | 0.283 | ||
| ≥ 400 (29) | 30.0 | |||
| Surgical approach | ||||
| Lobectomy (47) | 37.3 | 0.001 | 0.002 | 0.156-0.669 |
| Pneumonectomy (18) | 15.0 | |||
| Clinical stage | ||||
| I (20) | 67.2 | 0.002 | ||
| II (15) | 17.1 | |||
| III (30) | 20.0 | |||
| Lymphatic metastasis | ||||
| Yes (26) | 20.0 | 0.001 | ||
| No (39) | 67.0 | |||
| VEGF | ||||
| + (33) | 31.0 | 0.423 | ||
| - (32) | 29.8 | |||
| N-cadherin | ||||
| + (23) | 31.0 | 0.781 | ||
| - (42) | 29.8 | |||
| CXCR4 | ||||
| + (31) | 19.0 | 0.004 | 0.003 | 1.447-5.963 |
| - (34) | 41.0 | |||
| MMP9 | ||||
| + (34) | 24.0 | 0.899 | ||
| - (31) | 34.0 | |||
| TGF-β1 | ||||
| + (28) | 31.0 | 0.142 | 0.035 | 0.220-0.946 |
| - (37) | 29.0 | |||
Univariate Analysis;
Multivariate Analysis.
Table 2.
Statistical analysis of factors predicting patients’ progression free survival (PFS)
| (Number of patients) | PFS (months) | Univariate* | Multivariate** | |
|---|---|---|---|---|
|
|
||||
| Median | P Value | P Value | 95% CI | |
| Sex | ||||
| Male (44) | 11.3 | 0.056 | ||
| Female (21) | 39.3 | |||
| Age | ||||
| < 65 years (44) | 20.0 | 0.120 | ||
| ≥ 65 years (21) | 10.5 | |||
| Smoking Index | ||||
| < 400 (36) | 14.0 | 0.212 | ||
| ≥ 400 (29) | 18.0 | |||
| Surgical approach | ||||
| Lobectomy (47) | 23.0 | 0.004 | 0.002 | 0.162-0.658 |
| Pneumonectomy (18) | 9.0 | |||
| Clinical stage | ||||
| I (20) | 67.2 | 0.007 | ||
| II (15) | 11.3 | |||
| III (30) | 10.0 | |||
| Lymphatic metastasis | ||||
| Yes (26) | 11.0 | 0.002 | 0.005 | 0.185-0.742 |
| No (39) | 67.2 | |||
| VEGF | ||||
| + (33) | 21.2 | 0.272 | ||
| - (32) | 10.5 | |||
| N-cadherin | ||||
| + (23) | 18.0 | 0.737 | ||
| - (42) | 14.0 | |||
| CXCR4 | ||||
| + (31) | 10.5 | 0.005 | 0.006 | 1.311-5.134 |
| - (34) | 37.0 | |||
| MMP9 | ||||
| + (34) | 12.0 | 0.807 | ||
| - (31) | 20.0 | |||
| TGF-β1 | ||||
| + (28) | 18.0 | 0.095 | 0.028 | 0.238-0.919 |
| - (37) | 14.0 | |||
Univariate Analysis;
Multivariate Analysis.
The relationship among the expression of CXCR4, MMP-9, TGF-β1, N-cadherin and VEGF to metastasis
There were significant differences between the high expression of CXCR4 and distant metastasis (P = 0.004), especially to bone metastasis (P = 0.004), but no differences to brain metastasis (P = 0.068) or lymph node metastasis (P = 0.085). The high expression of MMP-9 was significantly associated with clinical staging (P = 0.048), but no differences were found with distant metastasis (P = 0.261) and lymph node metastasis (P = 0.085); no significantly differences were found among the following indicators of age, sex, local recurrence and lymphatic metastasis (P > 0.05) (Table 3), While no correlation were found between metastasis and the expression of TGF-β1, N-cadherin and VEGF (P > 0.05).
Table 3.
Relations among CXCR4, MMP9, TGF-β1, N-cadherin and VEGF expression to clinical pathological factors
| N | CXCR4 | P | MMP-9 | P | TGF-β1 | P | VEGF | P | N-cadherin | P | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||||||||||
| + | - | + | - | + | - | + | - | + | - | |||||||||
| Sex | ||||||||||||||||||
| Male | 44 | 22 | 22 | 0.590 | 23 | 21 | 0.993 | 18 | 26 | 0.609 | 21 | 23 | 0.164 | 15 | 29 | 0.206 | ||
| Female | 9 | 12 | 11 | 10 | 10 | 11 | 12 | 9 | 8 | 13 | ||||||||
| Age | ||||||||||||||||||
| < 65 years | 44 | 21 | 23 | 0.993 | 22 | 22 | 0.590 | 20 | 24 | 0.575 | 25 | 19 | 0.079 | 17 | 27 | 0.164 | ||
| ≥ 65 yesrs | 21 | 10 | 11 | 12 | 9 | 8 | 13 | 8 | 13 | 6 | 15 | |||||||
| Clinical stage | ||||||||||||||||||
| I | 20 | 7 | 13 | 0.322 | 12 | 8 | 0.048 | 8 | 12 | 0.312 | 10 | 10 | 0.335 | 8 | 12 | 0.191 | ||
| II | 15 | 7 | 8 | 11 | 4 | 9 | 6 | 10 | 5 | 7 | 8 | |||||||
| III | 30 | 17 | 13 | 11 | 19 | 11 | 19 | 13 | 17 | 8 | 22 | |||||||
| Lymphatic metastasis | ||||||||||||||||||
| Yes | 39 | 22 | 17 | 0.085 | 17 | 22 | 0.085 | 16 | 23 | 0.683 | 19 | 20 | 0.184 | 13 | 26 | 0.190 | ||
| No | 26 | 9 | 17 | 17 | 9 | 12 | 14 | 14 | 12 | 10 | 16 | |||||||
| Local recurrence | ||||||||||||||||||
| Yes | 17 | 9 | 8 | 0.614 | 8 | 9 | 0.614 | 8 | 9 | 0.700 | 8 | 9 | 0.208 | 6 | 11 | 0.231 | ||
| No | 48 | 22 | 26 | 26 | 22 | 20 | 28 | 25 | 23 | 17 | 31 | |||||||
| Distant Metastasis | ||||||||||||||||||
| Yes | 32 | 21 | 11 | 0.004 | 19 | 13 | 0.261 | 12 | 20 | 0.371 | 15 | 17 | 0.163 | 11 | 21 | 0.202 | ||
| No | 33 | 10 | 23 | 15 | 18 | 16 | 17 | 18 | 15 | 12 | 21 | |||||||
| Brain Metastasis | ||||||||||||||||||
| Yes | 11 | 8 | 3 | 0.068 | 7 | 4 | 0.409 | 5 | 6 | 0.861 | 5 | 6 | 0.240 | 4 | 7 | 0.267 | ||
| No | 54 | 23 | 31 | 27 | 27 | 23 | 31 | 28 | 26 | 19 | 35 | |||||||
| Bone Metastasis | ||||||||||||||||||
| Yes | 15 | 12 | 3 | 0.004 | 8 | 7 | 0.928 | 7 | 8 | 0.749 | 7 | 8 | 0.217 | 7 | 8 | 0.14 | ||
| No | 50 | 19 | 31 | 26 | 24 | 21 | 29 | 26 | 24 | 16 | 34 | |||||||
Discussion
Distant metastasis continues to be a significant threat to quality of life in patients with lung cancer. The risk of developing BM in SCLC is higher than with other histologies. Seute et al. reported the cumulative risk of BM at 2 years after the diagnosis to be 49% to 65% in SCLC [9]. Immunohistochemistry plays an important role in pathological study and diagnosis. In this study, we detected and analyzed the expressions of CXCR4, MMP-9, TGF-β1, N-cadherin and VEGF in SCLC, explored their correlations with brain metastasis and prognostic significance by immunohistochemistry, and we found high expression of CXCR4, MMP-9, TGF-β1 and VEGF were found in SCLC. High expression of MMP-9 was significantly associated with pathological staging, and high expression of CXCR4 was correlated with bone metastasis and also might be correlated with brain metastasis.
Matrix metalloproteinases-9 (MMP-9) has been identified to have negative correlation with NSCLC and promotes its distant metastasis [10-12], while few studies explore its correlations with metastasis and prognostic significance in SCLC. In our study of 65 SCLCs, the high expression of MMP-9 was significantly associated with clinical staging, which was coincide with the results of El-Badrawy [13] and Zheng et al [14] who found that the expression of MMP-9 correlated with pathologic stage, lymph node metastasis, and survival. But no differences were found among the high expression of MMP-9 to distant metastasis, lymph node metastasis and survival, which maybe resulted from our small sample size. Significantly higher expression and activity of MMP-9 in tumor tissue supports the important role of this metalloproteinase in the growth of SCLC, however further studies are needed to understand its clear mechanism during the progress of SCLC.
Over-expression of TGF-β1 was demonstrated as a negative prognosis marker in a variety of tumors such as head and neck squamous cell carcinoma, gastric carcinoma, breast cancer, pancreatic cancer, colorectal carcinoma, prostate cancer, human melanoma cells and bladder cancer [15-17], whereas some study suggested it as a favorable prognostic factor at the early stage of NSCLC [18,19]. These inconsistent results might be due to discrepancies in sample size, different techniques of detection, and unequal cutoff points between these studies. In our cohort, multivariate analysis suggested TGF-β1 was an independent protective prognostic factor for PFS; positive expression of TGF-β1 had a relative longer median (18.0 months) PFS for patient’s than the negative ones (14.0 months). However, the molecular mechanism by which TGF-β1 associated with prognosis has not been elucidated, and whether it’s a favorable prognostic factor or it has a prognostic or predictive values still needs to be further studied.
In our study of 65 SCLCs, the positive expression rates of CXCR4 was 93.8%, which was similar to a previous study [20]. To analyze the relationship among the expression of CXCR4, MMP-9 and TGF-β1 with patients’ prognosis, we found the high expression of MMP-9 only associated with the postoperative pathological staging of patients; and a high expression of CXCR4 is associated with distant metastasis, especially to bone metastases (P = 0.004) while no significance was found to TGF-β1, N-cadherin and VEGF. All the results suggested that the high expression of CXCR4 had positive correlation to distant metastasis in SCLC, especially to bone metastases. Sun et al [21] confirmed that CXCLl2/CXCR4 axis was activated in bone metastases of prostate cancer, neutralizing CXCR4 antibody or a specific peptide that blocked CXCR4 also decreased the size of the tumors and the total metastatic load compared with controls in vivo. Although few reports about CXCR4 in bone metastases of SCLC, our results indicated CXCR4 may be an effective predictor of bone metastases in SCLC. Our results showed that patients with high expression of CXCR4 had less shortened OS (19.0 months to 41.0 months) and DFS (10.5 months to 37.0 months) significantly. Univariate analysis suggested that surgical approach, clinical stage, lymphatic metastasis was significantly associated with prognosis, and high expression of CXCR4 was significantly correlated with OS and PFS, which showed CXCR4 may become a potentially effective predictor for patient’s OS and PFS.
CXCR4 could mediate cancer cell migration and adhesion to the stromal cells in SCLC [20]. Some studies had found that cancer cell could escape the necrosis caused by chemotherapy because of the migration of cancer cells mediated by CXCR4 and integrin [1,22]. Some studies indicated that blocking CXCR4 by neutralizing CXCR4 antibody or siRNA interference could inhibit the growth and metastasis of breast and prostate cancer cells. According to the regulation function of CXCR4 in distant metastasis and resistance against chemotherapeutic agents in SCLC, many studies devoted to the research of CXCR4 antagonists for treatment of SCLC, and CXCR4 was considered to be an important taget and a new hope for targeted therapy of SCLC [20,22].
In conclusion, high expression of CXCR4, MMP-9, TGF-β1 and VEGF were found in SCLC. High expression of MMP-9 was significantly associated with pathological staging, and high expression of CXCR4 was correlated with bone metastasis and poor survival in SCLC. CXCR4 were independent prognostic factor for survival in SCLC and expanded samples should be further explored in the future.
Acknowledgements
The authors acknowledge the funding received from The Project of National Natural Science Foundation of China (81372518).
Disclosure of conflict of interest
None.
References
- 1.Hartmann TN, Burger JA, Glodek A, Fujii N, Burger M. CXCR4 chemokine receptor and integrin signaling co-operate in mediating adhesion and chemoresistance in small cell lung cancer (SCLC) cells. Oncogene. 2005;24:4462–4471. doi: 10.1038/sj.onc.1208621. [DOI] [PubMed] [Google Scholar]
- 2.Xu Y, Lu S. Transforming growth factor-beta1-induced epithelial to mesenchymal transition increases mitochondrial content in the A549 non-small cell lung cancer cell line. Mol Med Rep. 2015;11:417–421. doi: 10.3892/mmr.2014.2678. [DOI] [PubMed] [Google Scholar]
- 3.Wang L, Wang Z, Liu X, Liu F. High-level C-X-C chemokine receptor type 4 expression correlates with brain-specific metastasis following complete resection of non-small cell lung cancer. Oncol Lett. 2014;7:1871–1876. doi: 10.3892/ol.2014.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miao ZF, Li WY, Wang ZN, Zhao TT, Xu YY, Song YX, Huang JY, Xu HM. Lung cancer cells induce senescence and apoptosis of pleural mesothelial cells via transforming growth factorbeta1. Tumour Biol. 2015;36:2657–65. doi: 10.1007/s13277-014-2888-7. [DOI] [PubMed] [Google Scholar]
- 5.Chen P, Zhu J, Liu DY, Li HY, Xu N, Hou M. Over-expression of survivin and VEGF in smallcell lung cancer may predict the poorer prognosis. Med Oncol. 2014;31:775. doi: 10.1007/s12032-013-0775-5. [DOI] [PubMed] [Google Scholar]
- 6.Hahn N, Heiden M, Seitz R, Salge-Bartels U. Inducible expression of tissue factor in small-cell lung cancer: impact on morphology and matrix metalloproteinase secretion. J Cancer Res Clin Oncol. 2012;138:695–703. doi: 10.1007/s00432-011-1139-1. [DOI] [PubMed] [Google Scholar]
- 7.Hazan RB, Phillips GR, Qiao RF, Norton L, Aaronson SA. Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J Cell Biol. 2000;148:779–790. doi: 10.1083/jcb.148.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X, Zhang H, Yu X, Song T, Huang P, Wang H, Song SA. The correlation of expression of VEGF and EGFR with SUV of (18)FDG-PET-CT in non-small cell lung cancer. Contemp Oncol (Pozn) 2014;18:334–339. doi: 10.5114/wo.2014.45308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seute T, Leffers P, ten Velde GP, Twijnstra A. Neurologic disorders in 432 consecutive patients with small cell lung carcinoma. Cancer. 2004;100:801–806. doi: 10.1002/cncr.20043. [DOI] [PubMed] [Google Scholar]
- 10.Safranek J, Pesta M, Holubec L, Kulda V, Dreslerova J, Vrzalova J, Topolcan O, Pesek M, Finek J, Treska V. Expression of MMP-7, MMP-9, TIMP-1 and TIMP-2 mRNA in lung tissue of patients with non-small cell lung cancer (NSCLC) and benign pulmonary disease. Anticancer Res. 2009;29:2513–2517. [PubMed] [Google Scholar]
- 11.Rydlova M, Holubec L Jr, Ludvikova M Jr, Kalfert D, Franekova J, Povysil C, Ludvikova M. Biological activity and clinical implications of the matrix metalloproteinases. Anticancer Res. 2008;28:1389–1397. [PubMed] [Google Scholar]
- 12.Iniesta P, Moran A, De Juan C, Gomez A, Hernando F, Garcia-Aranda C, Frias C, Diaz-Lopez A, Rodriguez-Jimenez FJ, Balibrea JL, Benito M. Biological and clinical significance of MMP-2, MMP-9, TIMP-1 and TIMP-2 in nonsmall cell lung cancer. Oncol Rep. 2007;17:217–223. [PubMed] [Google Scholar]
- 13.El-Badrawy MK, Yousef AM, Shaalan D, Elsamanoudy AZ. Matrix metalloproteinase-9 expression in lung cancer patients and its relation to serum mmp-9 activity, pathologic type, and prognosis. J Bronchology Interv Pulmonol. 2014;21:327–334. doi: 10.1097/LBR.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 14.Zheng S, Chang Y, Hodges KB, Sun Y, Ma X, Xue Y, Williamson SR, Lopez-Beltran A, Montironi R, Cheng L. Expression of KISS1 and MMP-9 in non-small cell lung cancer and their relations to metastasis and survival. Anticancer Res. 2010;30:713–718. [PubMed] [Google Scholar]
- 15.Booth C, Harnden P, Selby PJ, Southgate J. Towards defining roles and relationships for tenascin-C and TGFbeta-1 in the normal and neoplastic urinary bladder. J Pathol. 2002;198:359–368. doi: 10.1002/path.1214. [DOI] [PubMed] [Google Scholar]
- 16.Yin M, Soikkeli J, Jahkola T, Virolainen S, Saksela O, Holtta E. TGF-beta signaling, activated stromal fibroblasts, and cysteine cathepsins B and L drive the invasive growth of human melanoma cells. Am J Pathol. 2012;181:2202–2216. doi: 10.1016/j.ajpath.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 17.Bian Y, Hall B, Sun ZJ, Molinolo A, Chen W, Gutkind JS, Waes CV, Kulkarni AB. Loss of TGF-beta signaling and PTEN promotes head and neck squamous cell carcinoma through cellular senescence evasion and cancer-related inflammation. Oncogene. 2012;31:3322–3332. doi: 10.1038/onc.2011.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao Y, Wang Y, Sun L, Meng Q, Cai L, Dong X. Expression of TGFbeta-1 and EHD1 correlated with survival of non-small cell lung cancer. Tumour Biol. 2014;35:9371–9380. doi: 10.1007/s13277-014-2164-x. [DOI] [PubMed] [Google Scholar]
- 19.Inoue T, Ishida T, Takenoyama M, Sugio K, Sugimachi K. The relationship between the immunodetection of transforming growth factorbeta in lung adenocarcinoma and longer survival rates. Surg Oncol. 1995;4:51–57. doi: 10.1016/s0960-7404(10)80031-3. [DOI] [PubMed] [Google Scholar]
- 20.Burger M, Glodek A, Hartmann T, Schmitt-Graff A, Silberstein LE, Fujii N, Kipps TJ, Burger JA. Functional expression of CXCR4 (CD184) on small-cell lung cancer cells mediates migration, integrin activation, and adhesion to stromal cells. Oncogene. 2003;22:8093–8101. doi: 10.1038/sj.onc.1207097. [DOI] [PubMed] [Google Scholar]
- 21.Sun YX, Schneider A, Jung Y, Wang J, Dai J, Wang J, Cook K, Osman NI, Koh-Paige AJ, Shim H, Pienta KJ, Keller ET, McCauley LK, Taichman RS. Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. J Bone Miner Res. 2005;20:318–329. doi: 10.1359/JBMR.041109. [DOI] [PubMed] [Google Scholar]
- 22.Hartmann TN, Burger M, Burger JA. The role of adhesion molecules and chemokine receptor CXCR4 (CD184) in small cell lung cancer. J Biol Regul Homeost Agents. 2004;18:126–130. [PubMed] [Google Scholar]


