Abstract
Sacral chordoma is a rare spine tumor with a high recurrence rate even after optimal therapy. Previous studies have demonstrated that the PI3K/AKT pathway plays a pivotal role in chordoma, and high expression of pAKT is associated with poor prognosis. Recently, PHLPP was recognized to be a tumor suppressor that targets AKT. We analyzed the expression of PHLPP1 and AKT2 in 37 chordoma samples and 11 fetal nucleus pulposus samples by immunohistochemical staining. Of the chordoma cases, 40.5% (15/37) showed strong cytoplasmic staining (score ≥3) for PHLPP1, which was significantly lower than the 90.9% (10/11) of fetal nucleus pulposus samples (P = 0.004). Conversely, strong immunohistochemical staining for AKT2 was observed in 75.7% (28/37) of chordoma samples, which was significantly higher than 36.4% (4/11) of fetal nucleus pulposus (P = 0.021). Kaplan-Meier survival curves and log-rank test showed that patients with high expression of PHLPP1 experienced longer progression free survival time than those with low PHLPP1 expression (P = 0.011). Further multivariate Cox regression analysis indicated that PHLPP1 expression level and surgical approaches were independent risk factors for chordoma recurrence (P = 0.023 and P = 0.022). However, PHLPP1 expression was not statistically related to patients’ total survival time. Conclusively, our results suggest that PHLPP1 plays a crucial role in sacral chordoma, and may be a promising biomarker for prognosis. Meanwhile, manipulation of PHLPP1 expression is also a potential therapeutic approach for the treatment of sacral chordoma.
Keywords: Sacral chordoma, PHLPP1, tumor suppressor, AKT2
Introduction
Chordoma is a rare bone cancer that is commonly located in the axial skeleton, with highest distribution in the sacrum and lower in the skull base and the mobile spine. It is normally classified into three histological types: classical, chondroid, and dedifferentiated. These tumors are low-grade, but tend to be aggressive and locally invasive with a high recurrence rate, which leads to poor life quality and death. Chordoma is highly resistant to conventional chemo- and radio-therapy; surgery, so far, is accepted as the most effective in management of this disease [1]. Therefore, development of specific therapeutic approaches is greatly needed for effective treatment for chordoma.
Chordoma is thought to arise from notochordal remnants [1,2], while its pathogenetic mechanism remains largely unknown. Presently, no biomarkers are available for predicting the clinical behavior of this rare tumor. A previous study demonstrated that phosphatidylinositide 3-kinase/protein kinase B (PI3K/AKT) and mammalian target of rapamycin (mTOR) signaling pathways play a crucial role in chordoma. Phosphorylated AKT (pAKT) was up-regulated in most chordoma cases, and high expression of pAKT was correlated with poor prognosis [3]. Furthermore, the indispensability of PI3K/AKT pathway was confirmed by an in vitro study where blocking its activation by PI-103 decreased proliferation and induced apoptosis of the U-CH1 chordoma cell line [4]. Thus, discovery of a component that inhibits the PI3K/AKT pathway may be significant in limiting chordoma development. Recent studies revealed a new family of protein phosphatase, pleckstrin homology domain leucine-rich repeat protein phosphatase (PHLPP), which is frequently deleted in a variety of cancers. PHLPP is thought to be a negative regulator of the PI3K/AKT pathway that acts by direct dephosphorylation and inactivation, which means that PHLPP may be a tumor suppressor [5]. As reported, the expression of PHLPP is down-regulated in different cancer types [6], indicating that PHLPP may be a novel therapeutic target and a candidate biomarker. To the best of our knowledge, the understanding of PHLPP in chordoma is still unclear. Hence, in order to identify the role that PHLPP plays in this bone tumor, we evaluated the expression of PHLPP1 in sacral chordoma and explored its correlation with clinicopathological parameters and patients’ prognosis.
Materials and methods
Patients and tissue samples
The chordoma samples were acquired from 37 patients (20 males and 17 females), all of whom were identified and treated by tumor resection surgeries at the Department of Orthopedics, The First Affiliated Hospital of Soochow University, between January 1998 and November 2012. The mean age at the time of surgery was 53.1 years (range 18 to 83 years). Eleven fetal nucleus pulposus specimens were obtained from aborted fetuses with a gestational age of 8-24 weeks in the Department of Gynecology and Obstetrics. Tumor and fetal nucleus pulposus samples were fixed in 10% formalin and embedded in paraffin. All tissue sections underwent hematoxylin and eosin staining, and were histologically confirmed to be sporadic chordomas and fetal nucleus pulposus, respectively, by two experienced pathologists. The clinical data of the patients including age, gender, tumor location, tumor size and locally invasion, etc. were obtained by reviewing their medical records. This study was conducted with the approval of the Ethics Committee in our hospital (No. 2014825042), and all the patients and aborted fetuses’ parents gave informed consent.
Immunohistochemical staining
All slides were processed under the same conditions. Immunohistochemical staining was carried out on 4-μm-thick tissue sections using the EnVision two-step staining method [7]. The sections were dewaxed in xylene and rehydrated in graded ethanol, followed with antigen retrieval by subjecting the sections to high temperature and high pressure in citrate buffer (pH 6.0). After immersion in 3% hydrogen peroxide, the sections were incubated with primary antibodies, rabbit monoclonal anti-PHLPP1 (ab71972, Abcam, Cambridge, UK) and anti-AKT2 (ab175354, Abcam, Cambridge, UK), at the dilution of 1:100. Subsequently, the ChemMateTMEnvisionTM Detection Kit (GK500710, Gene Tech, Shanghai, China) used the multilink concentrated biotinylated anti-IgG as the secondary antibody and was used according to the manufacturer’s protocol. Finally, diaminobenzidine was applied as the chromogen before counterstaining with hematoxylin. Immunohistochemical staining of human cerebellum was used as the positive control for PHLPP1, while the staining of esophageal cancer tissue was used as the positive control for AKT2. For negative control, the primary antibody was substituted by PBS.
The stained tissue sections were scored by two independent experienced pathologists who had no prior knowledge of the patients’ clinical information. The sections were reviewed by randomly selecting five high power fields and evaluating semi-quantitatively as described previously [8]. Slides were scored based on the percentage of positive cells per tumor as follows: 0 (0-9%), 1 (10-25%), 2 (26-50%), 3 (51-100%). Immunostaining was also scored based on intensity as 0 (no staining), 1 (light yellow staining), 2 (yellow staining), and 3 (brownish-yellow staining). The final score was derived by combining the two scoring systems together, where 0-2 was defined as low expression, and 3-6 was regarded as high expression.
Real-time quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from 19 frozen chordoma samples and 11 frozen fetal nucleus pulposus samples with TRIzol RNA Isolation Reagents (DP405-02, Tiangen, Beijing, China). Reverse transcription and RT-qPCR were performed using the RT reagent Kit (RR037A, Takara, Dalian, China), and RT-qPCR was carried out by Roche LightCycler 480 (Roche, Mannheim, Germany). The primers used for PHLPP1 were: sense: 5’-GCCACATAATCCCCTGGAAC-3’ and antisense: 5’-CCATTGCAGTGGGGCTTC-3’ [9]. GAPDH was amplified and quantitated as an internal control. The primers for GAPDH were: sense: 5’-CGAGATCCCTCCAAAATCAA-3’ and antisense: 5’-TTCACACCCATGACGAACAT-3’.
Follow-up
Follow-up data were obtained by telephone, letters, and the clinical database of in- and out-patients. The follow-up time was every 3 months for the first 2 years and every 6 months for subsequent years. All data concerning demographic and clinical information, including progression free survival (PFS), and image data, was collected individually.
Statistical analysis
Statistical analysis was performed with SPSS 19.0 statistical software (SPSS Inc., Chicago, IL, USA). Quantitative data from two groups were compared by Student’s t test. The association between PHLPP1 expression and clinicopathological parameters was evaluated by Fisher’s exact test. The correlation between PHLPP1 and AKT2 expression was estimated by Spearman’s rank correlation test. The influence of PHLPP1 expression on PFS was described by Kaplan-Meier survival curves, and differences were detected by log-rank test. Cox multivariate regression analysis was used to determine independent prognostic factors. P<0.05 was considered to be statistically significant.
Results
Expression of PHLPP1 and AKT2 in sacral chordomas
Immunohistochemical staining of the tumors showed a cytoplasmic staining pattern with diverse intensity for PHLPP1 and AKT2. The mean immunohistochemical score for PHLPP1 was 2.3, while the mean score for AKT2 was 3.6 (P<0.001). Of the 37 samples, 40.5% (15/37) had high expression of PHLPP, which was significantly lower than the 90.9% (10/11) observed in the fetal nucleus pulposus group (P = 0.004) (Figure 1; Table 1). The high expression of AKT2 was in 75.7% (28/37) of samples, which was significantly higher than 36.4% (4/11) observed in the fetal nucleus pulposus samples (P = 0.021) (Figure 1). To validate the RNA level expression of PHLPP1 in chordoma samples, RT-qPCR was performed. Compared with fetal nucleus pulposus, PHLPP1 expression was approximately 4-fold lower in chordoma samples (P<0.001) (Figure 2). The results showed that PHLPP1 was expressed at a lower level in 17 out of the 19 chordoma samples.
Figure 1.
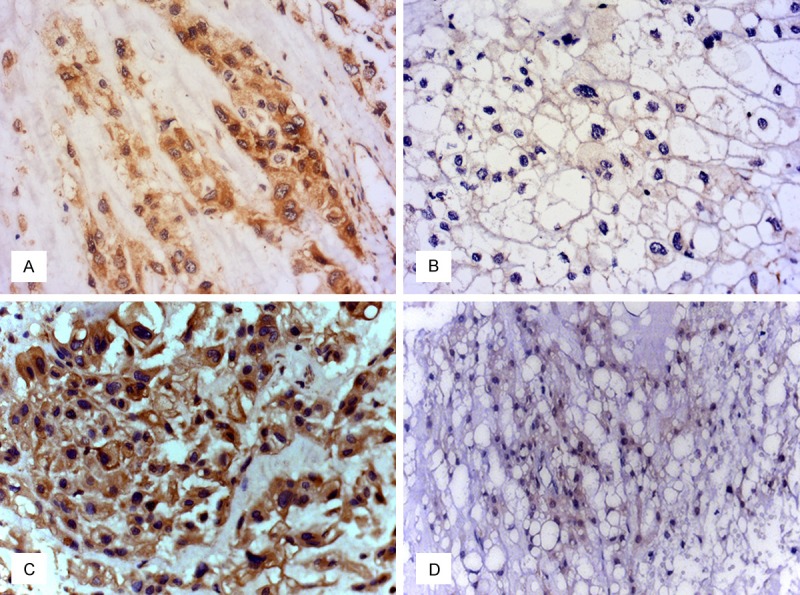
Representative expression of PHLPP1 and AKT2 in chordoma tissues. A. High expression of PHLPP1 in chordoma. B. Low expression of PHLPP1 in chordoma. C. High expression of AKT2 in chordoma. D. Low expression of AKT2 in chordoma. (magnification ×400).
Table 1.
Expression of PHLPP1 in sacral chordoma and fetal nucleus pulposus
| Sacral chordoma | Fetal nucleus pulposus | P value | ||
|---|---|---|---|---|
| PHLPP1 expression | High (%) | 15 (40.5%) | 10 (90.9%) | 0.004 |
| Low (%) | 22 (59.5%) | 1 (9.1%) | ||
| AKT2 expression | High (%) | 28 (75.7%) | 4 (36.4%) | 0.021 |
| Low (%) | 9 (24.3%) | 7 (63.6%) |
Figure 2.
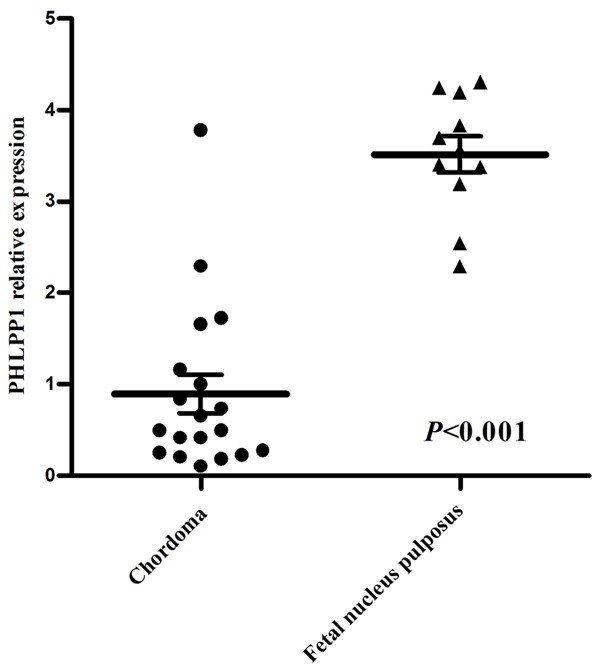
Comparison of PHLPP1 expression in chordoma and fetal nucleus pulposus tissue. PHLPP1 is expressed at a level approximately 4-fold lower in chordoma than in fetal nucleus pulposus tissue, as shown by RT-qPCR.
Correlations between PHLPP1 expression and clinicopathological parameters
To explore the relevance between clinicopathologic information and the candidate prognostic factors (Table 2), Fisher’s exact test was performed. PHLPP1 expression was statistically related to tumor recurrence. However, the expression of PHLPP1 was not associated with patients’ age, gender, tumor size, tumor location, histological subtypes, radiotherapy or surrounding muscle invasion.
Table 2.
Association of PHLPP1 expression with clinicopathological parameters in sacral chordoma
| n | PHLPP1 expression | P value | ||
|---|---|---|---|---|
|
|
||||
| High | Low | |||
| Age (years) | ||||
| <50 | 15 | 7 | 8 | 0.386 |
| ≥50 | 22 | 8 | 14 | |
| Gender | ||||
| Male | 20 | 10 | 10 | 0.175 |
| Female | 17 | 5 | 12 | |
| Tumor location | ||||
| Above S3 | 22 | 9 | 13 | 0.614 |
| S3 and below | 15 | 6 | 9 | |
| Tumor size (mm) | ||||
| <90 | 14 | 4 | 10 | 0.209 |
| ≥90 | 23 | 11 | 12 | |
| Histological subtypes | ||||
| Classical | 35 | 15 | 20 | 0.347 |
| Chondroid | 2 | 0 | 2 | |
| Dedifferentiated | 0 | 0 | 0 | |
| Radiotherapy | ||||
| Yes | 12 | 6 | 6 | 0.323 |
| No | 25 | 9 | 16 | |
| Surrounding muscle invasion | ||||
| Yes | 16 | 5 | 11 | 0.253 |
| No | 21 | 10 | 11 | |
| Recurrence | ||||
| Yes | 18 | 4 | 14 | 0.030 |
| No | 19 | 11 | 8 | |
Prognostic significance of PHLPP1 expression
Follow-up data were obtained for all 37 patients. The median PFS was 42 months (range 3-158 months). Eighteen patients (48.6%) developed local recurrence with a median time of 29.5 months (range 3-92 months). Of the 15 patients with high expression of PHLPP1, only four patients had recurrence, whereas 14 out of 22 patients with low PHLPP1 expression experienced recurrence. Kaplan-Meier survival curves indicated better prognosis for patients with high PHLPP1 expression. Patients with high PHLPP1 expression possessed a mean PFS of 121.93 months, which was longer than the 52.47 months observed in patients with low PHLPP1 expression (P = 0.011) (Figure 3). Further multivariate Cox regression analysis indicated that PHLPP1 expression level, along with surgical approaches, were independent risk factors for chordoma recurrence (Table 3). Meanwhile, at the time of last follow-up, five patients had died of chordoma, 13 patients were alive with disease, 17 patients had no evidence of chordoma, and two patients had died of other causes. By Kaplan-Meier survival curve analysis, no significant correlation was identified between PHLPP1 expression and total survival time (data not shown).
Figure 3.
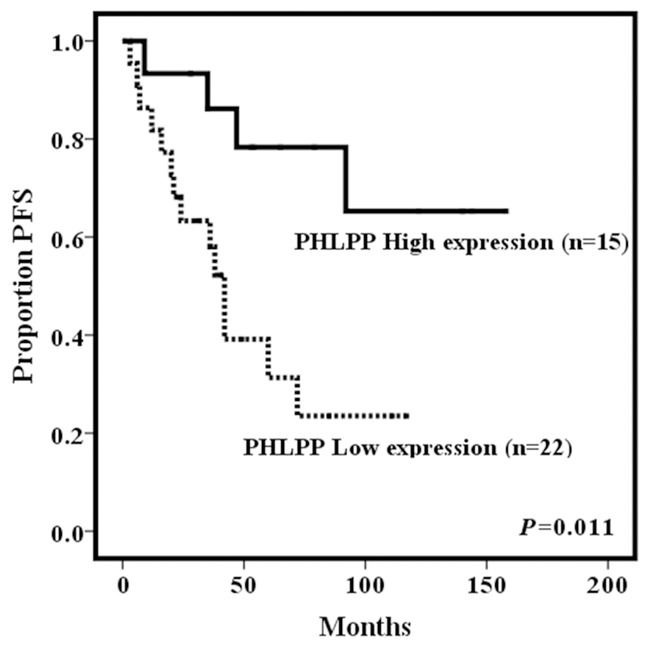
Relationship of PFS to PHLPP1 expression. Kaplan-Meier survival analysis curves showed that patients with low expression of PHLPP1 relapsed more easily.
Table 3.
Multivariate analyses of individual parameters for correlations with recurrence rate
| Odd ratio | 95.0% Confidence interval | P value | ||
|---|---|---|---|---|
|
|
||||
| Lower | Upper | |||
| Age (<50 or ≥50) | 1.755 | 0.492 | 6.257 | 0.386 |
| Gender (Male or female) | 0.392 | 0.123 | 1.248 | 0.113 |
| Tumor location (Above or below S3) | 2.147 | 0.598 | 7.707 | 0.241 |
| Tumor size (<90 or ≥90) | 0.887 | 0.251 | 3.129 | 0.852 |
| Radiotherapy (Yes or no) | 1.015 | 0.243 | 4.241 | 0.984 |
| Histological subtypes (Classical or chondroid) | 0.277 | 0.015 | 5.218 | 0.392 |
| Surgical approaches | ||||
| 1 versus 3* | 0.118 | 0.019 | 0.738 | 0.022 |
| 2 versus 3* | 0.500 | 0.083 | 3.010 | 0.449 |
| PHLPP1 (Positive or negative) | 0.151 | 0.030 | 0.771 | 0.023 |
| AKT2 (Positive or negative) | 1.214 | 0.227 | 6.509 | 0.821 |
1 = Radical resection, 2 = Perilesional resection, 3 = Intralesional resection.
Discussion
Sacral chordoma is relatively slow growing, but locally aggressive and invasive with poor prognosis. Our previous studies indicated that many factors, including tumor location, tumor size, surgical approaches, and muscle invasion, etc. may be potentially associated with patients’ prognosis [10]. Furthermore, several molecular factors, such as matrix metalloproteinase 9(MMP9) [11], insulin-like growth factor II mRNA-binding protein 3(IMP3) [12], and phosphatase and tensin homolog (PTEN) [13] have also been demonstrated to be correlated with patients’ outcome. However, due to the rarity of chordoma, the definitive characteristic of those bio-markers remains unclear. In this current work, we found that PHLPP1 was also a potential bio-marker in indicating patients’ outcome. However, stronger work should be conducted in defining the most reliable prognostic factor by detecting the known features simultaneously in the future.
In this study, we explored the expression of PHLPP1 in chordoma samples. PHLPP1, together with PHLPP2, comprise the PHLPP family. Chromosomal regions that contain PHLPP genes are commonly lost in a variety of cancers. PHLPP1 is located on chromosome 18q21.33, where the loss-of-heterozygosity is high for colon cancers [14]. Previous study has revealed that PHLPP1 dephosphorylates AKT2 andAKT3, but not AKT1, whereas PHLPP2 dephosphorylates AKT1 and AKT3, but not AKT2 [15]. Therefore, the PHLPP1 and AKT2 isoforms were chosen in our study. Expression of PHLPP1 was notably reduced in chordoma samples as determined by immunohistochemical staining and RT-qPCR compared to fetal nucleus pulposus. Correspondingly, other studies revealed that PHLPP1 expression was often down-regulated in different human cancers, including ductal breast carcinoma [16], chronic lymphocytic leukemia [17], colon cancer [14], esophageal adenocarcinoma [18], pancreatic ductal adenocarcinoma [19], and prostate cancer [20]. Although PHLPP1 was observed to be expressed at a lower level in many tumors, the mechanism of its down-regulation has not been well explained. Epigenetic modification, such as DNA methylation, was identified to be possibly responsible in reducing PHLPP1 expression in melanoma [9]; however, the exact mechanism remains to be further investigated.
As local recurrence is one of the most reliable predictors of chordoma patients’ mortality [21], we analyzed the correlation between PHLPP1 expression and PFS by Kaplan-Meier survival curve analysis. As expected, we found that patients with low PHLPP1 expression tended to have shorter PFS and a higher recurrence rate than those with high PHLPP1 expression, suggesting that low PHLPP1 expression was associated with poor prognosis of patients with chordoma. However, there was no statistical significance for any association between PHLPP1 expression and total survival time in chordoma patients. The results were not according with previous study revealing that recurrence was a strong indicator of chordoma patients’ survival, while this fact might be caused by the limitation of case load and follow-up time in our study. In contrast, inverse relationship between PHLPP1 expression and patients’ survival was observed in several other tumor types. Nitsche et al. [19] revealed that a high level of PHLPP1 was associated with longer survival time of patients with pancreatic ductal adenocarcinoma. Wang et al. [8] claimed that loss of PHLPP1 expression was observed in most gastric cancer samples, which correlated with lymph node metastasis. From the above, those results imply that low expression of PHLPP1 is potentially indicating poor prognosis.
All PHLPP isozymes share a similar domain structure including an N-terminal pleckstrin homology (PH) domain, a leucine-rich repeat region, a protein phosphatase 2C (PP2C) phosphatase domain, and a C-terminal PDZ-binding motif, which has been demonstrated to dephosphorylate a key regulatory site named the hydrophobic motif on AKT, and then inactivating the kinase [22]. AKT, however, is a member of PI3K/AKT signaling cascade, which promotes cell growth and survival. The expression of AKT is often increased in primary and metastatic tumors, and its activation is thought to be crucial for driving human cancers. In chordoma, a study conducted by Carolina et al. showed that 82% of chordoma cases collected were pAKT-positive, and the expression of pAKT was significantly correlated with overall survival of chordoma patients. The 5-year survival rate for patients with pAKT-negative chordomas was 100%, whereas, the rate for pAKT-positive patients was only 45% [3]. Similarly, our findings demonstrated that AKT2 was highly expressed in 85.3% chordoma samples, and patients with high expression of AKT2 tend to be more easily for relapse, which was consist with previous research, indicating a potential therapeutic target in chordoma treatment. Taken together, these results suggest that AKT pathway is activated in chordoma development, and the low level of PHLPP1 could be potentially responsible for this activity.
To further investigate the prognostic factors for chordoma patents, we conducted the multivariate analysis of individual parameters for correlations with recurrence rate, showing that PHLPP1 was an independent prognostic factor among those involved, which revealed that PHLPP1 could be a potential indicator of chordoma recurrence. Besides, surgical approach was found to be another independent factor in predicting relapse of this tumor, which was in accordance with previous studies indicating that radical resection of chordoma leads to lower recurrence rate [23,24]. It might be caused by the damage of the tumor walls in the procedures of perilesional and intralesional resection of the tumor. Therefore, making a clean sweep of the tumor lesion is also significantly critical in prevention of recurrence.
In this study, we demonstrated that the expression of PHLPP1 was down-regulated in chordoma, which was correlated with tumor relapse. Combined with AKT2 detection, we discovered that PHLPP1 was negatively associated with AKT2. The results suggest that PHLPP1 is a promising biomarker for sacral chordoma, which is also valuable as a prognostic indicator. Furthermore, as an on-off switch of the AKT pathway, manipulation of PHLPP1 expression may be a potential therapeutic approach for the treatment of sacral chordoma in the future.
Acknowledgements
Our study was funded by Jiangsu Provincial Special Program of Medical Science (BL2012004). We also thank the Chordoma Foundation, Durham NC, USA, for their kindness in providing us the U-CH1 chordoma cell line.
Disclosure of conflict of interest
None.
References
- 1.Walcott BP, Nahed BV, Mohyeldin A, Coumans JV, Kahle KT, Ferreira MJ. Chordoma: current concepts, management, and future directions. Lancet Oncol. 2012;13:e69–76. doi: 10.1016/S1470-2045(11)70337-0. [DOI] [PubMed] [Google Scholar]
- 2.Chugh R, Tawbi H, Lucas DR, Biermann JS, Schuetze SM, Baker LH. Chordoma: the nonsarcoma primary bone tumor. Oncologist. 2007;12:1344–50. doi: 10.1634/theoncologist.12-11-1344. [DOI] [PubMed] [Google Scholar]
- 3.de Castro CV, Guimaraes G, Aguiar S Jr, Lopes A, Baiocchi G, da Cunha IW, Campos AH, Soares FA, Begnami MD. Tyrosine kinase receptor expression in chordomas: phosphorylated AKT correlates inversely with outcome. Hum Pathol. 2013;44:1747–55. doi: 10.1016/j.humpath.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 4.Schwab J, Antonescu C, Boland P, Healey J, Rosenberg A, Nielsen P, Iafrate J, Delaney T, Yoon S, Choy E, Harmon D, Raskin K, Yang C, Mankin H, Springfield D, Hornicek F, Duan Z. Combination of PI3K/mTOR inhibition demonstrates efficacy in human chordoma. Anticancer Res. 2009;29:1867–71. [PubMed] [Google Scholar]
- 5.Newton AC, Trotman LC. Turning off AKT: PHLPP as a drug target. Ann Rev Pharmacol Toxicol. 2014;54:537–58. doi: 10.1146/annurev-pharmtox-011112-140338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Neill AK, Niederst MJ, Newton AC. Suppression of survival signalling pathways by the phosphatase PHLPP. FEBS J. 2013;280:572–83. doi: 10.1111/j.1742-4658.2012.08537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabattini E, Bisgaard K, Ascani S, Poggi S, Piccioli M, Ceccarelli C, Pieri F, Fraternali-Orcioni G, Pileri SA. The EnVision++ system: a new immunohistochemical method for diagnostics and research. Critical comparison with the APAAP, ChemMate, CSA, LABC, and SABC techniques. J Clin Pathol. 1998;51:506–11. doi: 10.1136/jcp.51.7.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Shu H, Li G, Cui J, Wu H, Cai S, He W, He Y, Zhan W. Loss expression of PHLPP1 correlates with lymph node metastasis and exhibits a poor prognosis in patients with gastric cancer. J Surg Oncol. 2013;108:427–32. doi: 10.1002/jso.23419. [DOI] [PubMed] [Google Scholar]
- 9.Dong L, Jin L, Tseng HY, Wang CY, Wilmott JS, Yosufi B, Yan XG, Jiang CC, Scolyer RA, Zhang XD, Guo ST. Oncogenic suppression of PHLPP1 in human melanoma. Oncogene. 2014;33:4756–66. doi: 10.1038/onc.2013.420. [DOI] [PubMed] [Google Scholar]
- 10.Chen KW, Yang HL, Lu J, Liu JY, Chen XQ. Prognostic factors of sacral chordoma after surgical therapy: a study of 36 patients. Spinal Cord. 2010;48:166–71. doi: 10.1038/sc.2009.95. [DOI] [PubMed] [Google Scholar]
- 11.Chen KW, Yang HL, Lu J, Wang GL, Ji YM, Wu GZ, Zhu LF, Liu JY, Chen XQ, Gu YP. Expression of vascular endothelial growth factor and matrix metalloproteinase-9 in sacral chordoma. J Neurooncol. 2011;101:357–63. doi: 10.1007/s11060-010-0263-0. [DOI] [PubMed] [Google Scholar]
- 12.Zhou M, Chen K, Yang H, Wang G, Lu J, Ji Y, Wu C, Chen C. Expression of insulin-like growth factor II mRNA-binding protein 3 (IMP3) in sacral chordoma. J Neurooncol. 2014;116:77–82. doi: 10.1007/s11060-013-1274-4. [DOI] [PubMed] [Google Scholar]
- 13.Chen K, Mo J, Zhou M, Wang G, Wu G, Chen H, Zhang K, Yang H. Expression of PTEN and mTOR in sacral chordoma and association with poor prognosis. Med Oncol. 2014;31:886. doi: 10.1007/s12032-014-0886-7. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Weiss HL, Rychahou P, Jackson LN, Evers BM, Gao T. Loss of PHLPP expression in colon cancer: role in proliferation and tumorigenesis. Oncogene. 2009;28:994–1004. doi: 10.1038/onc.2008.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brognard J, Sierecki E, Gao T, Newton AC. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell. 2007;25:917–31. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 16.Richardson AL, Wang ZC, De Nicolo A, Lu X, Brown M, Miron A, Liao X, Iglehart JD, Livingston DM, Ganesan S. X chromosomal abnormalities in basal-like human breast cancer. Cancer Cell. 2006;9:121–32. doi: 10.1016/j.ccr.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 17.Basso K, Margolin AA, Stolovitzky G, Klein U, Dalla-Favera R, Califano A. Reverse engineering of regulatory networks in human B cells. Nature Genet. 2005;37:382–90. doi: 10.1038/ng1532. [DOI] [PubMed] [Google Scholar]
- 18.Hao Y, Triadafilopoulos G, Sahbaie P, Young HS, Omary MB, Lowe AW. Gene expression profiling reveals stromal genes expressed in common between Barrett’s esophagus and adenocarcinoma. Gastroenterology. 2006;131:925–33. doi: 10.1053/j.gastro.2006.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nitsche C, Edderkaoui M, Moore RM, Eibl G, Kasahara N, Treger J, Grippo PJ, Mayerle J, Lerch MM, Gukovskaya AS. The phosphatase PHLPP1 regulates Akt2, promotes pancreatic cancer cell death, and inhibits tumor formation. Gastroenterology. 2012;142:377–87. e1–5. doi: 10.1053/j.gastro.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen M, Pratt CP, Zeeman ME, Schultz N, Taylor BS, O’Neill A, Castillo-Martin M, Nowak DG, Naguib A, Grace DM, Murn J, Navin N, Atwal GS, Sander C, Gerald WL, Cordon-Cardo C, Newton AC, Carver BS, Trotman LC. Identification of PHLPP1 as a tumor suppressor reveals the role of feedback activation in PTEN-mutant prostate cancer progression. Cancer Cell. 2011;20:173–86. doi: 10.1016/j.ccr.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen KW, Yang HL, Kandimalla Y, Liu JY, Wang GL. Review of current treatment of sacral chordoma. Orthop Surg. 2009;1:238–44. doi: 10.1111/j.1757-7861.2009.00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell. 2005;18:13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Sundaresan N, Huvos AG, Krol G, Lane JM, Brennan M. Surgical treatment of spinal chordomas. Arch Surg. 1987;122:1479–82. doi: 10.1001/archsurg.1987.01400240127024. [DOI] [PubMed] [Google Scholar]
- 24.Fuchs B, Dickey ID, Yaszemski MJ, Inwards CY, Sim FH. Operative management of sacral chordoma. J Bone Joint Surg Am. 2005;87:2211–6. doi: 10.2106/JBJS.D.02693. [DOI] [PubMed] [Google Scholar]


