Abstract
Chemokine receptor CXCR3 has been proved to play an important role in tumorigenesis and tumor progression in many malignancies, but its precise efficacy on gastric cancer (GC) has not been evaluated yet. The present study was aimed to explore the correlation of chemokine receptor CXCR3 with tumor-infiltrating lymphocytes (TILs) and prognosis in advanced gastric cancer (GC). Expression of CXCR3 and CD4+, CD8+ TILs was conducted in 192 advanced GC specimens and 48 corresponding paracancerous tissues by immunohistochemical (IHC) analysis. CXCR3 expression in GC tissues was significantly higher than that in paracancerous tissues (P<0.001) and CD8+, CD4+ TILs infiltration increased with high CXCR3 expression (P=0.032 and P<0.001, respectively). Our study showed significantly lower CXCR3 expression in patients with greater tumor invasion depth and lymph node metastasis compared with patients with lesser tumor invasion depth and without lymph node metastasis (P=0.002 and P=0.001, respectively). Univariate analysis indicated that patients with high CXCR3 expression and high CD8+ TILs infiltration had longer overall survival (OS) (log-rank test, P<0.001 and P=0.002, respectively). Univariate and multivariate analyses indicated that CXCR3 expression was an independent prognostic factor for OS (P=0.002). The present study suggested that CXCR3 expression was upregulated in advanced GC and was associated with increased CD4+, CD8+ TILs infiltration and improved OS. Therefore, CXCR3 overexpression is implicated as a favorable prognostic biomarker in human advanced GC.
Keywords: Gastric cancer, CXCR3, CD8, CD4, prognosis, biomarker
Introduction
Although the incidence of gastric cancer (GC) has been declining for decades, it is still the fourth most common malignancy in the world and is currently the second leading cause of cancer deaths worldwide [1]. Most patients who diagnosed with GC were at their advanced stage, with local extension or lymph node metastasis, and the 5-year survival rate for this malignancy is below 25% [2,3]. While for those who diagnosed with early GC, the 5-year survival rates exceeds 90% with curative resection. Thus, the identification of prognostic markers and new therapeutic targets for advanced GC is urgently required.
Recently, an increasing number of studies have been focused on the role of chemokines and chemokine receptors in tumorigenesis and tumor progression. It has become clear that chemokines play a role in mediating the regulation of tumor growth, invasion and metastasis [4].
Currently four major subgroups have been identified within the chemokine family (CXC, CC, CX3C and C), defined according to the positioning of the conserved cysteines in the amino-terminal region of these small, inducible proteins. The CXC chemokine family is further subdivided into ELR+ and ELR- chemokines, based on the presence or absence of the tripeptide glutamic acid-leucine-arginine (the ‘ELR’ motif) preceding the CXC domain. The ELR+ CXC chemokines, such as interleukin-8 (IL-8/CXCL8) enhance tumor growth by inducing chemoattraction of neutrophilic granulocytes. In contrast, ELR- CXC chemokines, such as CXCL9/CXCL10/CXCL11 (MIG/IP-10/I-TAC), which bind to CXCR3, attract anti-tumoural lymphocytes [5-7].
However, less is known about the precise role of CXC chemokine receptor 3 (CXCR3) in cancer. Research has shown that high CXCR3 expression in human melanoma [8], colorectal [9], and breast [10] carcinoma facilitates tumor metastasis to lymph nodes and leads to a poor prognosis, while other studies have indicated that high CXCR3 expression in clear cell renal carcinoma [11], prostate cancer [12], and chronic lymphocytic leukemia [13] is associated with a favorable prognosis. However, the correlation between CXCR3 expression and prognosis in GC patients has not yet been reported.
It has been reported that CXC chemokine ligands such as CXCL9, CXCL10 and CXCL11 attract anti-tumor T lymphocytes, therefore mediating tumor progression through binding to CXCR3 [14]. Increased expression of CXCR3 ligands in gastric adenocarcinoma results in chemoattraction and activation of cytotoxic T lymphocytes (CTLs) favoring tumor regression [15]. However, little is known about the correlation between CXCR3 expression and tumor-infiltrating lymphocytes (TILs) in GC patients and whether CXCR3 can be used as an independent molecular marker for predicting the prognosis of advanced GC patients.
In the present study, the correlations of CXCR3 protein expression with CD4+, CD8+ TILs infiltration, clinicopathologic features and overall survival (OS) of GC patients were evaluated. We further explored the potential of CXCR3 overexpression as an independent prognostic factor and a potential therapeutic target in advanced GC patients.
Materials and methods
Patients
192 patients with advanced GC who received surgical resection between 2008 and 2013 in Zhongnan Hospital of Wuhan University, China, were retrospectively evaluated. The diagnosis of advanced GC was confirmed in all patients by gastroscopic examination. None of these patients had received preoperative chemotherapy. 48 relevant adjacent (≥5 cm) non-cancerous tissues were obtained. The type of tumor was identified histologically as intestinal or diffuse according to Lauren’s classification [16] and TNM staging of GC was performed according to American Joint Committee on Cancer [17]. The study was approved by the scientific research ethics committee of Zhongnan Hospital of Wuhan University. Written informed consent for the tissues use for ex vivo experimentation was obtained from all patients prior to surgery. Follow-up duration (months) was defined as the time of diagnosis until the final visit of GC. The OS was defined as the time from the diagnosis of GC until the patient’s death or the final visit.
Immunohistochemical staining
A conventional S-P immunohistochemical (IHC) staining protocol was used in this study. Briefly, paraffin-embedded tumor tissue blocks were cut into 4 μm thick sections, then dried, deparaffinised, and dehydrated in a graded series of ethanol. Tissue sections were treated with 1% hydrogen peroxide to block endogenous tissue peroxidase activity for 10 min, followed by treatment with bovine serum for 30 min to reduce nonspecific binding. Antigen retrieval was then accomplished by using citrate buffer (pH 6.0) as follows: high heat microwave processing for 5 min followed by low heat microwave processing for 20 min. All the slides were incubated with primary rabbit anti-human CXCR3 polyclonal antibody (BA0759, 1:200 dilution; Wuhan Boster Biological Technology, Ltd., Wuhan, China), rabbit anti-human CD8 monoclonal antibody (1:50 dilution, SP16, Zhongshan Golden Bridge Biotechnology, Beijing, China) and mouse anti-human CD4 monoclonal antibody (1:50 dilution, UMAB64, Zhongshan Golden Bridge Biotechnology, Beijing, China) for one hour at 37°C or overnight at 4°C, followed by a 30-min incubation in Ultra-Sensitive S-P Kit (Maixin-Bio, Fuzhou, China). All slides were rinsed with phosphate-buffered saline before color development using 3, 3’-diaminobenzidine substrate kit, and then counterstained with haematoxylin.
Slides were read by two senior pathologists who were blinded to the clinicopathologic data. Cytoplasmic staining with CXCR3 antibody in tumor cells and membranous or cytoplasmic staining with CD8 or CD4 antibodies in TILs was defined as positive. IHC staining of CXCR3, CD8 and CD4 proteins was assessed in terms of staining intensity and percentage of positive cells as follows: 0 (negative, ≤5% of cells staining positive), 1+ (weak staining, 6-25% of cells staining positive), 2+ (moderate staining, 26-50% of cells staining positive), and 3+ (strong staining, >50% of cells staining positive). The final score for each slide was represented by the average of three representative high-power fields (HPF, ×400). Scores ≤1+ was defined as low expression and scores ≥2+ were defined as high expression.
Statistical analysis
Statistical analysis was performed using SPSS software (Version 17.0). The differences of CXCR3 expression in gastric cancerous tissues and paracancerous tissues were analyzed by Wilcoxon signed-rank tests. The correlation between CXCR3 expression and CD4+, CD8+ TILs infiltration, as well as clinicopathologic parameters were analyzed using chi-square and Mann-Whitney U tests. OS were analyzed using Kaplan-Meier method by the log-rank test. The Cox proportional hazards regression model was used to identify the prognostic factors that influenced OS. All two-sided P-values <0.05 were considered to be statistically significant.
Results
CXCR3 expression and CD4+, CD8+ TILs in GC tissues and paracancerous tissues
IHC analysis of 192 cases of GC tissue and 48 corresponding paracancerous tissue samples was performed. As shown in Figure 1, positive staining of the CXCR3 protein was mainly located in the cytoplasm of tumor cells in advanced GC tissues. The results of IHC staining of CXCR3, CD8 and CD4 proteins in GC tissues were shown in Table 1. CXCR3 expression in GC tissues was significantly higher than that in paracancerous tissues (P<0.001).
Figure 1.
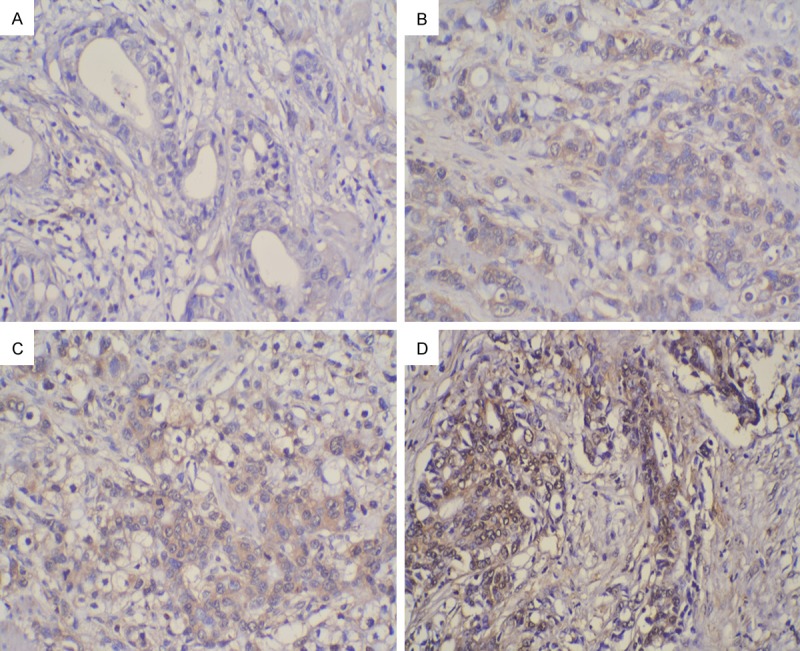
Imumunohistochemical staining of CXCR3 in advanced gastric cancer (GC) lesions. The staining of CXCR3 protein was mainly located in the cytoplasm of GC tumor cells (×200): A. Negative staining (0); B. Weak staining (1+); C. Moderate staining (2+); D. Strong staining (3+).
Table 1.
CXCR3 expression in gastric cancer and paracancerous tissues
| CXCR3 expression | P-value | ||||
|---|---|---|---|---|---|
|
| |||||
| 0 | 1+ | 2+ | 3+ | ||
| Gastric cancerous tissue (n = 192) | 36 (18.75%) | 44 (22.92%) | 60 (31.25%) | 52 (27.08%) | 0.000 |
| Paracancerous tissue (n = 48) | 26 (54.17%) | 15 (31.25%) | 5 (10.41%) | 2 (4.17%) | |
CD4 and CD8 protein expression was located mainly in the membrane and cytoplasm of TIL in advanced GC tissues (Figure 2). According to the IHC analysis, high-CXCR3 expressing GC tissues were associated with greater infiltration by CD4+ and CD8+ TILs compared with that associated with low-CXCR3 expressing GC tissues.
Figure 2.
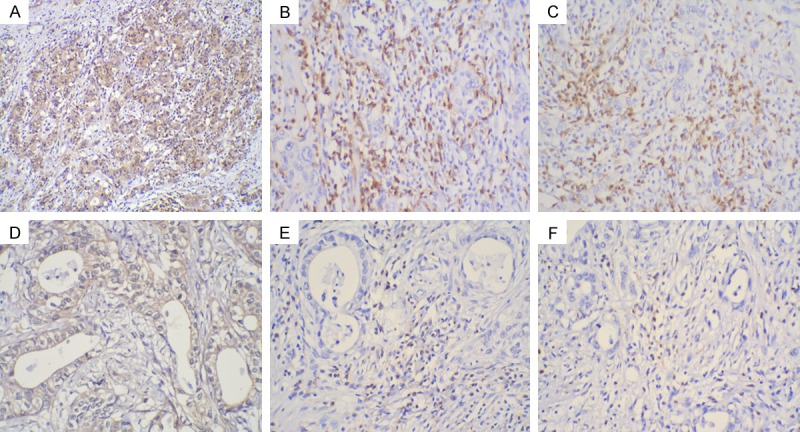
Immunohistochemical staining for CXCR3, CD4, and CD8 in gastric cancer lesions(×100): High CXCR3 protein expression (A) with Greater CD4 (C) and CD8 (E) TIL infiltration; Low CXCR3 protein expression (B) with Lesser CD4 (D) and CD8 (F) TIL infiltration.
Correlations of CXCR3 expression, infiltration by CD4+, CD8+ TILs and clinicopathologic features in GC tissues and paracancerous tissues
According to the CXCR3 immunoreactivity of GC tissues, 112 (58.3%) were classified as high-CXCR3 expression and 80 (41.7%) as low-CXCR3 expression, while among the paracancerous tissues, 7 (14.58%) were classified as high-CXCR3 expression and 41 (85.42%) as low-CXCR3 expression.
The correlation between CXCR3 expression and CD4+, CD8+ TILs in paracancerous tissues is shown in Table 2. There was no significant difference between CXCR3 expression and infiltration by CD4+ or CD8+ TILs (P=0.146 and P=0.211, respectively).
Table 2.
CXCR3 expression and CD4+ and CD8+ TILs in paracancerous tissues
| High-CXCR3 expression (n = 7) | Low-CXCR3 expression (n = 41) | P-value | |
|---|---|---|---|
| CD8 TIL | |||
| High | 1 (5.88%) | 16 (94.12%) | 0.211 |
| Low | 6 (19.35%) | 25 (80.65%) | |
| CD4 TIL | |||
| High | 0 (0.00%) | 10 (100.00%) | 0.146 |
| Low | 7 (18.42%) | 31 (81.58%) |
The correlation between CXCR3 expression and CD4+, CD8+ TILs, as well as the clinicopathologic features of advanced GC patients are shown in Table 3. It was found that CD4+, CD8+ TILs infiltration increased with high CXCR3 expression (P=0.032 and P<0.001, respectively). In GC patients with greater invasion depth and lymph node metastasis, CXCR3 expression was lower than that in patients with lesser invasion depth and without lymph node metastasis (P=0.002 and P=0.001, respectively). CXCR3 expression was not associated with sex (P=0.143), age (P=0.079), Lauren’s classification (P=0.692) and TNM stage (P=0.177).
Table 3.
CXCR3 expression, clinicopathologic parameters and infiltration by CD4+ and CD8+ TILs
| High-CXCR3 expression (n = 112) | Low-CXCR3 expression (n = 80) | P-value | |
|---|---|---|---|
| CD8 TILs | |||
| High | 88 (84.62%) | 16 (15.38%) | 0.000 |
| Low | 24 (27.27%) | 64 (72.73%) | |
| CD4 TILs | |||
| High | 61 (66.30%) | 31 (33.70%) | 0.032 |
| Low | 51 (51.00%) | 49 (49.00%) | |
| Sex | |||
| Male | 76 (55.07%) | 62 (44.93%) | 0.143 |
| Female | 36 (66.67%) | 18 (33.33%) | |
| Age | |||
| <55 | 62 (64.58%) | 34 (35.42%) | 0.079 |
| ≥55 | 50 (52.08%) | 46 (47.92%) | |
| Lauren’s classification | |||
| Intestinal | 48 (60.00%) | 32 (40.00%) | 0.692 |
| Diffuse | 64 (57.14%) | 48 (42.86%) | |
| Invasion depth | |||
| T2 | 46 (74.19%) | 16 (25.81%) | 0.002 |
| T3/T4 | 66 (50.77%) | 64 (49.23%) | |
| TNM stage | |||
| I+II | 32 (66.67%) | 16 (33.33%) | 0.177 |
| III+IV | 80 (55.56%) | 64 (44.44%) | |
| Lymph node metastasis | |||
| No | 48 (75.00%) | 16 (25.00%) | 0.001 |
| Yes | 64 (50.00%) | 64 (50.00%) |
T2, tumor invasion of the muscularis propria or subserosa; T3, tumor invasion extends to or beyond the serosa; T4, tumor invasion of adjacent structures.
Association between OS and CXCR3 expression, infiltration by CD8+ TILs and other parameters
The median survival time of the patients was 19.5 months (range: 1-54 months). The Kaplan-Meier and Cox proportional hazards regression methods were used to evaluate the association of CXCR3 expression with the prognosis of advanced GC patients. Kaplan-Meier analysis showed that patients with high CXCR expression and more CD8+ TILs infiltration had a longer OS (Figure 3A) than patients with low CXCR3 expression and less CD8+ TILs infiltration (Figure 3B) (log-rank test, P<0.001 and P=0.002, respectively) (Table 4). Patients aged over 55 had shorter OS (log-rank test, P=0.026). There were no significant differences between patient OS and CD4+ TILs infiltration (log-rank test, P=0.422), Lauren’s classification (log-rank test, P=0.951) invasion depth (log-rank test, P=0.064) and TNM stage (log-rank test, P=0.234).
Figure 3.
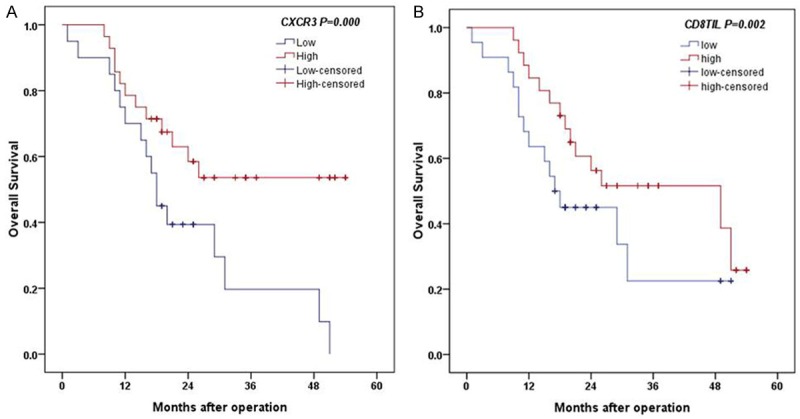
Analysis of OS between CXCR3 expression and CD8 expression level: A. High CXCR3 expression had longer OS (log-rank test, P<0.001). B. High CD8 expression is associated with longer OS (log-rank test, P=0.002).
Table 4.
Univariate and multivariate analysis of OS
| n | P-value univariate | P-value multivariate | Hazard ratio, 95% CI | |
|---|---|---|---|---|
| CXCR3 expression | ||||
| High | 112 | 0.000 | 0.002 | 0.464 (0.284-0.757) |
| Low | 80 | |||
| CD8 TILs | ||||
| High | 104 | 0.002 | 0.334 | 0.764 (0.443-1.318) |
| Low | 88 | |||
| CD4 TILs | ||||
| High | 92 | 0.422 | 0.574 | 1.133 (0.733-1.753) |
| Low | 100 | |||
| Age | ||||
| <55 | 96 | 0.026 | 0.473 | 1.184 (0.746-1.879) |
| ≥55 | 96 | |||
| Lauren’s classification | ||||
| Intestinal | 80 | 0.951 | 0.665 | 1.106 (0.702-1.743) |
| Diffuse | 112 | |||
| Invasion depth | ||||
| T2 | 62 | 0.064 | 0.339 | 1.304 (0.756-2.249) |
| T3/T4 | 130 | |||
| TNM stage | ||||
| I+II | 48 | 0.234 | 0.549 | 0.848 (0.495-1.453) |
| III+IV | 144 |
T2, tumor invasion of the muscularis propria or subserosa; T3, tumor invasion extends to or beyond the serosa; T4, tumor invasion of adjacent structures.
The factors found to have an effect on OS in advanced GC were then analyzed using the Cox proportional hazards regression method. Among the variables, CXCR3 expression was identified as an independent factor for OS. Patients with advanced GC with low CXCR3 expression had a 0.5-fold increased risk of death [hazard ratio (HR): 0.464 (0.284-0.757); P=0.002].
Discussion
Chemokines are a large family of heparin-binding proteins that modulate leukocyte trafficking and the targeting of immune cells [18]. They interact with their receptors to direct cells to specific sites throughout the body during development and other physiological processes, and involved in different steps of tumorigenesis, tumor growth, invasion and metastasis [19,20]. In cancer, chemokines and their receptors modulate tumor behavior by regulation of angiogenic or angiostatic activity, migration of leukocytes to intratumoral tissues, activation of a tumor-specific immune response and stimulation of tumor cell proliferation, invasion and metastasis [6,7]. CXCR3, which belongs to ELR- CXC chemokines receptors, could be produced by many tumor cells, including colorectal, breast and clear cell renal carcinoma, and prostate cancer, and played a complex role in tumorigenesis and tumor progression.
Teppei et al. [21] demonstrated that high CXCR3 expression is associated with lymph nodes metastasis of colorectal carcinoma and leads to a poor prognosis, while Tobias et al. [11] suggested that high CXCR3 expression in clear renal carcinoma results in a favorable prognosis. Furthermore, Takanobu et al. [22] revealed that high CXCR3 expression is associated with renal cell carcinoma migration, invasion and metastasis. This divergent outcome may be attributed to the inconsistent expression of CXCR3 in different types of tumors. However, limited data are available for the expression of CXCR3 in advanced GC, and still less is known about the correlation of CXCR3 with the clinicopathologic characteristics and prognosis of advanced GC patients. In our present study, we showed that CXCR3 expression was significantly higher in advanced GC tissues than that in corresponding paracancerous tissues. We further demonstrated that high CXCR3 expression was inversely and significantly associated with invasion depth and lymph node metastasis. These data indicated that high CXCR3 expression plays a vital role in the progression of tumor in GC.
It is generally believed that T lymphocytes represent the major population of tumor-infiltrating immune cells, among which CD8+ T cells (CTLs) and CD4+ T cells (Th) comprise the primary immune cells and are responsible for anti-tumor immunity [23]. Oldham et al. [24] demonstrated that CXCR3 plays a role in recruiting TILs in renal cell carcinoma. In our present study, we found that CD4+ and CD8+ TILs infiltration increased with CXCR3 expression. These observations support a role for CXCR3 in mediating T cell recruitment to GC and promoting anti-tumor immunity. Furthermore, a direct correlation was observed between increased CXCR3 expression and improved OS and multivariate analysis showed that high CXCR3 expression is an independent prognostic factor for GC patients. Consequently, CXCR3 is implicated in recruiting TILs in GC resulting in an improved OS.
To the best of our knowledge, this is the first report exploring the correlation between CXCR3 and TILs in GC, and this is the first report demonstrating the prognostic role of CXCR3 in GC patients associated with anti-tumor immunity; however, the mechanisms by which CXCR3 influences GC progression require further investigation to identify. In addition, due to the limited number of patients in this study, a larger study is required with the inclusion of prolonged follow-up to allow analysis of the 5-year OS rates.
In conclusion, the current study indicated that CXCR3 was overexpressed in advanced GC patients. Our data revealed that high CXCR3 expression in advanced GC patients was associated with greater CD4+ and CD8+ TILs infiltration, tumor progression and an improved prognosis. Therefore, this study demonstrated that, similar to CXCR3 expression in some other cancers, CXCR3 is an indicator of a good prognosis and a promising target for cancer therapy in GC. Further studies are required to clarify the mechanisms of which CXCR3 participates in the progression of GC.
Acknowledgements
This work was supported by Natural Foundation of Hubei Province (NO. 2013CFB267) and Wuhan Science and Technology Key Project (NO. 2013060602010248).
Disclosure of conflict of interest
None.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Lochhead P, El-Omar EM. Gastric cancer. Br Med Bull. 2008;85:87–100. doi: 10.1093/bmb/ldn007. [DOI] [PubMed] [Google Scholar]
- 3.Chen R, He Q, Cui J, Bian S, Chen L. Lymph node metastasis in early gastric cancer. Chin Med J. 2014;127:560–567. [PubMed] [Google Scholar]
- 4.Ma Y, Adjemian S, Galluzzi L, Zitvogel L, Kroemer G. Chemokines and chemokine receptors required for optimal responses to anticancer chemotherapy. Oncoimmunology. 2014;3:e27663. doi: 10.4161/onci.27663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vandercappellen J, Van Damme J, Struyf S. The role of CXC chemokines and their receptors in cancer. Cancer Lett. 2008;267:226–244. doi: 10.1016/j.canlet.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 6.Zhu Q, Han X, Peng J, Qin H, Wang Y. The role of CXC chemokines and their receptors in the progression and treatment of tumors. J Mol Histol. 2012;43:699–713. doi: 10.1007/s10735-012-9435-x. [DOI] [PubMed] [Google Scholar]
- 7.Lee HJ, Song IC, Yun HJ, Jo DY, Kim S. CXC chemokines and chemokine receptors in gastric cancer: from basic findings towards therapeutic targeting. WJG. 2014;20:1681–1693. doi: 10.3748/wjg.v20.i7.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amatschek S, Lucas R, Eger A, Pflueger M, Hundsberger H, Knoll C, Grosse-Kracht S, Schuett W, Koszik F, Maurer D, Wiesner C. CXCL9 induces chemotaxis, chemorepulsion and endothelial barrier disruption through CXCR3-mediated activation of melanoma cells. Br J Cancer. 2011;104:469–479. doi: 10.1038/sj.bjc.6606056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Z, Han X, Yan J, Pan Y, Gong J, Di J, Cheng Z, Jin Z, Wang Z, Zheng Q, Wang Y. The prognostic significance of chemokine receptor CXCR3 expression in colorectal carcinoma. Biomed Pharmacother. 2012;66:373–377. doi: 10.1016/j.biopha.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Li L, Chen J, Lu ZH, Yu SN, Luo YF, Zhao WG, Ma YH, Jia CW. Significance of chemokine receptor CXCR3 expression in breast cancer. Zhonghua Bing Li Xue Za Zhi. 2011;40:85–88. [PubMed] [Google Scholar]
- 11.Klatte T, Seligson DB, Leppert JT, Riggs SB, Yu H, Zomorodian N, Kabbinavar FF, Strieter RM, Belldegrun AS, Pantuck AJ. The chemokine receptor CXCR3 is an independent prognostic factor in patients with localized clear cell renal cell carcinoma. J Urol. 2008;179:61–66. doi: 10.1016/j.juro.2007.08.148. [DOI] [PubMed] [Google Scholar]
- 12.Nagpal ML, Davis J, Lin T. Overexpression of CXCL10 in human prostate LNCaP cells activates its receptor (CXCR3) expression and inhibits cell proliferation. Biochim Biophys Acta. 2006;1762:811–818. doi: 10.1016/j.bbadis.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 13.Ocana E, Delgado-Perez L, Campos-Caro A, Muñóz J, Paz A, Franco R, Brieva JA. The prognostic role of CXCR3 expression by chronic lymphocytic leukemia B cells. Haematologica. 2007;92:349–356. doi: 10.3324/haematol.10649. [DOI] [PubMed] [Google Scholar]
- 14.Billottet C, Quemener C, Bikfalvi A. CXCR3, a double-edged sword in tumor progression and angiogenesis. Biochim Biophys Acta. 2013;1836:287–295. doi: 10.1016/j.bbcan.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Verbeke H, Geboes K, Van Damme J, Struyf S. The role of CXC chemokines in the transition of chronic inflammation to esophageal and gastric cancer. Biochim Biophys Acta. 2012;1825:117–129. doi: 10.1016/j.bbcan.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Lauren P. The Two Histological Main Types of Gastric Carcinoma: Diffuse and so-called Intestina-type Carcinoma. An Attempt at a Histo-clinical Classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 17.Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077–3079. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 18.Keeley EC, Mehrad B, Strieter RM. CXC chemokines in cancer angiogenesis and metastases. Adv Cancer Res. 2010;106:91–111. doi: 10.1016/S0065-230X(10)06003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balkwill FR. The chemokine system and cancer. J Pathol. 2012;226:148–157. doi: 10.1002/path.3029. [DOI] [PubMed] [Google Scholar]
- 20.Koizumi K, Hojo S, Akashi T, Yasumoto K, Saiki I. Chemokine receptors in cancer metastasis and cancer cell-derived chemokines in host immune response. Cancer Sci. 2007;98:1652–1658. doi: 10.1111/j.1349-7006.2007.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murakami T, Kawada K, Iwamoto M, Akagami M, Hida K, Nakanishi Y, Kanda K, Kawada M, Seno H, Taketo MM, Sakai Y. The role of CXCR3 and CXCR4 in colorectal cancer metastasis. Int J Cancer. 2013;132:276–287. doi: 10.1002/ijc.27670. [DOI] [PubMed] [Google Scholar]
- 22.Utsumi T, Suyama T, Imamura Y, Fuse M, Sakamoto S, Nihei N, Ueda T, Suzuki H, Seki N, Ichikawa T. The Association of CXCR3 and Renal Cell Carcinoma Metastasis. J Urol. 2014;192:567–574. doi: 10.1016/j.juro.2014.01.100. [DOI] [PubMed] [Google Scholar]
- 23.Zamarron BF, Chen W. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci. 2011;7:651–658. doi: 10.7150/ijbs.7.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oldham KA, Parsonage G, Bhatt RI, Wallace DMA, Deshmukh N, Chaudhri S, Adams DH, Lee SP. T lymphocyte recruitment into renal cell carcinoma tissue: a role for chemokine receptors CXCR3, CXCR6, CCR5, and CCR6. Eur Urol. 2012;61:385–394. doi: 10.1016/j.eururo.2011.10.035. [DOI] [PubMed] [Google Scholar]


