Abstract
CD39/ectonucleoside triphosphate diphosphohydrolase-1 (ENTPD1) is a cell surface-located, rate-limiting enzyme in the generation of adenosine, and plays a crucial role in tumor development. We examined co-expression of CD39 and CD8in gastric cancer (GC) and showed that the expression of CD39 and CD8 increased significantly in tumor tissues compared to paired peritumor tissues. The expression of tumoral CD39 (tCD39), but not tumoral CD8 (tCD8), was related to overall survival. Furthermore, the CD39+/CD8+ ratio was associated with poor prognosis in resected GC patients. Taken together, our data indicate that highCD39 expression and high tCD39+/CD8+ ratio in GC is a predictor of poor prognosis for GC patients after radical resection. Moreover, CD39 could serve as a potential target for cancer immunotherapy.
Keywords: CD39, tCD39+/CD8+ ratio, gastric cancer, prognosis
Introduction
Gastric cancer (GC) is the fourth most common cancer in the world [1]. Because of improved diagnosis and treatment strategies, such as resection-based surgery and chemotherapy regimens, the overall survival rate has markedly improved in GC patients. However, GC still causes millions of death worldwide, especially in China, which accounts for 42% of cases globally [2]. Thus, it is urgent to explore the molecular mechanisms regulating gastric tumorigenesis and discover new diagnostic and prognostic markers.
The immune system plays a crucial role in controlling tumor immunogenicity. Immune cells often infiltrate tumors and the infiltration is frequently considered to be the result of interplay between host immune reactions and tumor cells [3]. Tumor-infiltrating lymphocytes (TILs) have been reported to be a significant prognostic factor in various tumors such as esophageal cancer, renal cell carcinoma, ovarian cancer, endometrial carcinoma and colorectal cancer [4-8], indicating a crucial role for immunological factors in evaluating the prognosis of patients.
Regulatory T cells (Tregs) are classified within the CD4 T lymphocyte, an important subset of TILs. Functionally, Tregs regulate T-cell immunity, block host antitumor responses by inhibiting natural killer (NK) lymphocytes or effector T cells in a tumor setting and are commonly believed to be the main obstacle in immunotherapy. Tregs express various surface antigens, including CD25, CD103 and CTLA-4, which are commonly used as Treg markers in immunology [9]. Recently, nucleoside triphosphate diphosphohydrolase-1 (ENTPD1, CD39), the rate-limiting enzyme in the generation of adenosine, was shown to be expressed by Tregs and is believed to be a novel surface marker of Tregs [10].
CD39, expressed on the cell surface of leukocytes and endothelial cells, hydrolyzes extracellular adenosine triphosphate (ATP) and adenosine diphosphate (ADP) into adenosine monophosphate (AMP), which is further processed into adenosine [11-13]. CD39 plays a crucial role in immune suppression in part because it serves as an integral component of the suppressive machinery of Tregs which inactivates and converts extracellular ATP into adenosine. Along with CD73, CD39 was used to efficiently distinguish Treg from other activated T cells in mice and humans [14]. In CD39-null mice, the differentiation of CD4-naive T cells into Th1 lineages was abolished, resulting in the spontaneous development of autoimmune diseases [15]. Moreover, CD39 dysregulation has been associated with many human cancer types, including leukemia, colon cancer, and pancreatic cancer [16-18].
The balance between CD8+ cytotoxic effector cells (CTLs) and Tregs has been shown to be important in tumor progression and prognosis. Tumor-infiltrating CD8+ T lymphocytes play important roles in anti-tumor immune responses and have prognostic value in various cancer types. Sato et al. found that intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/Treg cell ratio were associated with a favorable prognosis in ovarian cancer [19]. In a study by Shen et al., greater intratumoral infiltration by Treg cells positive for the forkhead box (Fox) transcription factor Foxp3 and a higher Foxp3+/CD8+ ratio were associated with poor prognosis in resectable gastric cancer [20].
In the present study, we examined the expression of CD39 and CD8 in 82 GC patients. Higher expression of CD39 was found in gastric tumor tissues compared to paired adjacent tissue. Moreover, increased CD39 expression and a higher tumor CD39+/CD8+ (tCD39+/CD8+) ratio were associated with poor prognosis in resected GC patients. Our findings suggest that combination therapy downregulating CD39 expression while stimulating CD8+ effector T cells could be a potential therapeutic strategy for resected GC immunotherapy.
Materials and methods
Patients
For this study, a total of 84 radical resection GC patients were collected in the Department of General Surgery, Zhongshan Hospital (Shanghai, China) between 2006 and 2010. No patients received any chemotherapy or radiation therapy before or after surgery as part of an adjuvant program. The resected specimens were evaluated according to the guidelines of the Chinese Anti-Cancer Association/Gastric Cancer Association. Stage classification was determined according to the TNM classification for gastric cancer. Formalin-fixed, paraffin-embedded specimens with complete clinicopathologic and follow-up data were selected. Written informed consent was obtained after approval by the institutional ethics committee of Zhongshan Hospital.
The final follow-up visits were completed by December 31, 2014. A minimum of 9 months follow-up was required with a median of 64.6 months (range 36-104 months). The interval between surgery and death or between surgery and the last observation of surviving patients was considered to be overall survival. Overall survival was censored at the final follow-up visit.
Immunohistochemistry
Tissue samples were fixed in formalin and embedded in paraffin. Sections (5 µm) were mounted on 3-amino-propyltriethoxysilane-coated slides. IHC was performed by the streptavidin-biotin method. Briefly, sections were deparaffinized in xylene (3 × 10 min) and dehydrated through graded alcohols (100% × 2, 95% × 2, 80% and 70%) to water. Antigen retrieval was performed by boiling the sections in citrate buffer for 10 min after blockade of endogenous peroxidase activity in 0.3% H2O2. The primary antibodies used were rabbit anti-human CD39 (1:200; Sigma-Aldrich, St Louis, MO, USA) and mouse anti-human CD8 (1:200; Sigma-Aldrich). After secondary antibody incubation, 3,3-diaminobenzidine (DAB) was used for color development and sections were counterstained in hematoxylin. Negative controls without the primary antibodies were included in all assays.
Quantification methods
For each immunolabeled slide, 10 images were randomly selected and scored by two experienced pathologists without any prior information on the clinical history of the patients. Positive staining of CD39 was evaluated by mean optical density in two computerized 200 × microscopic fields. The total number of CD8+ cells was determined in a 1.5 mm-diameter cylinder of 10 digital images using image analysis software and then averaged to calculate the final value for one computerized 400 × microscopic field. Slides were examined without knowledge of the corresponding clinicopathologic data.
Statistical analysis
Because we had no accepted standard cutoff points to determine clinical outcome, we selected the median intratumoral CD39+ cell count as the cutoff for definition of possible risk factors. Chi-squared and paired t-tests were carried out as appropriate. The overall survival was calculated by the Kaplan-Meier method and analyzed by log-rank test. Cox multivariate analysis was used to adjust for potentially confounding variables and to determine independent prognostic factors. P<0.05 was considered to be statistically significant. All analyses were performed using SPSS statistical software (SPSS version 13.0 for Windows; SPSS Inc., Chicago, IL, USA).
Results
Patient clinical profiles
A total of 84 patients were included in the analysis. The average age of the patients was 60 years (range, 32-80 years; SD = 10.25 years). Of the 84 patients, 2 patients (2.38%) were diagnosed at stage I, 31 patients (36.90%) at stage II, and 51 patients (60.71%) at stage III. By the final follow-up visit on December 31, 2014, 22 patients (26.19%) had died of their disease, while 62 (73.81%) were still alive. Overall survival was 69 months (range 5-121 months, SD = 14.13 months). The mean follow-up period was 5.38 years (range 0.69-10.06 years, SD = 3.8 years).
Correlation between CD39 expression and clinicopathological factors
Among the various clinicopathological factors, age, gender, location, histology, and Lauren score were not significantly associated with CD39 expression in univariate analysis. Moreover, tumor size (risk ratio 4.2; 95% confidence interval 0.3-9.0; P = 0.1546)and tumor differentiation (I-III; P = 0.6166) were significantly unfavorable factors (Table 1).
Table 1.
Univariate analysis of clinicopathological characteristics in gastric cancer (n = 84)
| OS | |
|---|---|
| Variables | Univariate |
| P-value | |
| TMA assays | |
| Age, year (≤60 vs. >60) | 0.1247 |
| Gender (male vs. female) | 0.9995 |
| Location (U/M/D) | 0.4691 |
| Tumor size, cm (≤3 vs. >3) | 0.1546 |
| Histology (good/poor) | 0.7321 |
| Lauren score (intestinal/diffuse) | 0.2331 |
| Tumor differentiation (I/II/III/) | 0.6166 |
| T (1/2/3/4) | 0.0004 |
| N (no/yes) | <0.0001 |
| M (no/yes) | <0.0001 |
| TNM stage (0/I/II/III/IV) | <0.0001 |
| Mean expression or counts (low vs. high) | |
| t-density-meanCD39 | 0.0316 |
| p-density-meanCD39 | 0.4313 |
| t-CD8 | 0.1931 |
We explored the correlation between clinicopathologic characteristics and tumor CD39 (tCD39) as well as peritumoral CD39 (pCD39) expression using the “minimum P-value” approach. As shown in Table 2, tCD39 expression was significantly associated with poor overall survival in GC patients.
Table 2.
Multivariate analysis demonstrating the independent risk factor for the cancer-specific death of the patients with gastric cancer (n = 84)
| The correlation between clinicopathologic characteristics and CD39 expression | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Characteristics | tCD39 | pCD39 | |||||
|
|
|
||||||
| low | high | P value | low | high | P value | ||
| Age | ≤60 | 9 | 33 | 1.000 | 18 | 24 | 1.000 |
| >60 | 9 | 33 | 17 | 25 | |||
| Gender | male | 15 | 39 | 0.094 | 19 | 35 | 0.114 |
| female | 3 | 27 | 16 | 14 | |||
| Tumor size | ≤3 cm | 10 | 39 | 0.794 | 24 | 25 | 0.122 |
| >3 cm | 8 | 27 | 11 | 24 | |||
| Histology | good | 9 | 28 | 0.601 | 14 | 23 | 0.656 |
| poor | 9 | 38 | 21 | 26 | |||
| Lanren | intestinal | 14 | 45 | 0.565 | 22 | 37 | 0.234 |
| diffuse | 4 | 21 | 13 | 12 | |||
| Tumor differentiation | I | 0 | 2 | 0.495 | 0 | 2 | 0.285 |
| II | 8 | 23 | 12 | 19 | |||
| III | 10 | 41 | 23 | 28 | |||
| Survival | yes | 17 | 45 | 0.032 | 24 | 38 | 0.431 |
| no | 1 | 21 | 11 | 11 | |||
CD39 expression and patient prognosis
The expression level of CD39 in tumor tissue was significantly higher than that in peritumor tissue (Figure 1A). We divided the 84 tissue samples into two groups according to the expression level of tCD39 or pCD39. In tumor tissue, the proportion of tCD39 high vs. low expression was 78.57% (66/84) vs. 21.43% (18/84), respectively. In peritumor tissue, the proportion of pCD39 high vs. low expression was 58.33% (49/84) vs. 41.67% (35/84), respectively.
Figure 1.
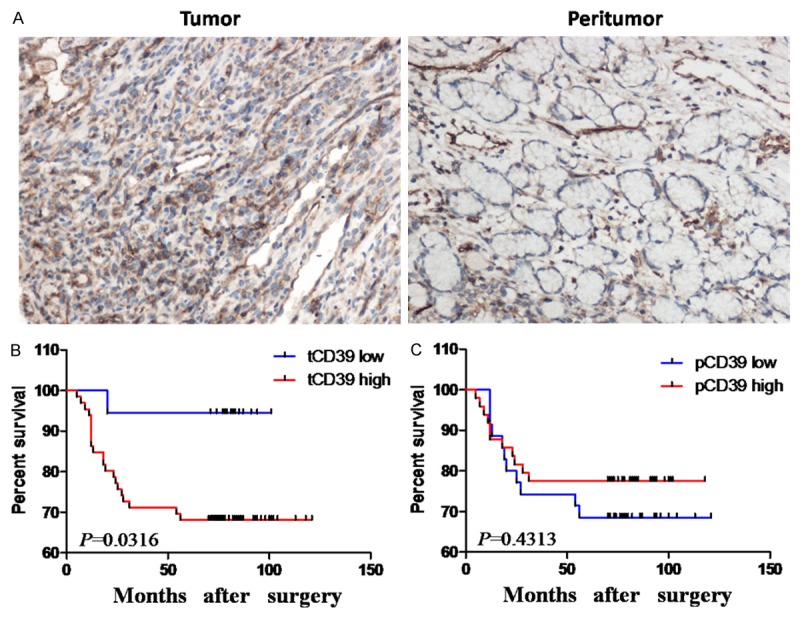
CD39 expression and overall survival analyses based on tumoral and peritumoral CD39 expression. (A) CD39 expression in tumor tissue was higher than in paired peritumor tissue. (B and C) Kaplan-Meier analysis of overall survival in relationship to tumoral CD39 (tCD39) expression (B) and peritumoral CD39 (pCD39) expression (C). Original magnification 200×.
Kaplan-Meier curves and log-rank tests showed that the overall survival of patients with high tCD39 expression was significantly lower than those with low expression (overall survival rate, low expression = 94.44% survival (17/18) vs. high expression = 68.18% survival (45/66), P = 0.032; Figure 1B and Table 2). The mean overall survival periods in tCD39 low vs. high groups were 79.22 months vs. 65.71 months.
The overall survival in patients with high pCD39 expression was better than in those with low pCD39expression (overall survival rate, low vs. high: 68.57% (24/35) vs. 77.55% (38/49), P = 0.431; Figure 1C and Table 2), but the difference was not statistically significant. The mean overall survival period of patients in the low vs. high pCD39 groups was 66.51 months vs. 70.10 months.
The overall survival rate of the tCD39 high expression group was less than that of the tCD39 low expression group. These results demonstrate that tCD39 expression was inversely correlated with prognosis of the patients with GC, indicating that tCD39 was an independent prognostic factor for poor overall survival in GC.
CD8 T lymphocytes and prognosis
CD8 expression was detected by immunohistochemistry. As shown in Figure 2A, tumor tissues had significantly higher CD8+ T cell densities compared to paired peritumor tissues, summarized in Figure 2B. Although Kaplan-Meier curve analysis and log rank tests (Figure 2C) showed longer survival of patients with high tCD8 expression, these results did not achieve statistical significance (overall survival rate, low vs. high: 70.59% (48/68) vs. 87.50% (14/16), P = 0.1931). Similarly, while the mean overall survival period was longer in the tCD8 high expression group (low vs. high, 64.22months vs. 87.25 months), the difference between groups was not significant. These results thus indicated that tCD8 expression was not significantly related to overall survival of GC patients.
Figure 2.
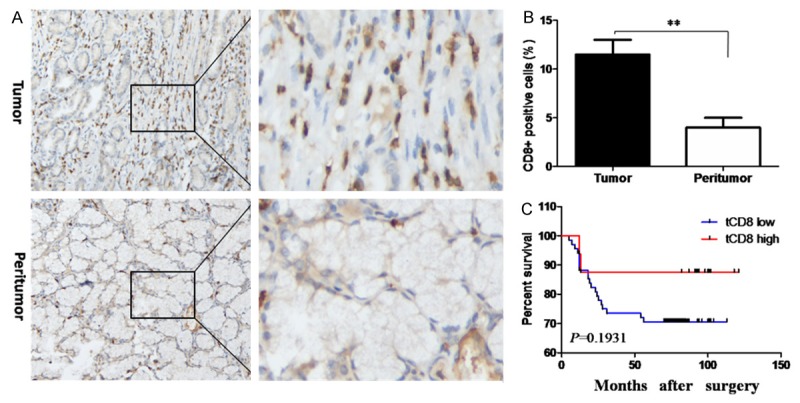
CD8 expression and overall survival analyses based on tumoral CD8 expression. A. Representative IHC staining of CD8 in tumor and peritumor tissues. Original magnification 200×, left panels; 600×, rightpanels. B. Mean number of CD8 positive cells in tumor and peritumor tissues. C. Kaplan-Meier analysis of overall survival in relationship to tumor CD8 expression.
Prognostic significance of tCD39+/CD8+ ratios in GC
The prognostic role of tCD39+/CD8+ and pCD39+/CD8+ ratios was also evaluated. As shown in Figure 3A, overall survival in patients with a high tCD39+/CD8+ ratio was significantly lower than in patients with a low ratio (overall survival rate, low vs. high: 84.00% (42/50) vs. 58.82% (20/34), P = 0.0081; Figure 3A). In contrast, overall survival in patients with high pCD39+/CD8+ ratios did not significantly differ from patients with a low pCD39+/CD8+ ratio (overall survival rate, low vs. high: 75.00% (42/56) vs. 71.43% (20/28; Figure 3B). These results demonstrate that the tCD39+/CD8+ ratio was a significant prognostic indicator for poor overall survival in GC.
Figure 3.
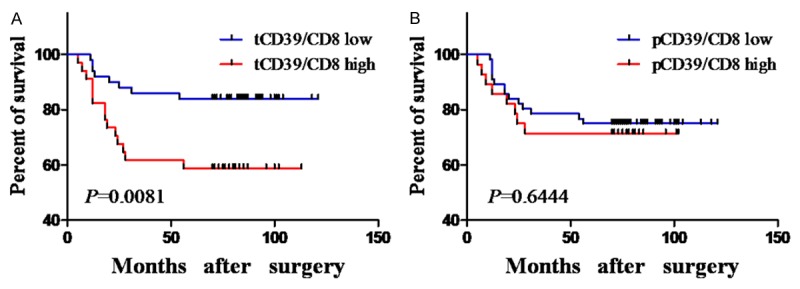
Overall survival analyses based on tCD39+/CD8+ and pCD39+/CD8+ ratios. (A and B) Kaplan-Meier analysis of overall survival in relationship to the tCD39+/CD8+ ratio (A) and pCD39+/CD8+ ratio (B).
Discussion
GC is one of the most common human cancers, and generally has a poor clinical outcome. The molecular mechanisms regulating GC tumorigenesis and progression remain poorly understood. Recent studies have shown that CD39, one member of the ecto-nucleoside triphosphate diphospohydrolase (E-NTPDase) family, plays a crucial role regulating cancer progression in many human cancers. In the present study, we examined expression of CD39 and CD8in tumor and peritumor tissues of GC patients. Tumor tissue had greater expression of CD39 and CD8 and a higher tCD39+/CD8+ ratio compared to paired peritumor tissues. High CD39 expression and tCD39+/CD8+ ratios were associated with poor prognosis in resected GC patients.
CD39 has been reported to be present on the surface of lymphocytes, endothelial cells, regulatory T cells [21,22], activated NK and T cells [11,23]. Dysregulation of CD39 was reported in various cancers, but few studies have been performed on theCD39 expression pattern in GC. In this study, we examined CD39 expression in GC using IHC and found that expression was upregulated in tumor tissues compared to paired peritumor tissues, suggesting a role for CD39 in GC.
CD39 plays a crucial role in hydrolyzing extracellular ATP and ADP to AMP, which is involved in multiple proinflammatory effects [24]. CD39 expression on FoxP3+ Treg cells is genetically driven and is increased at inflammation sites, where it plays an important role in mediating immune suppression [25]. In mice, the expression level of CD39 on CD4+CD25+ Treg cells is significantly enhanced by T-cell receptor (TCR) ligation, but its expression in humans is confined to Foxp3+ Treg cells [26]. Aided by co-expression of surface CD73, CD4+CD39+ Treg cells in humans produce adenosine, which is important in immunosuppressive reactions [2-7]. The tissue FoxP3+ Treg cells expressing CD39/CD73 were suggested to be potential therapeutic targets in patients with psoriasis [28]. In our study, we found high CD39 expression was related to poor prognosis in resected GC.
In patients with follicular lymphoma, CD39+ infiltrating T cells were greatly increased compared to healthy subjects and contributed to T cell hyporesponsiveness mediated by adenosine [29]. The ratio of CD39+FoxP3+ to FoxP3+ Treg cells in peripheral blood mononuclear cells (PBMC) of patients with head and neck cancer was significantly higher than in normal subjects [30]. Wang and colleagues showed that sepsis patients had a higher percentage and increased mean fluorescence intensity of circulating CD39+ Treg cells in PBMC when compared with healthy subjects. Moreover, the number of CD39+ Treg cells was positively correlated with the severity of sepsis and survival of sepsis patients, revealing a prognostic role for CD39 in sepsis [31]. Our study confirmed the importance of tCD39, but not pCD39, as a prognostic factor in GC.
Previous studies have shown that intratumoral CD8+ T lymphocytes could serve as prognostic factors in various cancers. Hamanishi et al. found that greater frequency of tumor-infiltrating CD8+ T lymphocytes predicted good overall survival and progression-free survival in ovarian cancer [6]. In prostate cancer, infiltration of CD8+ T lymphocytes has been used as an independent prognostic factor for biochemical failure-free survival [32]. Moreover, the prognostic role of tumor-infiltrating CD8+ T lymphocytes was also demonstrated in breast cancer, renal cell carcinoma and esophageal carcinomas [4,33]. In the present study, we found that patients with greater numbers of tumor-infiltrating CD8+ T lymphocytes had better overall survival, but the effect was not significant, possibly because of an insufficient number of cases. However, our results suggested that the tCD39+/CD8+ ratio could serve as an independent prognostic factor in GC patients. To our knowledge, this is the first report that demonstrates the prognostic value of the tCD39+/CD8+ ratio in GC.
Taken together, our data demonstrate that CD39 is highly expressed in GC. CD39 expression and the tCD39+/CD8+ ratio maybe useful biomarkers for predicting the outcome of GC. Finally, CD39 may be a potential therapeutic target for GC.
Acknowledgements
This work was supported by grants from Science and Technology Commission Foundation of Pudong New Area, Shanghai, China (Grant No. PKJ2011-Y02).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Zhou Z, Ji Z, Wang Y, Li J, Cao H, Zhu HH, Gao WQ. TRIM59 is up-regulated in gastric tumors, promoting ubiquitination and degradation of p53. Gastroenterology. 2014;147:1043–1054. doi: 10.1053/j.gastro.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 3.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 4.Schumacher K, Haensch W, Roefzaad C, Schlag PM. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res. 2001;61:3932–3936. [PubMed] [Google Scholar]
- 5.Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, Suzuki Y, Shintaku I, Nagura H, Ohtani H. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res. 2001;61:5132–5136. [PubMed] [Google Scholar]
- 6.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, Honjo T, Fujii S. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kondratiev S, Sabo E, Yakirevich E, Lavie O, Resnick MB. Intratumoral CD8+ T lymphocytes as a prognostic factor of survival in endometrial carcinoma. Clin Cancer Res. 2004;10:4450–4456. doi: 10.1158/1078-0432.CCR-0732-3. [DOI] [PubMed] [Google Scholar]
- 8.Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–3494. [PubMed] [Google Scholar]
- 9.Piccirillo CA, Thornton AM. Cornerstone of peripheral tolerance: naturally occurring CD4+CD25+ regulatory T cells. Trends Immunol. 2004;25:374–380. doi: 10.1016/j.it.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marcus AJ, Broekman MJ, Drosopoulos JH, Islam N, Alyonycheva TN, Safier LB, Hajjar KA, Posnett DN, Schoenborn MA, Schooley KA, Gayle RB, Maliszewski CR. The endothelial cell ecto-ADPase responsible for inhibition of platelet function is CD39. J Clin Invest. 1997;99:1351–1360. doi: 10.1172/JCI119294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plesner L. Ecto-ATPases: identities and functions. Int Rev Cytol. 1995;158:141–214. doi: 10.1016/s0074-7696(08)62487-0. [DOI] [PubMed] [Google Scholar]
- 13.Enjyoji K, Sevigny J, Lin Y, Frenette PS, Christie PD, Esch JS 2nd, Imai M, Edelberg JM, Rayburn H, Lech M, Beeler DL, Csizmadia E, Wagner DD, Robson SC, Rosenberg RD. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med. 1999;5:1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- 14.Dwyer KM, Deaglio S, Gao W, Friedman D, Strom TB, Robson SC. CD39 and control of cellular immune responses. Purinergic Signal. 2007;3:171–180. doi: 10.1007/s11302-006-9050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erdmann AA, Gao ZG, Jung U, Foley J, Borenstein T, Jacobson KA, Fowler DH. Activation of Th1 and Tc1 cell adenosine A2A receptors directly inhibits IL-2 secretion in vitro and IL-2-driven expansion in vivo. Blood. 2005;105:4707–4714. doi: 10.1182/blood-2004-04-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pulte D, Furman RR, Broekman MJ, Drosopoulos JH, Ballard HS, Olson KE, Kizer JR, Marcus AJ. CD39 expression on T lymphocytes correlates with severity of disease in patients with chronic lymphocytic leukemia. Clin Lymphoma Myeloma Leuk. 2011;11:367–372. doi: 10.1016/j.clml.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunzli BM, Bernlochner MI, Rath S, Kaser S, Csizmadia E, Enjyoji K, Cowan P, d’Apice A, Dwyer K, Rosenberg R, Perren A, Friess H, Maurer CA, Robson SC. Impact of CD39 and purinergic signalling on the growth and metastasis of colorectal cancer. Purinergic Signal. 2011;7:231–241. doi: 10.1007/s11302-011-9228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunzli BM, Berberat PO, Giese T, Csizmadia E, Kaczmarek E, Baker C, Halaceli I, Buchler MW, Friess H, Robson SC. Upregulation of CD39/NTPDases and P2 receptors in human pancreatic disease. Am J Physiol Gastrointest Liver Physiol. 2007;292:G223–230. doi: 10.1152/ajpgi.00259.2006. [DOI] [PubMed] [Google Scholar]
- 19.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen Z, Zhou S, Wang Y, Li RL, Zhong C, Liang C, Sun Y. Higher intratumoral infiltrated Foxp3+ Treg numbers and Foxp3+/CD8+ ratio are associated with adverse prognosis in resectable gastric cancer. J Cancer Res Clin Oncol. 2010;136:1585–1595. doi: 10.1007/s00432-010-0816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun X, Wu Y, Gao W, Enjyoji K, Csizmadia E, Muller CE, Murakami T, Robson SC. CD39/ENTPD1 expression by CD4+Foxp3+ regulatory T cells promotes hepatic metastatic tumor growth in mice. Gastroenterology. 2010;139:1030–1040. doi: 10.1053/j.gastro.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ring S, Oliver SJ, Cronstein BN, Enk AH, Mahnke K. CD4+CD25+ regulatory T cells suppress contact hypersensitivity reactions through a CD39, adenosine-dependent mechanism. J Allergy Clin Immunol. 2009;123:1287–1296. e1282. doi: 10.1016/j.jaci.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 23.Favaloro EJ. Differential expression of surface antigens on activated endothelium. Immunol Cell Biol. 1993;71:571–581. doi: 10.1038/icb.1993.63. [DOI] [PubMed] [Google Scholar]
- 24.Dwyer KM, Hanidziar D, Putheti P, Hill PA, Pommey S, McRae JL, Winterhalter A, Doherty G, Deaglio S, Koulmanda M, Gao W, Robson SC, Strom TB. Expression of CD39 by human peripheral blood CD4+ CD25+ T cells denotes a regulatory memory phenotype. Am J Transplant. 2010;10:2410–2420. doi: 10.1111/j.1600-6143.2010.03291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rissiek A, Baumann I, Cuapio A, Mautner A, Kolster M, Arck PC, Dodge-Khatami A, Mittrucker HW, Koch-Nolte F, Haag F, Tolosa E. The expression of CD39 on regulatory T cells is genetically driven and further upregulated at sites of inflammation. J Autoimmun. 2015;58:12–20. doi: 10.1016/j.jaut.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Hopner S, Centonze D, Bernardi G, Dell’Acqua ML, Rossini PM, Battistini L, Rotzschke O, Falk K. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 27.Schuler PJ, Saze Z, Hong CS, Muller L, Gillespie DG, Cheng D, Harasymczuk M, Mandapathil M, Lang S, Jackson EK, Whiteside TL. Human CD4+ CD39+ regulatory T cells produce adenosine upon co-expression of surface CD73 or contact with CD73+ exosomes or CD73+ cells. Clin Exp Immunol. 2014;177:531–543. doi: 10.1111/cei.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang HY, Yan KX, Huang Q, Ma Y, Fang X, Han L. Target tissue ectoenzyme CD39/CD73-expressing Foxp3+ regulatory T cells in patients with psoriasis. Clin Exp Dermatol. 2015;40:182–191. doi: 10.1111/ced.12497. [DOI] [PubMed] [Google Scholar]
- 29.Hilchey SP, Kobie JJ, Cochran MR, Secor-Socha S, Wang JC, Hyrien O, Burack WR, Mosmann TR, Quataert SA, Bernstein SH. Human follicular lymphoma CD39+-infiltrating T cells contribute to adenosine-mediated T cell hyporesponsiveness. J Immunol. 2009;183:6157–6166. doi: 10.4049/jimmunol.0900475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandapathil M, Lang S, Gorelik E, Whiteside TL. Isolation of functional human regulatory T cells (Treg) from the peripheral blood based on the CD39 expression. J Immunol Methods. 2009;346:55–63. doi: 10.1016/j.jim.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang H, Xu R, Lin F, Bao C, Wang S, Ji C, Li K, Jin L, Mu J, Wang Y, Li L, Sun L, Xu B, Zhang Z, Wang FS. High circulating CD39(+) regulatory T cells predict poor survival for sepsis patients. Int J Infect Dis. 2015;30:57–63. doi: 10.1016/j.ijid.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Ness N, Andersen S, Valkov A, Nordby Y, Donnem T, Al-Saad S, Busund LT, Bremnes RM, Richardsen E. Infiltration of CD8+ lymphocytes is an independent prognostic factor of biochemical failure-free survival in prostate cancer. Prostate. 2014;74:1452–1461. doi: 10.1002/pros.22862. [DOI] [PubMed] [Google Scholar]
- 33.Mella M, Kauppila JH, Karihtala P, Lehenkari P, Jukkola-Vuorinen A, Soini Y, Auvinen P, Vaarala MH, Ronkainen H, Kauppila S, Haapasaari KM, Vuopala KS, Selander KS. Tumor infiltrating CD8 T lymphocyte count is independent of tumor TLR9 status in treatment naive triple negative breast cancer and renal cell carcinoma. Oncoimmunology. 2015;4:e1002726. doi: 10.1080/2162402X.2014.1002726. [DOI] [PMC free article] [PubMed] [Google Scholar]


