Abstract
Lung cancer is the leading cause of cancer-related death in both men and women and consists of different histological types. Histopathological examination and accurate subtype diagnosis has become increasingly important in guiding patient management and, as such, is the most important currently available lung cancer “biomarker”. In this study, we examined the expression of PAX2 and PAX5 by immunohistochemistry in 47 cases of lung cancer and 13 cases of pneumonia. The results demonstrated that PAX2 were detected in 82.8% (24/29) of NSCLC, 0% (0/18) of SCLC and 7.7% (1/13) of pneumonia, respectively; However, PAX5 were detected in 15/18 cases (83.3%) of SCLC, 6.8% (2/29) of NSCLC and 7.7% (1/13) of pneumonia. Further, the samples with lymphatic metastasis had remarkable higher positive PAX2 or PAX5 than that without metastases. Overall, our data indicated that PAX2 and PAX5 differentially expressed in NSCLC and SCLC. Thus, PAX2 and PAX5 are useful biomarker in the differential diagnosis of lung cancer.
Keywords: Lung cancer, immunohistochemistry, PAX2, PAX5, biomarker
Introduction
Lung cancer is the leading cause of cancer death in both men and women worldwide. WHO classifies lung cancer into two broad histological subtypes: non-small-cell lung cancer (NSCLC) and small-cell lung cancer (SCLC). NSCLC could be further subdivided into adenocarcinoma, squamous cell carcinoma, and large cell carcinoma. The overall five year survival of NSCLC, which accounts for 85% of all lung cancers, is 16%. Whereas, SCLC comprises 14% of all lung cancers and the prognosis is only 6% over five years. Even with the current therapeutic agents, the response is poor in a majority of patients [1]. In order to further identify potential therapeutic targets in lung cancer, we examined the expression profile and the role of PAX2 and PAX5 in lung cancer.
PAX (Paired Box) genes are a family of nine nuclear transcription factors that play a crucial role in various developmental programs. Although all the nine PAX genes are expressed during various stages of embryogenesis and development in humans, most of the PAX genes are silent in adults. However, they become selectively active during tissue repair and regeneration. Interestingly, several PAX genes have been reported to be expressed in various cancers and likely to contribute to tumorigenesis. Usually, expressions of PAX genes in cancers appear to be related to tissue lineage thereby suggesting a process of de-differentiation [2].
Although these nine transcription factors control organs’ development, PAX2 is known to control the development of the central nervous system, thyroid gland, kidney, eye, and others [3-5]. Specifically, PAX2 has been most intensively studied in the kidney where it has been shown to play a crucial role in renal development [6]. Recently, the expression of PAX2 has also been reported in the female genital tract [7-9]. These transcription factors may reexpress in an organ-specific manner during neoplastic transformation [10]. PAX2 is abundantly expressed in renal blastemal cells during nephrogenesis, but are noted in only a few renal parenchymal cells in mature kidney, however, then are identified again in renal cell carcinoma [11-14]. Tissue expression of PAX transcription factors thus have been used as specific markers for tumor diagnosis. Indeed, several groups have reported the diagnostic utility of PAX2 immunohistochemistry in identifying primary and metastatic renal tumors [15-17].
PAX5 is essential for B cell development and its expression has been noted at all stages of B cell development except in the terminally differentiated plasma cells. PAX5 knockout mice predictably lack B cells and thereby any humoral immunity [18]. Significant PAX5 expression has been noted in most of the B cell lymphomas but the T and null- cell lymphomas, as well as multiple myeloma, and plasmacytomas lack PAX5 expression [19]. Deregulated expression of PAX5 has also been noted in pediatric cancers such as medulloblastomas and its expression in normal cells is inversely correlated with neuronal differentiation [20]. Most importantly, significant PAX5 expression has been noted in tumors of neuroendocrine origin such as neuroblastoma and SCLC [21]. PAX5 was found to be overexpressed in aggressive neuroblastoma, N-type, as opposed to the less aggressive S-type. A similar scenario has been reported with respect to highly metastatic SCLC cell lines. Significant amounts of PAX5 transcripts were found to be present in several SCLC cell lines but not in NSCLC cell lines [21]. PAX5 is believed to not only support cancer cell survival but also contribute to metastasis.
Several studies, each of which is devoted exclusively to either PAX2 or PAX5, have documented their expression in lung cancer [22,23]. Nevertheless, there are no reports to date that examine the role of PAX2 and PAX5 in the differential diagnosis of NSCLC and SCLC, we sought to investigate diagnostic utility of PAX2 and PAX5 in lung cancer.
Materials and methods
Patients and tissue samples
60 patients included in the study were from the First Affiliated Hospital of Zhengzhou University (Zhengzhou, Henan, PR China) from December 2014 to May 2015. Patients who had preoperative diagnosis and did not receive preoperative chemoradiation treatment were selected for this study on the basis of the availability of archived paraffin-embedded tissue blocks for immunohistochemical analysis. Ethical approval was obtained from the hospital and fully informed consent from all patients before sample collection. Tumor histological type was based on currently used histopathological criteria.
Hematoxylin and eosin (HE) staining and immunohistochemical analysis
The study group consisted of 36 men and 11 women with lung cancer and the control group consisted of 9 men and 4 women with pneumonia. The tissues were processed routinely stained with hematoxylin and eosin. The histological characteristics were reviewed by two pathologists. The PAX2 and PAX5 levels in the formalin-fixed, paraffin-embedded tissue sections were measured by immunohistochemical analysis, as previously described [24]. Briefly, the specimens were sectioned (4 μm thick), mounted on Superfrost/Plus slides (Fisher Scientific, Pittsburgh, PA), and then deparaffinized in two xylene and rehydrated through graded alcohols to distilled water. For antigen retrieval, the slides were boiled in a pressure cooker at maximum heat for 3 minutes containing 0.01 mol/L sodium citrate (pH 6.0) and cooling for 30 minutes at room temperature. Endogenous peroxidase activity was quenched in 0.3% H2O2 for 8 minutes at room temperature. The slides were washed 3 times in PBS for 2 minutes before being incubated with the primary antibodies PAX2 (Abcam, ab79389, Hongkong) and PAX5 (Abcam, ab109443, Hongkong) at 1:100 dilution for 1 hour at 37°C. The sections were incubated with a ready-to-use secondary antibody kit (DAKO Corp) for 30 minutes at room temperature and the chromogenic substrate 3,3-diaminobenzidine tetrahydro-chloride (DAB). The slides were then washed in distilled water, counterstained with hematoxylin, dehydrated, and mounted with permanent media. Expression levels of PAX2 and PAX5 were evaluated by counting at least 500 tumor cells in representative high-power fields. Tumor cells were considered positive for PAX2 and PAX5 when nuclear or cytoplasmic staining was present. For each tumor, we determined a proportion score. The proportion score represented the estimated fraction of positively stained cells (-, <10%; +, >10%).
Statistical analysis
Statistical analysis for the results was analyzed using Mann-Whitney test when only two groups, or Kruskal-Wallis test when more than two groups. Chi-Square test was used to compare the expression of PAX2 and PAX5 with various clinicopathologic variables. Differences between groups were stated to be statistically significant when P<0.05. All computations were carried out with SPSS 16.0 (SPSS, Chicago, IL, USA).
Results
Patient characteristics and demographics
To analyze the PAX2 and PAX5 expression patterns, we used 47 lung cancer tissue samples (mean age, 61; range, 35-78 years) and 13 pneumonia tissue samples (mean age, 56; range, 25-75 years). HE staining of the patients’ tissue is showed in Figure 1 and the clinicopathologic characteristics of the patients are summarized in Table 1.
Figure 1.
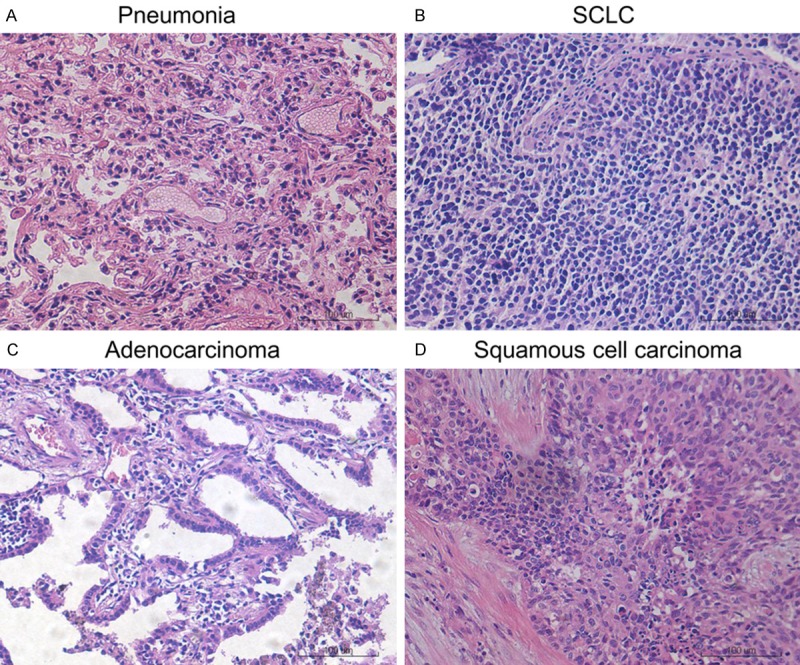
HE staining of pneumonia and lung cancer tissue. A. Pneumonia. B. SCLC. C. Adenocarcinoma. D. Squamous cell carcinoma.
Table 1.
Expression of PAX2 and PAX5 in patients
| Characteristics | No. patients | PAX2 positive | P value | PAX5 positive | P value |
|---|---|---|---|---|---|
| Gender | |||||
| M | 46 | 41.3% (19/46) | 0.918 | 26.1% (12/46) | 0.231 |
| F | 14 | 42.9% (6/14) | 42.9% (6/14) | ||
| Age (Y) | |||||
| ≥60 | 34 | 44.1% (15/34) | 0.660 | 26.5% (9/34) | 0.495 |
| <60 | 26 | 38.5% (10/26) | 34.6% (9/26) | ||
| Tissue type | |||||
| NSCLC | 29 | 82.8% (24/29) | 6.8% (2/29) | <0.01 | |
| SCLC | 18 | 0% (0/18) | <0.01 | 83.3% (15/18) | |
| Pneumonia | 13 | 7.7% (1/13) | 7.7% (1/13) | ||
| Smoking | |||||
| Yes | 37 | 43.2% (16/37) | 0.753 | 24.3% (9/37) | 0.224 |
| No | 23 | 39.1% (9/23) | 39.1% (9/23) |
PAX2 and PAX5 expression patterns in tissue samples from patients with lung cancer and benign pneumocyte hyperplasia
Immunohistochemical analysis of lung tissue samples showed that PAX2 was expressed in 82.8% (24/29) NSCLC (Table 2). In contrast, there was no PAX2 expression in SCLC (0/18), and only 1 of 13 pneumonia tissue had positive PAX2 staining (Figure 2). The data suggest that the PAX2 expression in NSCLC was much higher than those in SCLC and pneumonia tissue.
Table 2.
Expression of PAX2 in NSCLC
| Characteristics | No. patients | PAX2 positive | P value |
|---|---|---|---|
| Gender | |||
| M | 23 | 78.3% (18/23) | 0.553 |
| F | 6 | 100% (6/6) | |
| Age (Y) | |||
| ≥60 | 18 | 88.9% (16/18) | 0.339 |
| <60 | 11 | 72.7% (8/11) | |
| Histologic type | |||
| Adenocarcinoma | 12 | 91.7% (11/12) | 0.370 |
| Squamous cellcarcinoma | 17 | 76.5% (13/17) | |
| Smoking | |||
| Yes | 19 | 84.2% (16/19) | 1.000 |
| No | 10 | 80% (8/10) | |
| Lymph node metastasis | |||
| Positive | 3 | 33.3% (1/3) | 0.068 |
| Negative | 26 | 88.5% (23/26) | |
| Stage | |||
| I+II | 16 | 75% (12/16) | 0.343 |
| III+IV | 13 | 92.3% (12/13) |
Figure 2.
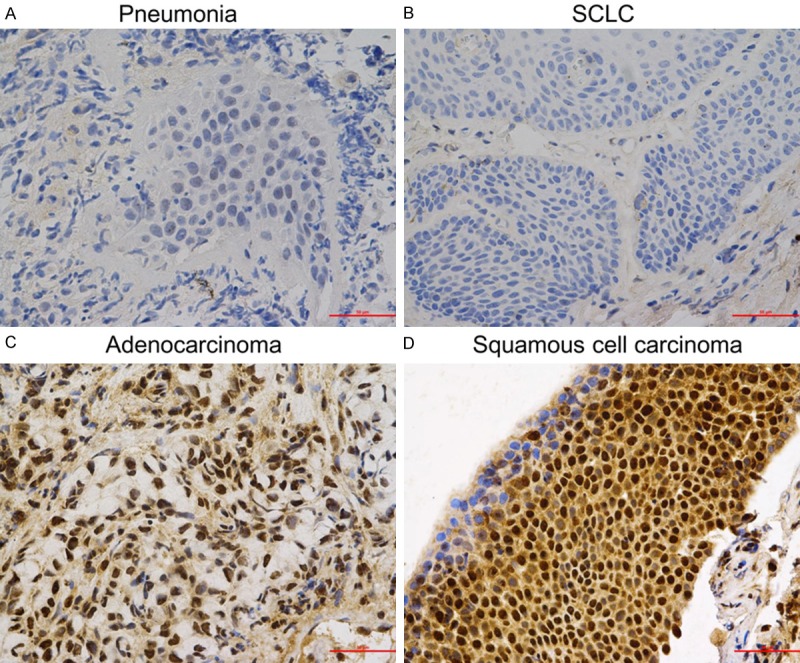
Expression patterns of PAX2 in pneumonia and lung cancer tissue. A. Pneumonia. B. SCLC. C. Adenocarcinoma. D. Squamous cell carcinoma.
In contrast, immunohistochemical analysis showed PAX5 expressed major in SCLC. PAX5 expression was found in 15/18 cases (83.3%) of SCLC (Table 3), but in most cases of NSCLC, the expression of PAX5 was negative, there was only 2 cases (6.8%) in NSCLC and only 1 case in pneumonia tissue (7.7%) were positive for PAX5 (Figure 3). These data indicated that the PAX5 expression in SCLC was much higher than those in NSCLC and pneumonia.
Table 3.
Expression of PAX5 in SCLC
| Characteristics | No. patients | PAX5 positive | P value |
|---|---|---|---|
| Gender | |||
| M | 13 | 76.9% (10/13) | 0.552 |
| F | 5 | 100% (5/5) | |
| Age (Y) | |||
| ≥60 | 11 | 90.9% (10/11) | 0.528 |
| <60 | 7 | 71.4% (5/7) | |
| Tissue type | |||
| Localized type | 9 | 88.9% (8/9) | 1.000 |
| Diffused type | 9 | 77.8% (7/9) | |
| Smoking | |||
| Yes | 10 | 80% (8/10) | 1.000 |
| No | 8 | 87.5% (7/8) | |
| Lymph node metastasis | |||
| Positive | 2 | 0% (0/2) | 0.020 |
| Negative | 16 | 93.8% (15/16) |
Figure 3.

Expression patterns of PAX5 in pneumonia and lung cancer tissue. A. Pneumonia. B. SCLC. C. Adenocarcinoma. D. Squamous cell carcinoma.
Correlation of PAX2 and PAX5 expression with clinic characteristics
In order to examine correlation between PAX2/PAX5 and clinic characteristics, we also analyzed the expression of PAX2 and PAX5 with various clinicopathologic variables. For the PAX2 expression in NSCLC, the samples with lymphatic metastasis had remarkable higher positive PAX2 than that without metastases, however, there were no statistic difference (P=0.068) between two groups. It could attribute to a relatively small number of patients. In contrast, for the PAX5 expression in SCLC, the positivity of PAX5 in patients with lymphatic metastasis was much higher than that without metastases (P=0.02). Our results, however, found no significant correlation between PAX2/PAX5 expression and sex, age and histopathological grading of the tumors (Tables 1, 2 and 3).
Discussion
The main purpose of this study was to understand PAX2 and PAX5 expression in various lung cancer tissues and to thus provide a scientific basis for the improvement of lung cancer diagnosis, treatment, and prognosis in the future. The highly specific PAX5 expression in SCLC may be associated with lymphatic metastasis.
Our previous studies demonstrated that PAX2 and PAX8 reliably distinguishes ovarian serous tumors from mucinous tumors [24]. Other researchers have also evaluated the potential utility of PAX2 for assisting in discriminating between papillary serous carcinoma and peritoneal mesothelioma [8,25]. It was reported PAX2 expression in 24 (67%) of 36 papillary serous carcinomas, whereas only 2 (12%) of 17 peritoneal mesotheliomas in women and none of 37 in men included in the investigation were positive for this marker [8]. Besides, another group [25] reported PAX2 expression in 21 (61%) of serous carcinomas, whereas all 25 mesotheliomas investigated were negative for this marker. These results suggest that PAX2 could be a useful marker for assisting in the differential diagnosis between these malignancies. We sought out as certain the sensitivity and specificity of PAX2 and PAX5 as a marker for lung cancer. The results of this study confirmed that PAX2 (82.8%) was specifically expressed in the NSCLC tissue but not in SCLC or benign pneumocyte hyperplasia tissue.
PAX5 is a transcription factor essential for B cell development, and is widely used in hematopathology practice as a specific marker to recognize B cell lineage. It was shown recently that PAX5 was also expressed in neuroendocrine tumors of the lung, especially SCLC and LCNEC [26], which is similar to the results of the present study. We discovered that PAX5 was expressed in the majority of SCLC patients (83.3%) but in only 2 of 29 NSCLC patients, suggesting that PAX2 and PAX5 may serve as a useful immunohistochemical marker for assisting in the differential diagnosis of NSCLC and SCLC. In addition, we found that either PAX2 or PAX5 was expressed in only 1 of 13 benign pneumocyte hyperplasia. Our findings suggest that PAX2- and PAX5- targeted treatment strategy may have high therapeutic efficacy for NSCLC and SCLC. The above findings may have some clinical value for the differential diagnosis of NSCLC and SCLC, and the detection of PAX2 and PAX5 in lung cancer tissues may contribute to early diagnosis.
In a previous study [27], most remarkably, the expression of PAX5 in neuroendocrine tumors seems to parallel the level of tumor aggressiveness. In the present study, a significantly higher percentage of lymphatic metastasis SCLC patients had PAX5 positive cells compared with those with no-metastasis SCLC. This finding is in accordance with the findings reported by other group [21], who reported that PAX5 is expressed in aggressive N-type neuroblastoma cell lines, whereas no expression was detected in S-type cells. The authors also reported that enforced overexpression of PAX5 in the S-type cell line CA-2E restored several malignant properties, in particular anchorage-independent growth. In contrast, down-regulation of PAX5 in several N-type cell lines significantly reduced their proliferation rate. It is speculated that reexpressed PAX genes promote tumor development and progression by increasing proliferation and motility while inhibiting apoptosis [28]. A similar scenario has been reported with respect to highly metastatic SCLC cell lines. Significant PAX5 transcripts were found to be present in several SCLC cell lines but not in NSCLC cell lines [21]. Since enforced expression of PAX5 in neuroblastoma S-type cells confers on them a more oncogenic phenotype while PAX5 knockdown results insignificant loss in cell viability, PAX5 is believed to not only support cancer cell survival but also contribute to metastasis.
In this study, we investigated the distribution of PAX2 and PAX5 in lung cancer tissues. However, our immunohistochemical results showed that the percentage of patients with PAX2 or PAX5 positive cells has no significant correlation with the tumor stage or tumor grade in NSCLC and SCLC, the clinical significance and value of PAX2 and PAX5 expression remain to be clarified and require further in-depth investigation and evaluation. For example, this study revealed that more than half of NSCLC patients expressed PAX2 in tumor cells but did not investigate the underlying mechanisms and clinical significance of this result. Moreover, the causes and clinical significance of the observation that there was a significant difference in PAX2 and PAX5 expression between SCLC and NSCLC tissues needs to be further explored in future studies.
In summary, we have shown that PAX2 and PAX5 differential expressed in lung cancer, indicating that PAX2 and PAX5 can serve as a useful immunohistochemical marker for assisting in the differential diagnosis of NSCLC and SCL.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81372816) and the Fundamental Research Funds for the Central Universities (JUSRP115A31) to YP.
Disclosure of conflict of interest
None.
References
- 1.Abidoye O, Ferguson MK, Salgia R. Lung carcinoma in African Americans. Nat Clin Pract Oncol. 2007;4:118–129. doi: 10.1038/ncponc0718. [DOI] [PubMed] [Google Scholar]
- 2.Robson EJ, He SJ, Eccles MR. A PANorama of PAX genes in cancer and development. Nat Rev Cancer. 2006;6:52–62. doi: 10.1038/nrc1778. [DOI] [PubMed] [Google Scholar]
- 3.Bouchard M, Souabni A, Mandler M, Neubuser A, Busslinger M. Nephric lineage specification by Pax2 and Pax8. Genes Dev. 2002;16:2958–2970. doi: 10.1101/gad.240102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grote D, Souabni A, Busslinger M, Bouchard M. Pax 2/8-regulated Gata 3 expression is necessary for morphogenesis and guidance of the nephric duct in the developing kidney. Development. 2006;133:53–61. doi: 10.1242/dev.02184. [DOI] [PubMed] [Google Scholar]
- 5.Narlis M, Grote D, Gaitan Y, Boualia SK, Bouchard M. Pax2 and pax8 regulate branching morphogenesis and nephron differentiation in the developing kidney. J Am Soc Nephrol. 2007;18:1121–1129. doi: 10.1681/ASN.2006070739. [DOI] [PubMed] [Google Scholar]
- 6.Dressler GR. The specification and maintenance of renal cell types by epigenetic factors. Organogenesis. 2009;5:73–82. doi: 10.4161/org.5.2.8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roh MH, Kindelberger D, Crum CP. Serous tubal intraepithelial carcinoma and the dominant ovarian mass: clues to serous tumor origin? Am J Surg Pathol. 2009;33:376–383. doi: 10.1097/PAS.0b013e3181868904. [DOI] [PubMed] [Google Scholar]
- 8.Tong GX, Chiriboga L, Hamele-Bena D, Borczuk AC. Expression of PAX2 in papillary serous carcinoma of the ovary: immunohistochemical evidence of fallopian tube or secondary Mullerian system origin? Mod Pathol. 2007;20:856–863. doi: 10.1038/modpathol.3800827. [DOI] [PubMed] [Google Scholar]
- 9.Tung CS, Mok SC, Tsang YT, Zu Z, Song H, Liu J, Deavers MT, Malpica A, Wolf JK, Lu KH, Gershenson DM, Wong KK. PAX2 expression in low malignant potential ovarian tumors and lowgrade ovarian serous carcinomas. Mod Pathol. 2009;22:1243–1250. doi: 10.1038/modpathol.2009.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maulbecker CC, Gruss P. The oncogenic potential of Pax genes. EMBO J. 1993;12:2361–2367. doi: 10.1002/j.1460-2075.1993.tb05890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ozcan A, Zhai J, Hamilton C, Shen SS, Ro JY, Krishnan B, Truong LD. PAX-2 in the diagnosis of primary renal tumors: immunohistochemical comparison with renal cell carcinoma marker antigen and kidney-specific cadherin. Am J Clin Pathol. 2009;131:393–404. doi: 10.1309/AJCPM7DW0XFHDHNY. [DOI] [PubMed] [Google Scholar]
- 12.Tong GX, Yu WM, Beaubier NT, Weeden EM, Hamele-Bena D, Mansukhani MM, O’Toole KM. Expression of PAX8 in normal and neoplastic renal tissues: an immunohistochemical study. Mod Pathol. 2009;22:1218–1227. doi: 10.1038/modpathol.2009.88. [DOI] [PubMed] [Google Scholar]
- 13.Sangoi AR, Karamchandani J, Kim J, Pai RK, McKenney JK. The use of immunohistochemistry in the diagnosis of metastatic clear cell renal cell carcinoma: a review of PAX-8, PAX-2, hKIM-1, RCCma, and CD10. Adv Anat Pathol. 2010;17:377–393. doi: 10.1097/PAP.0b013e3181f89400. [DOI] [PubMed] [Google Scholar]
- 14.Truong LD, Shen SS. Immunohistochemical diagnosis of renal neoplasms. Arch Pathol Lab Med. 2011;135:92–109. doi: 10.5858/2010-0478-RAR.1. [DOI] [PubMed] [Google Scholar]
- 15.McKnight R, Cohen C, Siddiqui MT. Utility of paired box gene 8 (PAX8) expression in fluid and fine-needle aspiration cytology: an immunohistochemical study of metastatic ovarian serous carcinoma. Cancer Cytopathol. 2010;118:298–302. doi: 10.1002/cncy.20089. [DOI] [PubMed] [Google Scholar]
- 16.Tong GX, Devaraj K, Hamele-Bena D, Yu WM, Turk A, Chen X, Wright JD, Greenebaum E. Pax8: a marker for carcinoma of Mullerian origin in serous effusions. Diagn Cytopathol. 2011;39:567–574. doi: 10.1002/dc.21426. [DOI] [PubMed] [Google Scholar]
- 17.Wiseman W, Michael CW, Roh MH. Diagnostic utility of PAX8 and PAX2 immunohistochemistry in the identification of metastatic Mullerian carcinoma in effusions. Diagn Cytopathol. 2011;39:651–656. doi: 10.1002/dc.21442. [DOI] [PubMed] [Google Scholar]
- 18.Cobaleda C, Schebesta A, Delogu A, Busslinger M. Pax5: the guardian of B cell identity and function. Nat Immunol. 2007;8:463–470. doi: 10.1038/ni1454. [DOI] [PubMed] [Google Scholar]
- 19.Krenacs L, Himmelmann AW, Quintanilla-Martinez L, Fest T, Riva A, Wellmann A, Bagdi E, Kehrl JH, Jaffe ES, Raffeld M. Transcription factor B-cell-specific activator protein (BSAP) is differentially expressed in B cells and in subsets of B-cell lymphomas. Blood. 1998;92:1308–1316. [PubMed] [Google Scholar]
- 20.Kozmik Z, Sure U, Ruedi D, Busslinger M, Aguzzi A. Deregulated expression of PAX5 in medulloblastoma. Proc Natl Acad Sci U S A. 1995;92:5709–5713. doi: 10.1073/pnas.92.12.5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baumann Kubetzko FB, Di Paolo C, Maag C, Meier R, Schafer BW, Betts DR, Stahel RA, Himmelmann A. The PAX5 oncogene is expressed in N-type neuroblastoma cells and increases tumorigenicity of a S-type cell line. Carcinogenesis. 2004;25:1839–1846. doi: 10.1093/carcin/bgh190. [DOI] [PubMed] [Google Scholar]
- 22.Song J, Li M, Tretiakova M, Salgia R, Cagle PT, Husain AN. Expression patterns of PAX5, c-Met, and paxillin in neuroendocrine tumors of the lung. Arch Pathol Lab Med. 2010;134:1702–1705. doi: 10.5858/2009-0664-oar1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muratovska A, Zhou C, He S, Goodyer P, Eccles MR. Paired-Box genes are frequently expressed in cancer and often required for cancer cell survival. Oncogene. 2003;22:7989–7997. doi: 10.1038/sj.onc.1206766. [DOI] [PubMed] [Google Scholar]
- 24.Wang M, Ma H, Pan Y, Xiao W, Li J, Yu J, He J. PAX2 and PAX8 reliably distinguishes ovarian serous tumors from mucinous tumors. Appl Immunohistochem Mol Morphol. 2015;23:280–287. doi: 10.1097/PAI.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 25.Gao FF, Krasinskas AM, Chivukula M. Is PAX2 a reliable marker in differentiating diffuse malignant mesotheliomas of peritoneum from serous carcinomas of mullerian origin? Appl Immunohistochem Mol Morphol. 2012;20:272–276. doi: 10.1097/PAI.0b013e3182366531. [DOI] [PubMed] [Google Scholar]
- 26.Kanteti R, Nallasura V, Loganathan S, Tretiakova M, Kroll T, Krishnaswamy S, Faoro L, Cagle P, Husain AN, Vokes EE, Lang D, Salgia R. PAX5 is expressed in small-cell lung cancer and positively regulates c-Met transcription. Lab Invest. 2009;89:301–314. doi: 10.1038/labinvest.2008.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong HY, Liu W, Cohen P, Mahle CE, Zhang W. B-cell specific activation protein encoded by the PAX-5 gene is commonly expressed in merkel cell carcinoma and small cell carcinomas. Am J Surg Pathol. 2005;29:687–692. doi: 10.1097/01.pas.0000155162.33044.4f. [DOI] [PubMed] [Google Scholar]
- 28.Schafer BW. Emerging roles for PAX transcription factors in cancer biology. Gen Physiol Biophys. 1998;17:211–224. [PubMed] [Google Scholar]


