Abstract
Via routinely used thyroid function tests, scintigraphy and ultrasonography (USG), important information is obtained in the clinical and diagnostic practice for thyroid nodules. However, the distinction between benign and malignant lesions cannot precisely be performed with these tests. Thyroid fine needle aspiration biopsy (FNAB) is considered the most reliable diagnostic method in the differentiation between benign and malignant thyroid nodules. It has recently been likely to perform aspiration from deeper nodules via the implemention of FNAB along with USG. Today, in cytopathological examination of thyroid FNAB, standardized Bethesda System for Reporting Thyroid Cytopathology (BSRTC) system is used. Here, FNAB was performed in 1096 patients with thyroid nodules in the Medical School of Selcuk University between January 2009 and July 2014. Patients consisted of 919 women and 177 men between 12 and 87 years of age. Evaluated via BSRTC, the results were classified as unsatisfactory, benign, atypia (or follicular lesions) of undetermined significance (AUS), follicular neoplasm or lesions suspicious for follicular neoplasm (FN), suspected malignant and malignant. After FNAB, 183 patients were operated and evaluated histopathologically. Histological malignancy rates of the categories were as follows: 16% (5), 15% (6) 14% (1) 60% (9), 72% (18) and 97% (63), respectively. In our study, we aimed to compare FNAB results of thyroid nodules with histopathology results after thyroidectomy and to show the sensitivity and specificity of FNAB technique to be higher in the follow-up and diagnosis of thyroid lesions. Given the malignancy detection rate in the follow-up of patients whose cytology was reported as inadequate, we also consider follow-ups are important in these patients.
Keywords: Thyroid, fine needle aspiration biopsy, bethesda
Introduction
As in the whole world, thyroid diseases are also considered to be one of the common diseases in our country, Turkey. Thyroid nodules are seen between 4-7% in the general population, and 5-10% of these cases are malignant [1,2]. Low as the rate seems, the early diagnosis of these cancers is very important because of their slow progression and patients’ longevity due to early treatment [3,4]. For cytological interpretation, fine-needle aspiration biopsy (FNAB) is an established diagnostic modality in the evaluation of thyroid nodules. Although nearly 75% of these modalities definitively produce benign or malignant diagnoses, and the accuracy of those diagnoses exceeds 95%, indeterminate or suspicious results are frequent and challenging in terms of defining an appropriate management strategy. While standardized Bethesda System for Reporting Thyroid Cytopathology (BSRTC) system has brought standardization to the diagnostic terminology of thyroid cytology, morphological interpretation still remains as an inherently subjective practice that leads to interobserver discrepancies [5-7].
Because FNAB results are reliable, cost effective, well tolerated by patients and cause very few complications, FNAB is the first choice in the diagnosis of thyroid nodules [8-10]. In our study, we aimed at comparing the cytological results of patients exposed to FNAB due to thyroid nodules with the histopathological findings of surgical specimens, and at determining the sensitivity and specificity of FNAB.
Materials and methods
In our study, the patients admitted to the general surgery and otolaryngology departments of the university hospital of The Medical School of Selcuk University between January 2009 and July 2014 and exposed to FNAB due to suspected nodular goitre, multinodular goitre, hyperthyroidy and throid cancer and then to surgery were retrospectively investigated. Of 1096 patients constituting the study population, 919 were women, and 177 were men between 12-87 years of age (average 49.5 years). All patients were exposed to FNAB. Cytological specimens were stained with the dyes of Papanikolaou (PAP), May-Grünwald Giemsa (MGG) and Hematoksilen-Eozin (HE). In histopathological evaluation performed via BSRTC, the results were classified as unsatisfactory, benign, atypia (or follicular lesions) of undetermined significance (AUS), follicular neoplasm or lesions suspicious for follicular neoplasm (FN), suspected malignant and malignant.
Cellular adequacy was considered insufficient, less than optimal or adequate. Insufficient cases had no atypical findings and fewer than 6 groups of 10 benign follicular cells [5].
Results
Among 1096 specimens of FNAB, 12% (132) were reported as unsatisfactory, 72% (789) as benign (Figure 1), 3% (29) as atypia (or follicular lesions) of undetermined significance (AUS), 3% (35) as follicular neoplasm or lesions suspicious for follicular neoplasm (FN) (Figure 2), 4% (46) as suspected malignant and 6% (65) as malignant (Figure 3). The gender and age distributions of the patients diagnosed cytologically are prsented in Tables 1 and 2. The specimens of 183 patients operated after FNAB were histopathologically evaluated, and the malignancy rates in these categories were determined as follows: 16% (5), 15% (6), 14% (1), 60% (9), 72% (18) and 97% (63), respectively. The age distributions of 102 patients (77 women and 25 men) determined as histologically malignant were 11 between 0-30 years, 68 between 31-60 and 23 over 60. The cytological and histological correlations of these patients are presented in Table 3.
Figure 1.
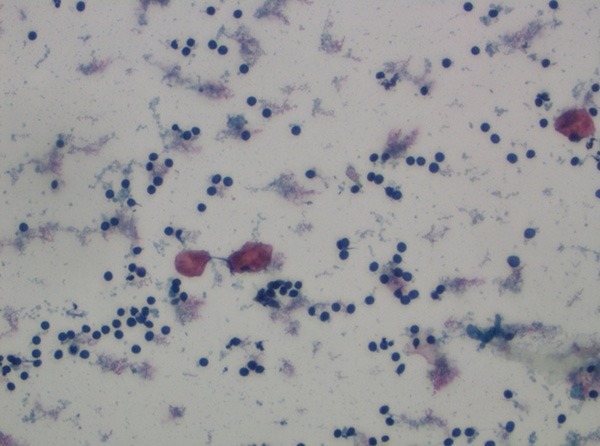
Benign cytology (PAP, ×400).
Figure 2.
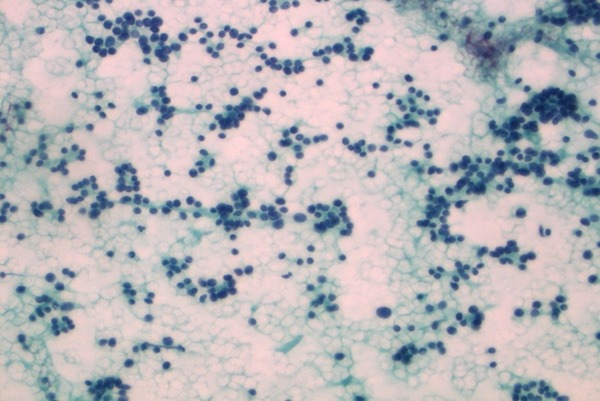
Follicular Neoplasia (PAP, ×400).
Figure 3.
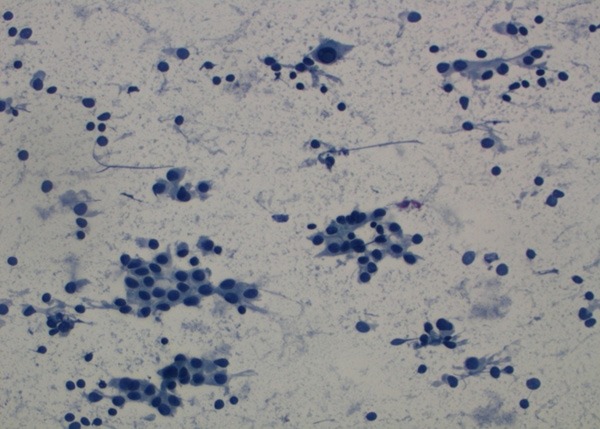
Malignant cytology (PAP, ×400).
Table 1.
Cytologic diagnosis sex distribution
| Sex | Total | Inadequate | Benign | Atypia of undetermined significance | Follicular Neoplasm/Suspicion | Suspicion of malignancy | Malignant |
|---|---|---|---|---|---|---|---|
| Women | 919 | 110 | 671 | 25 | 27 | 38 | 48 |
| Men | 177 | 22 | 118 | 4 | 8 | 8 | 17 |
Table 2.
Age distribution of cytological diagnosis
| Age | Total | Inadequate | Benign | Atypia of undetermined significance | Follicular Neoplasm/Suspicion | Suspicion of malignancy | Malignant |
|---|---|---|---|---|---|---|---|
| 0-30 | 86 | 21 | 44 | 2 | 8 | 7 | 4 |
| 31-60 | 773 | 81 | 571 | 21 | 24 | 28 | 48 |
| >60 | 237 | 30 | 174 | 6 | 3 | 11 | 13 |
| Total | 1096 | 132 | 789 | 29 | 35 | 46 | 65 |
Table 3.
Cytologic-histologic correlation
| Cytological diagnosis | Number of patients operated with | Benign | Papillary Carcinoma | Follicular carcinoma | Medullary Carcinoma |
|---|---|---|---|---|---|
| Inadequate | 31 | 26 | 5 | 0 | 0 |
| Benign | 40 | 34 | 5 | 1 | 0 |
| AUS | 7 | 6 | 1 | 0 | 0 |
| FN | 15 | 6 | 4 | 4 | 1 |
| Suspicion of malignancy | 25 | 7 | 18 | 0 | 0 |
| Malignant | 65 | 2 | 63 | 0 | 0 |
Those with unsatisfactory FNAB cytology of thyroid nodules were excluded out of the criteria. In our comparison, those reported as the suspected were included into the malignancy group. The sensitivity and specivity of preoperative thyroid FNAB on postoperative diagnosis, and the negative and positive predictive values were investigated. The rates of validity, sensitivity, specifity, positive predictive and negative predictive values in our study were detected as 88%, 93%, 79%, 90% and 85%, respectively.
Discussion
Thyroid nodules are among commonly encountered endocrine pathologies. In western countries, the rate of thyroid nodules detected palpably ranges between 3-8% [11]. In Turkey, the prevalence of thyroid nodules was determined as 2-6% with palpable examination and 18% with ultrasonography (USG) [12,13]. Thyroid cancers consist of 1% of all malignant neoplasms and are seen three or four times more frequently in women, compared to men. In our study, as to the detection of thyroid malignancy, the rate was women/men: 77/25=3.08, and our rate is consistent with that reported in literature [14]. Age and gender are definitive criteria in terms of the malignancy of nodules. While the likeliness of nodules to be observed as malignant in women between tha ages of 20-40 is lower, this rate increases in nodulles witnessed in men at advanced ages. Although the likeliness of malignancy was increased in both genders with advanced age in our study, no significant association was determined between malignancy, and gender and age.
Upon yhe diagnosis of thyroid nodules, the main problem is to distinguish between whether nodules are benign or malignant. FNAB is known to be the most accurate and cost-effective test in order to evaluate thyroid nodules [15]. The malignant and benign diagnoses peformed by pathologists are easily understood and managed by clinicians. However, indeterminate diagnoses may cause confusion and uncertainty of the management among patients and clinicians [16]. BSRTC provides a consensus in diagnostic terminology and morphological criteria for pathologists, related to implied risks of malignancy and recommendations in patient management [17-20]. In literature, the rate of malignancy was reported as 80-90% for papillary carcinomas, as 5-15% for follicular carcinomas, as 3-5% for medullary carcinomas, as 0.4-10% for hurtle cell carcinomas, as 1-3% for anaplastic carcinomas and as 1-2% for other malignancies [21,22]. In our study, of 102 malignant cases, 96 (94%) were detected to be papillary carcinomas, 5 (5%) to be follicular carcinomas, and only one (1%) to be medullary carcinomas. While papillary carcinomas are encountered a little more frequently, the distribution of these cancers was consistent with that reported in other studies, and no anaplastic carcinomas, seen less than other types, were encountered in our study.
It may sometimes be impossible to diagnose cytologically medullary thyroid carcinomas with more agressive nature. In a meta-analysis study, it was reported that while 56% of histologically proven medullary thyroid carcinomas were correctly detected by cytologic evaluation, the remaining medullary thyroid carcinomas were defined as benign, indeterminate, nondiagnostic or as other types of neoplasms by FNAB [23].
In our study, of 46 cases where FNAB results were evaluated as suspected malignant, 25 were operated on, and 18 were determined with malignancy. Due to risk of malignancy, surgery is recommended for the certainity of diagnosis in cases with suspected malignancy [24].
The target of FN category is to determine nodules that may be follicular carcinoma and to triage them for surgical lobectomy. Based on BSRTC, 15-30% of cases classified as FN prove to be malignant [17,25]. Among 15 patients whose FNABs were indicated as follicular neoplasms in our study, surgical pathology findings of nine cases were reported as malignant. We consider that the patients reported as follicular neoplasms should be operated on [26].
In our study, of 35 (3%) patients in AUS category, only one (14%) was determined as malignant. According to BSRTC, the rate of AUS interpretations in a laboratory should range between 3 and 6% of all thyroid FNABs, and should not be over 7%, because higher rates most probably represent an overuse of this category, and other interpretations may be more appropriate [17,27-30].
The rate of unsatisfactory materials in FNAB reported in literature ranges between 10% and 28.2%. This rate, however, was found as 12% in our study. In previous studies, the reasons decreasing the productivity of FNAB were reported as unsatisfactory sampling, unexperienced cytopathologists and difficulty in distinguishing between follicular lesions. Because the follicular variants of hyperplastic adenomatiod nodules, follicular adenomas, well-differentiated follicular carcinomas and papillary carcinomas are of similar criteria to each other, it is difficult to differentiate between these lesions via FNAB [31]. In order to achieve FNAB of thyroids, providing satisfactory specimens and existence of experienced cytopathologists are two important factors. Patients with unsatisfactory specimens should undergo repeated FNAB, preferably under sonographic guidance [13,32]. The current study demonstrated that among patients with initial unsatisfactory specimens exposed to repeated thyroid FNAB, 83% had a diagnostic repeated FNAB allowing appropriate follow-ups. The malignancy rate (8.5%) in repeated FNABs because of prior unsatisfactory aspiration was comparable to the malignancy rate for the patients with a diagnostic initial FNAB (7.6%) [33].
In conclusion, the sensitivity and specifity rates of FNAB used as the first step modality in the evaluation of thyroid nodules were reported to change between 65-99%, 72-100% and 76-100% in literature, respectively [10,14,34]. Given that the cytology of suspected FNABs were accepted as malignant in our study, the sensivity and specifity rates were found as 93% and 79%, as consistent with the findings given in literature.
In summary, our study demonstrated that thyroid FNAB is an accurate and relatively precise tool in the diagnosis of thyroid malignancy. Six diagnostic categories of thyroid cytology are very useful for triaging patients with thyroid nodules for clinical management [33,35,36]. Additionally, considering that the rate of malignancy was determined as 16% in the follow-ups of patients reported as unsatisfactory, the importance of repeating FNABs is quite remarkable.
Disclosure of conflict of interest
None.
References
- 1.Choi YJ, Jung I, Min SJ, Kim HJ, Kim J, Kim S, Park JS, Shin JH, Sohn YM, Yoon JH, Kwak JY. Thyroid Nodule with Benign Cytology: Is Clinical Follow-Up Enough? PLoS One. 2013;8:e63834. doi: 10.1371/journal.pone.0063834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rossi ED, Straccia P, Martini M, Revelli L, Lombardi CP, Pontecorvi A, Fadda G. The Role of Thyroid Fine-Needle Aspiration Cytology in the Pediatric Population. Cancer Cytopathol. 2014;122:359–367. doi: 10.1002/cncy.21400. [DOI] [PubMed] [Google Scholar]
- 3.Naz S, Hashmi AA, Khurshid A, Faridi N, Edhi MM, Kamal A, Khan M. Diagnostic accuracy of Bethesda system for reporting thyroid cytopathology: an institutional perspective. Int Arch Med. 2014;7:46. doi: 10.1186/1755-7682-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Abbadi MA, Shareef SQ, Ali JA, Yousef MM. Application of the Bethesda System for Reporting Thyroid Cytopathology in the Eastern Province of Saudi Arabia: Phase I Pilot Retrospective Analysis. Acta Cytologica. 2013;57:481–488. doi: 10.1159/000351612. [DOI] [PubMed] [Google Scholar]
- 5.Olson MT, Boonyaarunnate T, Han PA, Umbricht CB, Ali SZ, Zeiger MA. A Tertiary Center’s Experience With Second Review of 3885 Thyroid Cytopathology Specimens. J Clin Endocrinol Metab. 2013;98:1450–1457. doi: 10.1210/jc.2012-3898. [DOI] [PubMed] [Google Scholar]
- 6.Shere SK, Kulkarni AS, Phulgirkar PP, Anjum S, Patil SP, Bindu R. Correlatıon of fıne needle aspıratıon cytology wıth hıstopathology ın dıagnosıs of thyroıd lesıons. Journal of Evolution of Medical and Dental Sciences. 2013;2:4826–4831. [Google Scholar]
- 7.Carlos AD, Mirasol R, Aquino ET, Goco ML, Toledo PR, Santos KC. Management and Malignancy Rate of Thyroid Nodules with a Cytologic Diagnosis of Atypia or Follicular Lesion of Undetermined Significance. JAFES. 2014;29:79–83. [Google Scholar]
- 8.Mamoon N, Jamy R, Khan AH. Evaluation of fine needle aspiration cytology as a screening tool in thyroid lesions. J Pak Med Assoc. 2013;63:1120–1123. [PubMed] [Google Scholar]
- 9.Poller DN, Kandaswamy P. Simplified economic approach to thyroid FNA cytology and surgical intervention in thyroid nodules. J Clin Pathol. 2013;66:583–588. doi: 10.1136/jclinpath-2012-201339. [DOI] [PubMed] [Google Scholar]
- 10.Kim S, Jang EJ, Jeong JY, Park JY. Clinical usefulness of fine needle aspiration cytology in patients less than 20 years old: a 10-year experience at a single institution. Int J Clin Exp Pathol. 2013;6:2962–7. [PMC free article] [PubMed] [Google Scholar]
- 11.Unal B, Sezer C. Diagnostic Value of Ultrasound-Guided Fine Needle Aspiration Biopsy in Malignant Thyroid Nodules: Utility for Micronodules. Asian Pac J Cancer Prev. 2014;15:8613–8616. doi: 10.7314/apjcp.2014.15.20.8613. [DOI] [PubMed] [Google Scholar]
- 12.Cheng P, Chen ED, Zheng HM, He QX, Li Q. Ultrasound Score to Select Subcentimetersized Thyroid Nodules Requiring Ultrasoundguided Fine Needle Aspiration Biopsy in Eastern China. Asian Pac J Cancer Prev. 2013;14:4689–4692. doi: 10.7314/apjcp.2013.14.8.4689. [DOI] [PubMed] [Google Scholar]
- 13.Chang TC. The Roles of Ultrasonography and Ultrasonography-guided Fine-needle Aspiration Cytology in the Planning of Management of Thyroid Cancers. Journal of Medical Ultrasound. 2014;7:1–8. [Google Scholar]
- 14.Patel MM, Patel K, Kaptan KR, Italiya SL, Saini G. Fıne needle aspıratıon cytology as a fırst lıne ınvestıgatıon ın thyroıd lesıons. Natıonal Journal of Medıcal Research. 2013;3:106–110. [Google Scholar]
- 15.Auger M, Nayar R, Khalbuss WE, Barkan GA, Benedict CC, Tambouret R, Schwartz MR, Howell LP, Souers RJ, Hartley DA, Thomas N, Moriarty AT. Emplementation of the Bethesda System for Reporting Thyroid Cytopathology. Arch Pathol Lab Med. 2013;137:1555–1559. doi: 10.5858/arpa.2012-0658-CP. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan PS, Hirschowitz SL, Fung PC, Apple SK. The Impact of Atypia/Follicular Lesion of Undetermined Significance and Repeat Fine-Needle Aspiration: 5 Years Before and After Implementation of the Bethesda System. Cancer Cytopathol. 2014;122:866–72. doi: 10.1002/cncy.21468. [DOI] [PubMed] [Google Scholar]
- 17.Tepeoglu M, Bilezikci B, Bayraktar SG. A histological assessment of the Bethesda system for reporting thyroid cytopathology (2010) abnormal categories: a series of 219 consecutive cases. Cytopathology. 2014;25:39–44. doi: 10.1111/cyt.12051. [DOI] [PubMed] [Google Scholar]
- 18.Mastorakis E, Meristoudis C, Margari N, Pouliakis A, Leventakos K, Chroniaris N, Panayiotides I, Karakitsos P. Fine needle aspiration cytology of nodular thyroid lesions: a 2-year experience of the Bethesda system for reporting thyroid cytopathology in a large regional and a university hospital, with histological correlation. Cytopathology. 2014;25:120–128. doi: 10.1111/cyt.12062. [DOI] [PubMed] [Google Scholar]
- 19.Kiernan CM, Broome JT, Solórzano CC. The Bethesda System for Reporting Thyroid Cytopathology: A Single-Center Experience over 5 Years. Ann Surg Oncol. 2014;21:3522–7. doi: 10.1245/s10434-014-3743-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brister KJ, Singh RS, Wang HH. Reporting Thyroid FNA Before and After Implementation of the Bethesda System One Institution’s Experience. Diagn Cytopathol. 2015;43:28–31. doi: 10.1002/dc.23182. [DOI] [PubMed] [Google Scholar]
- 21.Manoli NN, Manoli NS, Patel S. Thyroid Cytopathology: It’s Significance in Surgical Management of Thyroid Nodule/Malignancies. Surgery. 2013;12:2–7. [Google Scholar]
- 22.Pandey P, Dixit A, Chaturvedi V, Chandra S, Dayal S, Sharma A. Usefulness of fine-needle aspiration in the diagnosis of thyroid lesions: an institutional experience of 340 patients. Otolaryngology. 2013;3:1–15. [Google Scholar]
- 23.Trimboli P, Treglia G, Guidobaldi L, Romanelli F, Nigri G, Valabrega S, Sadeghi R, Crescenzi A, Faquin WC, Bongiovanni M, Giovanella L. Detection rate of FNA cytology in medullary thyroid carcinoma: a meta-analysis. Clin Endocrinol (Oxf) 2015;82:280–5. doi: 10.1111/cen.12563. [DOI] [PubMed] [Google Scholar]
- 24.Mahajan A, Lin X, Nayar R. Thyroid Bethesda reporting category, ‘suspicious for papillary thyroid carcinoma’, pitfalls and clues to optimize the use of this category. Cytopathology. 2013;24:85–91. doi: 10.1111/j.1365-2303.2012.00966.x. [DOI] [PubMed] [Google Scholar]
- 25.Boonyaarunnate T, Matthew TO, Ali SZ. ‘Suspicious for a Follicular Neoplasm’ before and after the Bethesda System for Reporting Thyroid Cytopathology: Impact of Standardized Terminology. Acta Cytologica. 2013;57:455–463. doi: 10.1159/000351664. [DOI] [PubMed] [Google Scholar]
- 26.Kasper KA, Stewart J, Das K. Fine-Needle Aspiration Cytology of Thyroid Nodules with Hürthle Cells: Cytomorphologic Predictors for Neoplasms, Improving Diagnostic Accuracy and Overcoming Pitfalls. Acta Cytologica. 2014;58:145–152. doi: 10.1159/000358264. [DOI] [PubMed] [Google Scholar]
- 27.Pathak P, Srivastava R, Singh N, Arora VK, Bhatia A. Implementation of the Bethesda System for Reporting Thyroid Cytopathology: ınterobserver concordance and reclassification of previously ınconclusive aspirates. Diagn Cytopathol. 2014;42:944–949. doi: 10.1002/dc.23162. [DOI] [PubMed] [Google Scholar]
- 28.McElroy MK, Mahooti S, Hasteh F. A Single Institution Experience with the New Bethesda System for Reporting Thyroid Cytopathology: correlation with existing cytologic, clinical, and histological data. Diagn Cytopathol. 2014;42:564–569. doi: 10.1002/dc.23071. [DOI] [PubMed] [Google Scholar]
- 29.Dincer N, Balci S, Yazgan A, Guney G, Ersoy R, Cakir B, Guler G. Follow-up of atypia and follicular lesions of undetermined significance in thyroid fine needle aspiration cytology. Cytopathology. 2013;24:385–390. doi: 10.1111/cyt.12021. [DOI] [PubMed] [Google Scholar]
- 30.Mathur A, Najafian A, Schneider EB, Zeiger MA, Olson MT. Malignancy risk and reproducibility associated with atypia of undetermined significance on thyroid cytology. Surgery. 2014;156:1471–1476. doi: 10.1016/j.surg.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 31.Harvey AM, Mody DR, Amrikachi M. Thyroid fine-needle aspiration reporting rates and outcomes before and after bethesda ımplementation within a Combined Academic and Community Hospital System. Arch Pathol Lab Med. 2013;137:1664–1668. doi: 10.5858/arpa.2012-0366-OA. [DOI] [PubMed] [Google Scholar]
- 32.Yadav A, Yadav P, Kulkarni CV, Tiwari NP. Thyroıd lesıons: sonologıcal, cytologıcal, and hıstopathologıcal correlatıon: a 3-year experıence. International Journal of Medical Science and Public Health. 2014;3:1–3. [Google Scholar]
- 33.Yang J, Schnadig V, Logrono R, Wasserman PG. Fine-needle aspiration of thyroid nodules: a study of 4703 patients with histologic and clinical correlations. Cancer. 2007;111:306–315. doi: 10.1002/cncr.22955. [DOI] [PubMed] [Google Scholar]
- 34.Pahuja N, Tambekar M, Dhar R, Borkar DB. Significance of cell pattern approach in fine needle aspiration cytology of thyroid lesions. International Journal of Advanced Research. 2014;2:1092–1101. [Google Scholar]
- 35.Ratour J, Polivka M, Dahan H, Hamzi L, Kania R, Dumuis ML, Cohen R, Laloi-Michelin M, Cochand-Priollet B. Diagnosis of follicular lesions of undetermined significance infine-needle aspirations of thyroid nodules. J Thyroid Res. 2013;2013:250347. doi: 10.1155/2013/250347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhasin TS, Mannan R, Manjari M, Mehra M, Gill Sekhon AK, Chandey M, Sharma S, Kaur P. Reproducibility of ‘The Bethesda System for reporting thyroid cytopathology’; a multicenter study with review of the literature. J Clin Diagn Res. 2013;7:1051–4. doi: 10.7860/JCDR/2013/5754.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]


