Abstract
Lung cancer is the leading cause of cancer-related mortality. This study was undertaken to investigate the efficacy and safety of adding regulatory T cell inhibitor cyclophosphamide to pemetrexed therapy for the second-line treatment of NSCLC with wild-type epidermal growth factor receptor (EGFR). A total of 70 patients were screened between March 2011 and December 2013, out of which 62 patients were enrolled in the study. Patients were randomized to receive 500 mg/m2 pemetrexed in combination with 20 mg/kg cyclophosphamide in a 21 day cycle (n=30) or 500 mg/m2 pemetrexed (n=32), and followed up for 30 months. Disease progression was observed in 23 patients in the pemetrexed plus cyclophosphamide arm and 27 patients in the pemetrexed monotherapy arm. Median progression-free survival was 3.6 months (95% confidence interval [CI], 1.3 to 5.9 months) in the pemetrexed plus cyclophosphamide arm and 2.2 months (95% CI, 1.3 to 3.1 months) in the pemetrexed monotherapy arm. The 6-month PFS rates were 22% (95% CI, 10 to 34) and 14.5% (95% CI, 6 to 23) in the pemetrexed plus cyclophosphamide arm and pemetrexed monotherapy arm, respectively. Median overall survival was 9.8 months for the pemetrexed combination therapy arm and 8.8 months for the pemetrexed arm, and the 1-year survival rates were 46% and 33%, respectively. The present study showed that pemetrexed in combination with low-dose cyclophosphamide may be a better treatment approach than pemetrexed monotherapy when considering second-line treatment for wild-type EGFR NSCLC.
Keywords: Non-small cell lung cancer, EGFR, second-line treatment, adenocarcinoma
Introduction
Lung cancer is the leading cause of cancer-associated death in the world [1]. Most patients with non-small cell lung cancer (NSCLC) are diagnosed at a relatively late stage, and platinum-based first-line chemotherapy is prescribed as a part of the standard treatment for advanced NSCLC. However, the factors that may predict survival and treatment response are limited. Approximately half of the patients with NSCLC are initially diagnosed with advanced disease. For patients with negative or unknown driver mutation status, platinum-based combination chemotherapy represents the standard of care in advanced NSCLC [2]. However, almost all patients eventually develop progressive disease that requires further treatments after the initial therapy.
Traditional first-line therapy for advanced NSCLC is platinum-doublet chemotherapy. Several randomized studies comparing first-line epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) with doublet chemotherapy in patients with advanced NSCLC harboring activating EGFR mutations have demonstrated better improvements in objective response rate (ORR), progression-free survival (PFS), and quality of life (QoL) with EGFR-TKIs than with platinum-based chemotherapy [3-5]. Therefore, EGFR-TKIs are recommended as a standard first-line treatment for patients with EGFR-mutant advanced NSCLC and EGFR mutation detection is mandatory to determine the use of first-line EGFR-TKIs. However, in second-line or greater settings, the role of EGFR mutation status in the guidance of EGFR-TKI use is unclear. Eight randomized trials [6-13] compared EGFR-TKIs with standard second-line chemotherapy (docetaxel or pemetrexed) in patients with advanced NSCLC, but none of these trials was initially designed to evaluate the effect of EGFR genotype. Therefore, the patients enrolled in these trials were unselected populations.
Cyclophosphamide is the most widely used alkylating agent and its antineoplastic and immunomodulating activities have been registered for early and advanced breast cancer [6,14]. Pemetrexed, a multitargeted antifolate, inhibits thymidylate synthase, dihydrofolate reductase (DHFR), and glycinamide ribonucleotide formyltransferase (GARFT), and has demonstrated antitumor activity in multiple tumor types [7,8]. Single-agent pemetrexed has showed activity in several phase II studies in patients with lung cancer [9]. On the basis of the folate-disrupting mechanism of pemetrexed, which is similar to methotrexate and 5-fluorouracil, and the tolerable safety profile evaluated in a phase I study, we designed this phase II, multicenter, randomized study to determine whether pemetrexed plus cyclophosphamide can be a valuable regimen for the treatment of locally advanced NSCLC [10]. The primary objective of this study was to assess the antitumor activity, measured by response rate, of two doses of pemetrexed in combination with cyclophosphamide versus pemetrexed monotherapy as a second-line treatment in patients with advanced non-squamous NSCLC with wild-type EGFR. Secondary objectives were time-to-event efficacy variables, safety, pharmacokinetics of pemetrexed and cyclophosphamide when given in combination, and assessment of patients’ baseline nutritional status on safety and efficacy as measured by homocysteine levels.
Materials and methods
Study design and patient eligibility
The study was undertaken in accordance with the Declaration of Helsinki, Good Clinical Practice guidelines, and the protocol was approved by local Ethics Committee of The First Affiliated Hospital of Nanchang University, China. A written informed consent for participation in the study was obtained from all patients.
This phase II, open-label, randomized study was undertaken at a single centre in China. The criteria for enrollment were as follows: patients must have histologically or cytologically proven advanced (stage IIIB or IV) or recurrent NSCLC, disease (AJCC/UICC version 6) without EGFR mutations in exons 18-21 in their tumor samples, as tested by direct sequencing, and previously received one chemotherapy regimen as palliative therapy for locally advanced or metastatic disease. The inclusion criteria are: disease progression after first-line or second-line chemotherapy; age ≥18 years; Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≤2; at least one measurable lesion; adequate bone marrow (absolute neutrophil count ≥1500/mL and platelet count ≥100,000/mL), normal hepatic (bilirubin ≤1.25 upper limit of normal [ULN] and hepatic transaminase ≤2.5 ULN), and renal (serum creatinine <1.5 mg/dL) functions; and an estimated life expectancy of at least 3 months. Patients with brain metastasis were also eligible if they were treated with radiation therapy and were clinically stable. Exclusion criteria were chronic diarrhea of any grade, inflammatory bowel disease, uncontrolled comorbid illness, or other malignancies.
Treatment
Patients were randomized to receive either pemetrexed plus cyclophosphamide or pemetrexed monotherapy, and were stratified according to histology (adenocarcinoma vs. others) and smoking history (current/ex-smokers vs. nonsmokers). Pemetrexed (Alimta, 500 mg/m2) was administered intravenously with concomitant administration of cyclophosphamide (Cytoxan) over 10 min on day 1 of every 21-day cycle (n=30). In the monotherapy arm, only 500 mg/m2 of i.v. pemetrexed was administered (n=32). Cycles were repeated until disease progression or unacceptable toxicity was observed or until the patient declined further treatment. Patients in the pemetrexed arm were instructed to take folic acid (1 mg orally daily) from 1 week before the administration of the first dose of pemetrexed until 3 weeks after the last dose. Intramuscular injection of Vitamin B12 (1 µg) was administered 1 week before the first dose of pemetrexed and was repeated after every 3 cycles.
Target lesions were assessed using computed tomography (CT) and magnetic resonance imaging (MRI). Tumor response and disease progression were assessed according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0. Patients were assessed for response and disease progression by investigators, together with input from radiologists at each center, and assessed independently by independent review committee (IRC). Adverse events (AEs) were classified according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 3.0. Patient’s QoL and symptoms were assessed every 6 weeks using Functional Assessment of Cancer Therapy-Lung (FACT-L) questionnaire, Lung Cancer Subscale (LCS), and Trial Outcome Index (TOI). Primary endpoint was IRC-assessed PFS. Secondary endpoints included investigator-assessed PFS, 4-month and 6-month PFS rates, overall survival (OS), ORR, disease control rate (DCR), QoL and safety.
Dose modification
Dose adjustments to new cycles were based on the consideration of the worst toxicity observed during the previous cycle. Treatment interruptions up to 14 days were allowed for recovery from adverse events. Pemetrexed treatment was started only when the neutrophil count was ≥1000/mL, the platelet count was ≥75,000/mL, and nonhematologic toxicities were grade ≤1. Pemetrexed doses were reduced by 25% in patients with grade ≥4 neutropenia, febrile neutropenia, grade ≥3 thrombocytopenia, or grade ≥3 nonhematologic toxicity, excluding nausea, vomiting, and alopecia. Patients who experienced grade ≥3 hypersensitivity reactions or required two successive dose reductions were withdrawn from the study.
Evaluation
The baseline evaluation included detailed medical history, physical examination, complete blood counts, blood chemistries, and imaging for tumor assessment. These evaluations were also made before each treatment cycle. Tumor responses were classified according to RECIST ver. 1.1. The response was evaluated based on the findings from the CT scan of the chest and upper abdomen. Clinical responses were assessed after every 2 cycles or earlier in case of clinical deterioration. The toxic effects were assessed according to the National Cancer Institute Common Toxicity Criteria version 3.0.
All enrolled patients were investigated for wild-type EGFR in exons 18-21, as tested in tumors by sequencing. For EGFR gene analysis, genomic DNA was extracted from paraffin-embedded tumor tissues and amplified using polymerase chain reaction (PCR). EGFR mutational status was examined in exons 18-21 through direct sequencing.
Statistical analysis
The primary endpoint of this study was PFS rate at 6 months, and secondary endpoints were PFS, toxicity, response rate (RR), and (OS). Statistical significance was accepted for P-values of <0.05. All analyses were performed using SPSS version 19.0 (SPSS Inc., Chicago, IL). Survival was estimated using Kaplan-Meier method and was presented as a median value with a range and a two-sided 95% CI. A two-sided log-rank test was the main method used to compare survival between two arms. The estimate of the treatment effect was expressed as a hazard ratio (HR) of pemetrexed plus cyclophosphamide versus pemetrexed monotherapy, with a two-sided 95% CI. ORR was analyzed using χ 2 test. Pre-planned subgroup analyses of PFS were performed using Cox proportional hazards model. QoL scores were calculated using logistic regression analysis.
Results
Patient characteristics
A total of 62 patients, enrolled between March 2011 and December 2013, were randomly assigned to the pemetrexed plus cyclophosphamide arm (n=30) or the pemetrexed monotherapy arm (n=32) (Figure 1). A summary of patient baseline characteristics is shown in Table 1. According to patient characteristics, 87.09% were male, 24% had squamous cell carcinoma, and 98% had wild-type EGFR status. The treatment groups were generally well balanced for baseline characteristics, except that the pemetrexed monotherapy group included a higher percentage of patients with pleural metastasis (50% vs. 29%), wild-type EGFR (58% vs. 38%), and no response to previous chemotherapy (42% vs. 33%) than the pemetrexed plus cyclophosphamide group.
Figure 1.
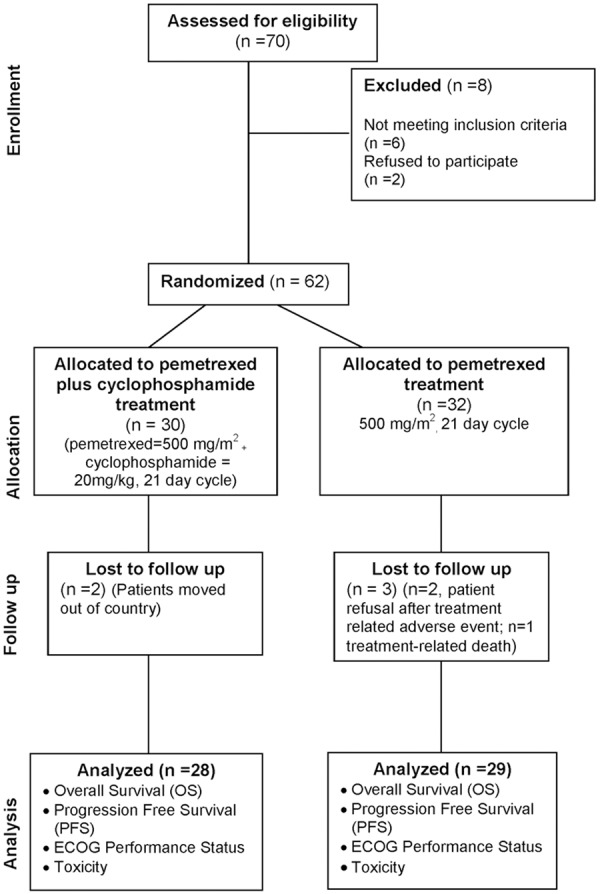
CONSORT diagram showing the flow of participants through each stage of a randomized trial.
Table 1.
Baseline patient characteristics
| Characteristics | Pemetrexed + cyclophosphamide (n=30) | Pemetrexed (n=32) | P-values |
|---|---|---|---|
| Gender | 0.77 | ||
| Male | 26 | 28 | |
| Female | 4 | 4 | |
| Median age (years) | 56 (31-81) | 42 (32-52) | |
| ≥60 | 8 | 9 | 0.26 |
| Performance status | 0.94 | ||
| 0 | 5 (17%) | 5 (16%) | |
| 1 | 13 (43%) | 13 (41%) | |
| 2 | 12 (40%) | 14 (44%) | |
| Smoking status | 0.88 | ||
| Current or ex-smokers | 21 (70%) | 24 (75%) | |
| Nonsmokers | 9 (30%) | 8 (25%) | |
| Pathological subtype | 0.99 | ||
| Adenocarcinoma | 12 (40%) | 14 (44%) | |
| Squamous cell carcinoma | 10 (33%) | 12 (37%) | |
| Large-cell neuroendocrine carcinoma | 8 (27%) | 6 (19%) | |
| Stage at treatment | |||
| IIIB | 3 (10%) | 6 (19%) | |
| IV | 27 (90%) | 26 (81%) | |
| Metastatic sites | 0.59 | ||
| Lung to lung | 21 (70%) | 21 (66%) | |
| Pleura | 16 (54%) | 26 (81%) | |
| Brain | 2 (7%) | 4 (12%) | |
| ≥2 sites | 23 (77%) | 18 (56%) | |
| Treatment sequence | 0.32 | ||
| 3rd line | 13 (43%) | 18 (56%) | 0.57 |
| 2nd line | 12 (40%) | 21 (66%) | 0.80 |
Values are presented as number (%, rounded off).
Efficacy
After a median follow-up period of 30.6 months, 25 patients in the pemetrexed plus cyclophosphamide arm and 29 patients in the pemetrexed monoth erapy arm showed progression. The 6-month PFS rates were 22% (95% CI, 10 to 34) in the pemetrexed plus cyclophosphamide arm and 14% (95% CI, 5 to 25) in the pemetrexed monotherapy arm. The median PFS was 3.55 months (95% CI, 1.4 to 5.7 months) and 2.0 months (95% CI, 1.2 to 2.8 months) in the pemetrexed plus cyclophosphamide arm and the pemetrexed monotherapy arm, respectively (Figure 2). Results of exploratory analyses showed no significant differences in the 6-month PFS rate (P=0.35) and PFS (P=0.71) between the two arms.
Figure 2.
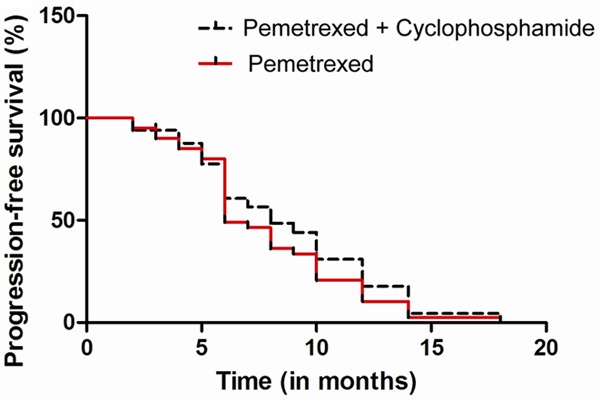
Kaplan-Meier curves for progression-free survival (PFS).
The observed median OS was 9.1 months for both arms, and 1-year survival rates were 44% for pemetrexed plus cyclophosphamide arm and 31% for pemetrexed monotherapy (Figure 3). For all patients, the results of a multivariable analysis showed that ECOG performance status (PS) 0 to 1 (HR, 0.43; 95% CI, 0.26 to 0.71) and adenocarcinoma (HR, 0.59; 95% CI, 0.35 to 0.98) were independent prognostic factors for longer OS. The response could not be assessed in five patients: two in the pemetrexed plus cyclophosphamide arm (patient refusal after the first cycle and follow-up loss) and three in the pemetrexed monotherapy arm (patient refusal after the first cycle and treatment-related death). The disease control rates for pemetrexed plus cyclophosphamide and pemetrexed monotherapy arms were 45% and 35%, respectively (P=0.36).
Figure 3.
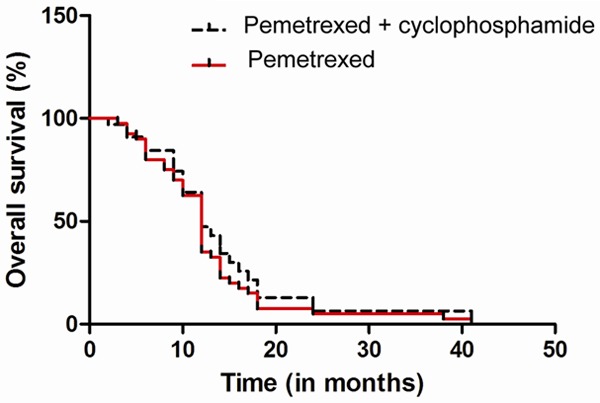
Kaplan-Meier curves for overall survival (OS).
Efficacy analysis according to EGFR mutation status
In 57 patients with wild-type EGFR, a trend for better RR was observed in the pemetrexed plus cyclophosphamide group than in the pemetrexed monotherapy group (39% vs. 9%, P=0.07); the median PFS and median OS were longer in the pemetrexed plus cyclophosphamide arm than in the pemetrexed monotherapy arm, but the differences were not statistically significant (median PFS: 6.6 months vs. 3.1 months, P=0.45; median OS: 34.3 months vs. 14.8 months, P=0.62). In patients with unknown EGFR mutation status, similar efficacy outcomes were observed between pemetrexed plus cyclophosphamide and pemetrexed monotherapy arms (median PFS: 3.0 months vs. 1.8 months, P=0.55; median OS: 6.9 months vs. 6 months, P=0.80; RR: 0% vs. 8%, P=0.17).
Toxicity
All patients were assessed for toxicity. A list of treatment-related hematologic and nonhematologic toxicities is shown in Table 2. The reasons for treatment discontinuation were disease progression (61% for pemetrexed plus cyclophosphamide arm and 83% for pemetrexed monotherapy arm), adverse events (9% and 10%, respectively), and follow-up loss (2% and 0%, respectively). The most common adverse events were anemia (51%) and fatigue (45%) in the pemetrexed plus cyclophosphamide arm and skin rash (49%) and anorexia (38%) in the pemetrexed monotherapy arm. Grade 3 or 4 adverse events occurred in 28% of patients in the pemetrexed plus cyclophosphamide arm and 19% in the pemetrexed monotherapy arm. There was 1 treatment-related death resulting from pneumonia in each arm. Interstitial lung disease was noted in 2 patients who received pemetrexed monotherapy.
Table 2.
Adverse events
| Adverse events | Pemetrexed + cyclophosphamide (n=30) | Pemetrexed monotherapy (n=32) | ||
|---|---|---|---|---|
|
| ||||
| All grade (%) | Grade 3 to 4 (%) | All grades (%) | Grade 3 to 4 (%) | |
| Hematologic toxicity | ||||
| Anemia | 12 (40%) | 2 (7%) | 10 (31%) | 1 (3%) |
| Leukocytopenia | 2 (6%) | - | - | - |
| Neutropenia | 3 (10%) | - | - | - |
| Thrombocytopenia | 3 (10%) | 1 (3%) | - | - |
| Nonhematologic toxicity | ||||
| Skin rash | 5 (17%) | - | 19 (59%) | - |
| Fatigue | 11 (36.6%) | - | 14 (44%) | - |
| Anorexia | 6 (20%) | - | 10 (31%) | - |
| Nausea | 10 (33%) | 1 (3%) | 18 (56%) | 1 (3%) |
| Vomiting | 4 (13%) | - | 7 (22%) | - |
| Stomatitis | 3 (10%) | - | 9 (28%) | - |
| Constipation | 2 (7%) | 4 (12%) | - | |
| Diarrhea | 4 (13%) | - | 8 (25%) | - |
| Infection | 2 (7%) | - | 2 (6%) | - |
| Edema | 2(7%) | - | 3 (9%) | - |
Values are presented as number (%, rounded off).
Discussion
To our knowledge, this is the first head-to-head study designed to explore the efficacy and safety of pemetrexed plus cyclophosphamide versus pemetrexed monotherapy as second-line treatment for patients with advanced non-squamous NSCLC harboring wild-type EGFR. In this study, patients showed significant improvements in PFS and DCR with pemetrexed in combination with cyclophosphamide compared with pemetrexed monotherapy, which was confirmed by IRC. Therefore, the data analysis was validated. There was also a trend towards improved OS in the pemetrexed plus cyclophosphamide arm, which may have achieved statistical significance given a sufficient sample size.
Both regimens demonstrated modest activity as a second-line treatment with a median PFS of 2.0 months and a median OS of 8.5 months in each arm. Both the regimens were generally well-tolerated and related toxicities were mild. The results of exploratory analyses for comparison of clinical outcomes between the two arms showed no significant difference in efficacy between pemetrexed plus cyclophosphamide and pemetrexed monotherapy in overall population and in patients with wild-type EGFR tumors. For patients with EGFR activating mutations, EGFR-TKIs are the favored second-line therapy if not used in the first-line setting [11,12]. However, the role of EGFR-TKIs as the second-line treatment of patients with wild-type EGFR or unknown EGFR status remains controversial. Both EGFR-TKIs and pemetrexed monotherapy are currently used in East Asia as the standard second-line treatment for advanced non-squamous NSCLC. Previously, two randomized trials compared EGFR-TKIs with pemetrexed monotherapy in a second-line treatment setting. The aim of the phase III KCSGLU08-01 study (n=135) was to compare pemetrexed monotherapy with gefitinib in a clinically selected population (nonsmoker Korean patients with pulmonary adenocarcinoma) [13].
Of all the randomized trials comparing EGFR-TKIs with second-line chemotherapy [13,15-17], CTONG0806 was the first study that was specifically designed to address the role of EGFR mutation as a predictive marker. In April 2014, 2 meta-analyses of EGFR-TKIs versus chemotherapy in wild-type EGFR NSCLC achieved same conclusion that chemotherapy significantly improved PFS but not OS [16,17]. CTONG0806 was the only trial that initially enrolled patients with wild-type EGFR. The other trials enrolled unselected patients and the conclusion about patients with wild-type EGFR was only from subgroup analysis.
These findings suggest that the exact mutational status should be determined in order to guide rational decision making for the second-line treatment. In the current study, no significant difference of efficacy was observed between pemetrexed plus cyclophosphamide and pemetrexed monotherapy among patients with wild-type EGFR. This observation may be explained by the small number of patients in our study who were confirmed to have wild-type EGFR tumors. In addition, we used direct sequencing for EGFR mutation analysis. Our study has some limitations. First, it was conducted at a single institution and in a small study population using an initial noncomparative design, which reduced the accuracy of comparison between the two treatment arms. Second, our study was initiated before the interaction between pemetrexed efficacy and histology was detected; therefore, patients with squamous cell carcinoma (20%) were included in the analyses, which could have biased the results. Although the results of a subgroup analysis of nonsquamous patients showed no significant differences in PFS (P=0.66) and OS (P=0.84) between the two arms, our results clearly demonstrate that pemetrexed in combination with cyclophosphamide is better than pemetrexed monotherapy.
The present study showed that pemetrexed in combination with low dose cyclophosphamide may be a better treatment approach than pemetrexed monotherapy when considering second-line treatment for EGFR-positive NSCLC.
Acknowledgements
This work was partly supported by research grants from the Central Hospital of Zibo, China.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Azzoli CG, Temin S, Aliff T, Baker S Jr, Brahmer J, Johnson DH, Laskin JL, Masters G, Milton D, Nordquist L, Pao W, Pfister DG, Piantadosi S, Schiller JH, Smith R, Smith TJ, Strawn JR, Trent D, Giaccone G. Focused Update of 2009 American Society of Clinical Oncology Clinical Practice Guideline Update on Chemotherapy for Stage IV Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2011;29:3825–3831. doi: 10.1200/JCO.2010.34.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, Nishiwaki Y, Ohe Y, Yang JJ, Chewaskulyong B, Jiang H, Duffield EL, Watkins CL, Armour AA, Fukuoka M. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 4.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Ogura T, Ando M, Miyazawa H, Tanaka T, Saijo Y, Hagiwara K, Morita S, Nukiwa T. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 5.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, Zhang S, Wang J, Zhou S, Ren S, Lu S, Zhang L, Hu C, Hu C, Luo Y, Chen L, Ye M, Huang J, Zhi X, Zhang Y, Xiu Q, Ma J, Zhang L, You C. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, openlabel, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 6.Joslin CA, Kunkler PB, Evans IH, Jones V, Wong K. Cyclophosphamide in the management of advanced breast cancer. Proc R Soc Med. 1970;63:81–84. [PMC free article] [PubMed] [Google Scholar]
- 7.Schilsky RL. Biochemical pharmacology of chemotherapeutic drugs used as radiation enhancers. Semin Oncol. 1992;19:2–7. [PubMed] [Google Scholar]
- 8.Adjei AA. Pemetrexed (Alimta): a novel multitargeted antifolate agent. Expert Rev Anticancer Ther. 2003;3:145–156. doi: 10.1586/14737140.3.2.145. [DOI] [PubMed] [Google Scholar]
- 9.Hwang KE, Kim YS, Hwang YR, Kwon SJ, Park DS, Cha BK, Kim BR, Yoon KH, Jeong ET, Kim HR. Pemetrexed induces apoptosis in malignant mesothelioma and lung cancer cells through activation of reactive oxygen species and inhibition of sirtuin 1. Oncol Rep. 2015;33:2411–2419. doi: 10.3892/or.2015.3830. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi T, Nakanishi T, Hayashi M, Uozu S, Okamura T, Morishita M, Takeyama T, Minezawa T, Morikawa S, Niwa Y, Mieno Y, Kato A, Hoshino T, Isogai S, Okazawa M, Imaizumi K. Efficacy and safety of cisplatin plus pemetrexed as a first-line treatment for Japanese patients with advanced non-squamous non-small-cell lung cancer-a retrospective analysis. Gan To Kagaku Ryoho. 2015;42:183–187. [PubMed] [Google Scholar]
- 11.Douillard JY, Shepherd FA, Hirsh V, Mok T, Socinski MA, Gervais R, Liao ML, Bischoff H, Reck M, Sellers MV, Watkins CL, Speake G, Armour AA, Kim ES. Molecular predictors of outcome with gefitinib and docetaxel in previously treated non-small-cell lung cancer: data from the randomized phase III INTEREST trial. J. Clin. Oncol. 2010;28:744–752. doi: 10.1200/JCO.2009.24.3030. [DOI] [PubMed] [Google Scholar]
- 12.Lee CK, Brown C, Gralla RJ, Hirsh V, Thongprasert S, Tsai CM, Tan EH, Ho JC, Chu dT, Zaatar A, Osorio Sanchez JA, Vu VV, Au JS, Inoue A, Lee SM, Gebski V, Yang JC. Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst. 2013;105:595–605. doi: 10.1093/jnci/djt072. [DOI] [PubMed] [Google Scholar]
- 13.Sun JM, Lee KH, Kim SW, Lee DH, Min YJ, Yun HJ, Kim HK, Song HS, Kim YH, Kim BS, Hwang IG, Lee K, Jo SJ, Lee JW, Ahn JS, Park K, Ahn MJ. Gefitinib versus pemetrexed as second-line treatment in patients with nonsmall cell lung cancer previously treated with platinum-based chemotherapy (KCSG-LU08-01): an open-label, phase 3 trial. Cancer. 2012;118:6234–6242. doi: 10.1002/cncr.27630. [DOI] [PubMed] [Google Scholar]
- 14.Fleming RA. An overview of cyclophosphamide and ifosfamide pharmacology. Pharmacotherapy. 1997;17:146S–154S. [PubMed] [Google Scholar]
- 15.Hayakawa K. [Revised by the Japan lung cancer society: guideline for diagnosis and treatment of lung cancer] . Gan To Kagaku Ryoho. 2011;38:1273–1276. [PubMed] [Google Scholar]
- 16.Ciuleanu T, Stelmakh L, Cicenas S, Miliauskas S, Grigorescu AC, Hillenbach C, Johannsdottir HK, Klughammer B, Gonzalez EE. Efficacy and safety of erlotinib versus chemotherapy in second-line treatment of patients with advanced, non-small-cell lung cancer with poor prognosis (TITAN): a randomised multicentre, open-label, phase 3 study. Lancet Oncol. 2012;13:300–308. doi: 10.1016/S1470-2045(11)70385-0. [DOI] [PubMed] [Google Scholar]
- 17.Lee DH, Park K, Kim JH, Lee JS, Shin SW, Kang JH, Ahn MJ, Ahn JS, Suh C, Kim SW. Randomized Phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinumbased chemotherapy. Clin Cancer Res. 2010;16:1307–1314. doi: 10.1158/1078-0432.CCR-09-1903. [DOI] [PubMed] [Google Scholar]


