Abstract
Peripheral blood-derived inflammation-based scores such as the neutrophil-lymphocyte ratio (NLR) have recently been proposed as prognostic markers in ulcerative colitis. In some previous serological markers are commonly used to detect the severity of the Crohn’s disease (CD), but their sensitivity and specificity are relatively low. So we want to use simple indicators which are easy to obtain to predict disease severity. Now, we investigated and compared the capacity of NLR and other inflammatory markers in detecting CD activity and differentiating CD patients from healthy controls. These CD patients had not received corticosteroid or immunosuppressive drugs within a defined period of time. Data from our hospital between 2010 and 2012 was used. Neutrophil-lymphocyte ratio (NLR), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), white blood cells (WBC), platelet count and albumin were measured in 44 patients with active CD, 66 patients with inactive CD, and 55 healthy blood donors. Disease activity was assessed by the Crohn’s Disease Activity Index. In the active CD group, NLR values were found to be elevated compared to inactive CD patients and controls (6.00±7.38, 5.53±6.18 and 1.84±0.85, respectively), but statistical difference was not found between active and inactive CD groups. The overall accuracy of NLR (cutoff: 2.13 fl), CRP (cutoff: 10.5 mg/dl), ESR (cutoff: 19.5 mm/hour) and WBC (cutoff: 9.2 × 109/l) in differentiating CD patients from healthy controls was 80.9%, 67.3%, 71% and 60% respectively. NLR values were found to be correlated with WBC and CRP levels. NLR increased in CD patients compared with healthy subjects. NLR had the best accuracy in determination of CD patients and healthy controls. NLR did not show a discriminative value in disease activity.
Keywords: Crohn’s disease, neutrophil-lymphocyte ratio, noninvasive monitoring
Introduction
Crohn’s disease (CD) is chronic relapsing and remitting diseases of the bowel, with an unknown etiology and appears to involve interaction between genetic susceptibility, environmental factors and the immune system. Previous studies suggested that early detection of disease activity could significantly reduce the mortality of CD [1]. Non-invasive tests, such as C reactive protein (CRP), erythrocyte sedimentation rate (ESR), white blood cells (WBC), acid glycoprotein, platelet count and albumin are therefore being increasingly recognized as important markers for initial diagnosis and disease activity detection [2].
A simple, inexpensive and effective marker of inflammation that has been linked with several inflammatory and neoplastic diseases is the neutrophil-lymphocyte ratio (NLR). NLR on the outcome of many kinds of malignancies, including colorectal cancer, ovarian cancer, gastric cancer, intrahepatic cholangiocarcinoma, hepatocellular carcinoma and pancreatic cancer has been well demonstrated [3-10].
Materials and methods
Patients and methods
We prospectively collected 110 CD patients and 55 healthy subjects between January 2010 and December 2011 (Figure 1). The control group consisted of 55 healthy, age and gender matched subjects (male/female: 32/23). The diagnosis of CD was based on standard clinical, radiological, endoscopic and histological criteria. The classification of patients with CD was based on the Vienna classification of Crohn’s disease [11]. The following data were extracted from the hospital database: age, sex, body mass index, smoking history, behavior, Extraintestinal manifestations, activity, treatment and localization of the disease. Complete blood count (CBC) ESR, and CRP were also recorded for each CD patient. All CBC analysis was performed in hematology laboratory of our hospital. CBC analysis was performed with the same analyzer within 2 hours after collection of blood samples with the use of a Beckman Coulter (High Wycombe, UK) Gen-S automated analyzer.
Figure 1.
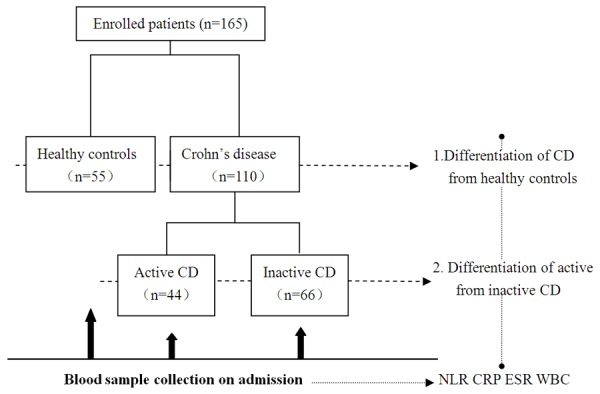
Study design. A total of 165 subjects were enrolled in the current study. 55 healthy controls were differentiated with 110 Crohn’s disease (CD) patients using neutrophil-lymphocyte ratio (NLR), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR) and white blood cells (WBC). Furthermore, the 110 CD patients were divided into active (n = 44) and inactive (n = 66) groups and distinguished using the same inflammatory biomarkers. All blood sample collections were obtained on admission (before any medication or procedure). Abbreviations: CD, Crohn’s disease; NLR, neutrophil-lymphocyte ratio; CRP, C-reative protein; ESR, erythrocyte sedimentation rate; WBC, white blood cell.
Exclusion criteria for the entry into the study can be summarized as prior treatment with corticosteroids, hematological or neoplastic disorders, and clinical evidence of active infection, since NLR may be affected by those conditions.
Disease activity
For CD patients, the disease activity was defined according to the Crohn’s Disease Activity Index (CDAI) [12]. Patients were further divided into an active CD group (CDAI > 150) and an inactive CD group (CDAI < 150) based on the number bloody stools per day, degree of abdominal pain, general health, complication, body temperature and hematocrit, abdominal mass.
Statistical analysis
The Statistical Package for Social Sciences (SPSS) 19.0 for Windows was used to analyze the data. Continuous variables were tested for normality by the Kolmogorov-Smirnov test. Values were presented as mean ± standard deviation or, in the case of non-normally distributed data, as median and range. For categorical variables, percentages were provided and the chi-squared test was used. Independent-samples t-test, paired t-test, one-way analysis of variance parametric tests, and Mann-Whitney U, Wilcoxon-t, and Kruskal-Wallis H nonparametric tests were used for the comparison of continuous variables. Receiver operating characteristic (ROC) curve analysis was used to identify optimal cut-off values of NLR and other inflammatory markers. The overall accuracy was also calculated by additional true-positive and true negative test results divided by all tests: (a + d)/(a + b +c + d).
Results
The demographic features of CD patients and healthy controls are shown in Table 1. The distributions of age, gender, smoking habit and body mass index were not statistically significant between groups.
Table 1.
Demographics of patients and controls
| Crohn’s disease (n = 110) | Control group (n = 55) | P | |
|---|---|---|---|
| Age (years) | 33.5±14.1 | 47.3±9.43 | NS |
| Male (%) | 58.2 | 64.5 | NS |
| Smoking | 35 (31.8%) | 15 (27.3%) | NS |
| Boby mass index (kg/m2) | 19.7±2.73 | 19.1±2.13 | NS |
| Active disease | 44 (66.7%) | - | - |
| Disease location (%) | |||
| A1 (ileal) | 42 (38.2%) | - | - |
| A2 (colonic) | 45 (40.9%) | - | - |
| A3 (ileocolonic) | 23 (20.9%) | - | - |
| +A4 (upper gastrointestinal tract) | 0 (0%) | - | - |
| Disease behavior (%) | |||
| B1 (inflammatory) | 68 (61.8%) | - | - |
| B2 (penetrating) | 15 (13.6%) | - | - |
| B3 (stricturing) | 27 (24.5%) | - | - |
| Treatment | |||
| 51 (46.4%) | - | - | |
| 16 (14.5%) | - | - | |
| 43 (39.1%) | - | - | |
| Intestinal manifestations | 25 (22.7%) | - | - |
Data are presented as median (range) or mean ± SD. NS: non-significant.
The mean NLR values of CD patients and controls were 5.72±6.66 and 1.84±0.85, respectively (P < 0.001). Mean NLR values of active CD patients and inactive CD patients were significantly higher than those of control CD patients (6.00±7.38 and 5.53±6.18 vs. 1.84±0.85) (P < 0.001) (Figure 2).
Figure 2.
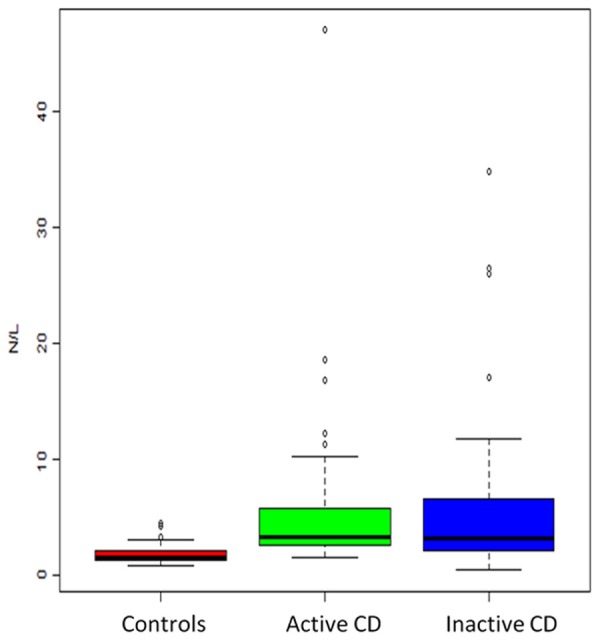
Box-plot representation of neutrophil to lymphocyte ratio (NLR) in patients with CD (active and inactive) and healthy controls.
Table 2 demonstrates that there is a significant decline in NLR of CD patients compared with healthy controls (5.72±6.66 vs. 1.84±0.85, P < 0.001). Meanwhile, CRP (36.05±45.2 mg/dl vs. 8.48±6.44 mg/dl, P < 0.001), ESR (27.41±19.24 mm/h vs. 11.79±5.81 mm/h, P < 0.001) and WBC (11.57±7.81 × 109/l vs. 7.35±3.57 × 109/l, P < 0.001) were statistically higher in the CD group than those in the control group. Mean NLR values of active CD patients were significantly higher than those of control group (6.00±7.38 vs. 1.84±0.85) (P < 0.001) (Figure 1). Table 3 shows mean NLR values and the other inflammatory markers of study participants at the onset of the study. No significant differences were observed with respect to NLR, WBC and ESR levels between study participants.
Table 2.
Comparison of NLR and other inflammatory markers between Crohn’s disease and control groups
| Crohn’s disease (n = 110) | Control group (n = 55) | P value | |
|---|---|---|---|
| NLR | 5.72±6.66 | 1.84±0.85 | < 0.001 |
| CRP (mg/dl) | 36.05±45.2 | 8.48±6.44 | < 0.001 |
| ESR (mm/h) | 27.41±19.24 | 11.79±5.81 | < 0.001 |
| WBC (× 109/l) | 11.57±7.81 | 7.35±3.57 | < 0.001 |
NLR, neutrophil-lymphocyte ratio; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; WBC, white blood cells.
Table 3.
Comparison of NLR and other inflammation markers between active and inactive CD patients
| Active CD (n = 44) | Inactive CD (n = 66) | P | |
|---|---|---|---|
| NLR | 6.00±7.38 | 5.53±6.18 | NS |
| CRP (mg/dl) | 48.53±52.10 | 27.74±38.23 | < 0.001 |
| ESR (mm/h) | 37.09±19.14 | 20.95±16.50 | NS |
| WBC (× 109/l) | 10.84±6.79 | 8.01±3.61 | NS |
CD: Crohn’s disease; WBC: white blood cells; NLR: neutrophil- lymphocyte ratio; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; NS: non-significant.
Although Spearman correlation analysis indicated a significant correlation of NLR with WBC (r = 0.493, P < 0.001) and CRP (r = 0.327, P < 0.001), no correlation was found with ESR (r = 0.137, P = 0.082).
ROC curve analysis suggested that the optimum NLR cut-off point for active UC was 2.13, with a sensitivity and specificity of 82.7%, 76.9% respectively (AUC: 0.85) (Figure 3). The overall accuracy of NLR in determination of active UC was 80.9%. The same analysis for other inflammation markers is summarized in Table 4.
Figure 3.
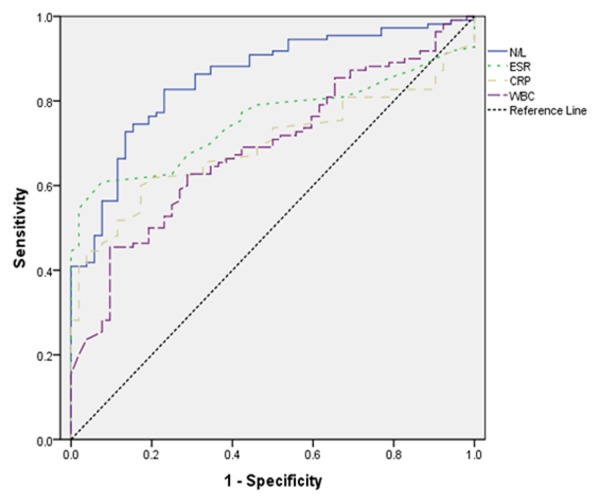
Receiver operating characteristic (ROC) curve of neutrophil to lymphocyte ratio (NLR) vs. other inflammation markers in predicting active disease for CD.
Table 4.
Accuracy and ROC analyses of NLR and other inflammatory markers in differentiate patients and controls
| AUC | Sensitivity (%) | Specificity (%) | Overall Accuracy (%) | |
|---|---|---|---|---|
| NLR (cutoff: 2.13) | 0.8545 | 82.7 | 76.9 | 80.9 |
| ESR (cutoff: 19.5) | 0.7540 | 60.9 | 92.3 | 71 |
| CRP (cutoff: 10.5) | 0.6961 | 60 | 82.6 | 67.3 |
| WBC (cutoff: 9.2) | 0.6892 | 45.4 | 90.3 | 60 |
AUC, area under the curve; NLR, neutrophil-lymphocyte ratio; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; ROC, receiver operating characteristic; WBC, white blood cells.
Discussion
Crohn’s disease (CD) is a chronic inflammatory bowel disease with a complex etiology involving genetic factors, priming by enteric microflora, environmental factors and an alteration in the immune-mediated response [13]. Previous studies have demonstrated that appropriate and effective therapy could significantly control symptoms, maintain remission, prevent relapse, improve quality of life and reduce mortality [14]. The early determination of diagnosis and detection of disease activity are therefore essential for tailoring therapy [15].
As invasive techniques, including endoscopic, radiological and histopathologic methods, are routinely used for diagnostic decision and disease activity supervision, an ideal non-invasive test is increasingly expected for initial diagnosis and identification of disease activity [16].
In CD, the intestinal inflammation is confined to the colon mucosa. In active disease, this results in specific symptomatology with frequent diarrhea and blood loss. The Truelove and Witts score was the first index used to quantify disease activity in UC. Disadvantages of this index are that the difficulty of classifying some patients in the appropriate disease category, and changes in disease activity over time are difficult to quantify [17]. Other activity indices have been proposed by various authors. Endoscopic assessment of disease activity is important because the complaints of the patients do not always correspond to the severity and extent of the disease. Rachmilewitz developed an endoscopic index, scoring for granularity, vascular pattern, vulnerability, and mucosal damage [18]. The Rachmilewitz score is numerical and has been used in clinical trials. To combine the advantages of the clinical Truelove and Witts index and the endoscopic Rachmilewitz score, the DAI score from the Mayo clinic was elaborated [19]. Currently, the Mayo score is the most used in clinical studies.
To monitor accurately intestinal inflammation, symptoms and clinical examination, combined with endoscopy and histology, are required. Because of the invasiveness of endoscopy, several laboratory markers have been evaluated.
Although there is no ideal single serum marker for predicting disease severity, white blood cell count, CRP and ESR are the most commonly used inflammatory indices in routine clinical practice for determining CD activity. These parameters can change according to the degree of the inflammatory state, but they do not adequately reflect disease activity because of their low sensitivity and specificity for intestinal inflammation [20].
C-reactive protein (CRP) is a marker of inflammation, and serum CRP concentration reflects disease activity in patients with CD [21]. CRP is a pentameric protein produced almost exclusively by hepatocytes in response to stimulation by Interleukin 6, Interleukin 1α, and tumor necrosis factor β [22]. CRP is the most important acute-phase protein. The baseline concentration of CRP is 1 mg/l and levels are partially genetically regulated. Levels of CRP increase dramatically in the presence of an acute-phase inflammation or infection. CRP concentrations also quickly decrease when the inflammation process is treated [23].
Recently, a series of stool tests, such as fecal lactoferrin, calprotectin and elastase, were investigated as novel inflammatory markers. Even though they may be superior to CRP or ESR with higher sensitivity and specificity in detecting gastrointestinal inflammation, they are not specific markers for IBD; and they are inconvenient and unpleasant for stool sampling [15,24,25]. NLR is a simple and inexpensive index of systemic inflammatory burden that correlates with prognosis in distinct disease states. It has been generally investigated in inflammatory and neoplastic diseases, such as acute pancreatitis, ulcerative colitis, colorectal cancer, hepatocellular, ovarian, nasopharyngeal, and metastatic renal cell carcinoma, recurrent optic neuritis, critical limb ischemia, esophageal squamous cell carcinoma, as a prognostic index [3-10,26-32]. The NLR is correlated with disease severity in patients with nonalcoholic fatty liver disease [33]. Elevated levels of NLR were also found to be associated with poor survival in patients after percutaneous coronary intervention and in those undergoing coronary artery bypass graft [34,35].
Neutrophils, one of the most abundant and important mediators of innate immunity, are professional phagocytes which mount the acute inflammatory response and act as the first line of defense against invading pathogens [36]. The role of neutrophils in CD pathology remains obscure. Impaired neutrophils function may result in limited bacterial clearance and fuel an on-going, chronic inflammatory response. Neutrophils accumulation within epithelial crypts and in the intestinal lumen directly correlates with clinical disease activity and epithelial injury. On the other hand, previous studies in patients with inflammatory bowel disease have strongly revealed that their lymphocyte function is abnormal at both the peripheral and mucosal level [37].
In conclusion, our study demonstrated increase of MPV in CD patients compared with healthy controls. We also compared NLR with other inflammatory markers including CRP, ESR and WBC. NLR had the best accuracy in determination of CD patients and healthy controls. NLR did not show a discriminative value in disease activity. Finally, we suggested that it should be cautious to use MPV as a marker in determination of CD activity. Large multicenter studies are expected to resolve the controversy.
Disclosure of conflict of interest
None.
References
- 1.Sandborn WJ, Loftus EV Jr, Colombel JF, Fleming KA, Seibold F, Homburger HA, Sendid B, Chapman RW, Tremaine WJ, Kaul DK, Wallace J, Harmsen WS, Zinsmeister AR, Targan SR. Evaluation of serologic disease markers in a population-based cohort of patients with ulcerative colitis and Crohn’s disease. Inflamm Bowel Dis. 2001;7:192–201. doi: 10.1097/00054725-200108000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Mack DR, Langton C, Markowitz J, LeLeiko N, Griffiths A, Bousvaros A, Evans J, Kugathasan S, Otley A, Pfefferkorn M, Rosh J, Mezoff A, Moyer S, Oliva-Hemker M, Rothbaum R, Wyllie R, delRosario JF, Keljo D, Lerer T, Hyams J Pediatric Inflammatory Bowel Disease Collaborative Research Group. Laboratory values for children with newly diagnosed inflammatory bowel disease. Pediatrics. 2007;119:1113–1119. doi: 10.1542/peds.2006-1865. [DOI] [PubMed] [Google Scholar]
- 3.Halazun KJ, Aldoori A, Malik HZ, Al-Mukhtar A, Prasad KR, Toogood GJ, Lodge JP. Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. Eur J Surg Oncol. 2008;34:55–60. doi: 10.1016/j.ejso.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Kishi Y, Kopetz S, Chun YS, Palavecino M, Abdalla EK, Vauthey JN. Blood neutrophil-tolymphocyte ratio predicts survival in patients with colorectal liver metastases treated with systemic chemotherapy. Ann Surg Oncol. 2009;16:614–622. doi: 10.1245/s10434-008-0267-6. [DOI] [PubMed] [Google Scholar]
- 5.Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y, Fukushima M. The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology. 2007;73:215–220. doi: 10.1159/000127412. [DOI] [PubMed] [Google Scholar]
- 6.Cho H, Hur HW, Kim SW, Kim SH, Kim JH, Kim YT, Lee K. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol Immunother. 2009;58:15–23. doi: 10.1007/s00262-008-0516-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez D, Morris-Stiff G, Toogood GJ, Lodge JP, Prasad KR. Impact of systemic inflammation on outcome following resection for intrahepatic cholangiocarcinoma. J Surg Oncol. 2008;97:513–518. doi: 10.1002/jso.21001. [DOI] [PubMed] [Google Scholar]
- 8.Gomez D, Farid S, Malik HZ, Young AL, Toogood GJ, Lodge JP, Prasad KR. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg. 2008;32:1757–1762. doi: 10.1007/s00268-008-9552-6. [DOI] [PubMed] [Google Scholar]
- 9.Halazun KJ, Hardy MA, Rana AA, Woodland DCt, Luyten EJ, Mahadev S, Witkowski P, Siegel AB, Brown RS Jr, Emond JC. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg. 2009;250:141–151. doi: 10.1097/SLA.0b013e3181a77e59. [DOI] [PubMed] [Google Scholar]
- 10.An X, Ding PR, Li YH, Wang FH, Shi YX, Wang ZQ, He YJ, Xu RH, Jiang WQ. Elevated neutrophil to lymphocyte ratio predicts survival in advanced pancreatic cancer. Biomarkers. 2010;15:516–522. doi: 10.3109/1354750X.2010.491557. [DOI] [PubMed] [Google Scholar]
- 11.Gasche C, Scholmerich J, Brynskov J, D’Haens G, Hanauer SB, Irvine EJ, Jewell DP, Rachmilewitz D, Sachar DB, Sandborn WJ, Sutherland LR. A simple classification of Crohn’s disease: report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998. Inflamm Bowel Dis. 2000;6:8–15. doi: 10.1097/00054725-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976;70:439–444. [PubMed] [Google Scholar]
- 13.Marks DJ, Rahman FZ, Sewell GW, Segal AW. Crohn’s disease: an immune deficiency state. Clin Rev Allergy Immunol. 2010;38:20–31. doi: 10.1007/s12016-009-8133-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong A, Bass D. Laboratory evaluation of inflammatory bowel disease. Curr Opin Pediatr. 2008;20:566–570. doi: 10.1097/MOP.0b013e32830d3aaf. [DOI] [PubMed] [Google Scholar]
- 15.Langhorst J, Elsenbruch S, Koelzer J, Rueffer A, Michalsen A, Dobos GJ. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol. 2008;103:162–169. doi: 10.1111/j.1572-0241.2007.01556.x. [DOI] [PubMed] [Google Scholar]
- 16.Bruining DH, Loftus EV. Current and future diagnostic approaches: from serologies to imaging. Curr Gastroenterol Rep. 2007;9:489–496. doi: 10.1007/s11894-007-0065-5. [DOI] [PubMed] [Google Scholar]
- 17.Naber AH, de Jong DJ. Assessment of disease activity in inflammatory bowel disease; relevance for clinical trials. Neth J Med. 2003;61:105–110. [PubMed] [Google Scholar]
- 18.Rachmilewitz D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ. 1989;298:82–86. doi: 10.1136/bmj.298.6666.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625–1629. doi: 10.1056/NEJM198712243172603. [DOI] [PubMed] [Google Scholar]
- 20.Khan K, Schwarzenberg SJ, Sharp H, Greenwood D, Weisdorf-Schindele S. Role of serology and routine laboratory tests in childhood inflammatory bowel disease. Inflamm Bowel Dis. 2002;8:325–329. doi: 10.1097/00054725-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Solem CA, Loftus EV Jr, Tremaine WJ, Harmsen WS, Zinsmeister AR, Sandborn WJ. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:707–712. doi: 10.1097/01.mib.0000173271.18319.53. [DOI] [PubMed] [Google Scholar]
- 22.Tall AR. C-reactive protein reassessed. N Engl J Med. 2004;350:1450–1452. doi: 10.1056/NEJMe048020. [DOI] [PubMed] [Google Scholar]
- 23.Vermeire S, Van Assche G, Rutgeerts P. Creactive protein as a marker for inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:661–665. doi: 10.1097/00054725-200409000-00026. [DOI] [PubMed] [Google Scholar]
- 24.Sutherland AD, Gearry RB, Frizelle FA. Review of fecal biomarkers in inflammatory bowel disease. Dis Colon Rectum. 2008;51:1283–1291. doi: 10.1007/s10350-008-9310-8. [DOI] [PubMed] [Google Scholar]
- 25.Peterson CG, Sangfelt P, Wagner M, Hansson T, Lettesjo H, Carlson M. Fecal levels of leukocyte markers reflect disease activity in patients with ulcerative colitis. Scand J Clin Lab Invest. 2007;67:810–820. doi: 10.1080/00365510701452838. [DOI] [PubMed] [Google Scholar]
- 26.Tamhane UU, Aneja S, Montgomery D, Rogers EK, Eagle KA, Gurm HS. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. 2008;102:653–657. doi: 10.1016/j.amjcard.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Bhutta H, Agha R, Wong J, Tang TY, Wilson YG, Walsh SR. Neutrophil-lymphocyte ratio predicts medium-term survival following elective major vascular surgery: a cross-sectional study. Vasc Endovascular Surg. 2011;45:227–231. doi: 10.1177/1538574410396590. [DOI] [PubMed] [Google Scholar]
- 28.Azab B, Jaglall N, Atallah JP, Lamet A, Raja-Surya V, Farah B, Lesser M, Widmann WD. Neutrophil-lymphocyte ratio as a predictor of adverse outcomes of acute pancreatitis. Pancreatology. 2011;11:445–452. doi: 10.1159/000331494. [DOI] [PubMed] [Google Scholar]
- 29.Guclu H, Ozal SA, Pelitli Gurlu V, Birgul R. Elevated Neutrophil Lymphocyte Ratio in Recurrent Optic Neuritis. J Ophthalmol. 2015;2015:758687. doi: 10.1155/2015/758687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belaj K, Pichler M, Hackl G, Rief P, Eller P, Hafner F, Brodmann M, Gary T. Association of the Derived Neutrophil-Lymphocyte Ratio With Critical Limb Ischemia. Angiology. 2015 doi: 10.1177/0003319715590701. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Duan H, Zhang X, Wang FX, Cai MY, Ma GW, Yang H, Fu JH, Tan ZH, Meng YQ, Fu XY, Ma QL, Lin P. Prognostic role of neutrophil-lymphocyte ratio in operable esophageal squamous cell carcinoma. World J Gastroenterol. 2015;21:5591–5597. doi: 10.3748/wjg.v21.i18.5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torun S, Tunc BD, Suvak B, Yildiz H, Tas A, Sayilir A, Ozderin YO, Beyazit Y, Kayacetin E. Assessment of neutrophil-lymphocyte ratio in ulcerative colitis: a promising marker in predicting disease severity. Clin Res Hepatol Gastroenterol. 2012;36:491–497. doi: 10.1016/j.clinre.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Alkhouri N, Morris-Stiff G, Campbell C, Lopez R, Tamimi TA, Yerian L, Zein NN, Feldstein AE. Neutrophil to lymphocyte ratio: a new marker for predicting steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease. Liver Int. 2012;32:297–302. doi: 10.1111/j.1478-3231.2011.02639.x. [DOI] [PubMed] [Google Scholar]
- 34.Duffy BK, Gurm HS, Rajagopal V, Gupta R, Ellis SG, Bhatt DL. Usefulness of an elevated neutrophil to lymphocyte ratio in predicting long-term mortality after percutaneous coronary intervention. Am J Cardiol. 2006;97:993–996. doi: 10.1016/j.amjcard.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 35.Gibson PH, Croal BL, Cuthbertson BH, Small GR, Ifezulike AI, Gibson G, Jeffrey RR, Buchan KG, El-Shafei H, Hillis GS. Preoperative neutrophil-lymphocyte ratio and outcome from coronary artery bypass grafting. Am Heart J. 2007;154:995–1002. doi: 10.1016/j.ahj.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 36.Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Selby WS, Janossy G, Bofill M, Jewell DP. Intestinal lymphocyte subpopulations in inflammatory bowel disease: an analysis by immunohistological and cell isolation techniques. Gut. 1984;25:32–40. doi: 10.1136/gut.25.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]


