Abstract
Background: MicroRNAs (miRNAs) play important roles in many important cellular processes and deregulation of miRNAs is linked to many human diseases including cancer. Although miR-424 has been demonstrated to inhibit progression of hepatocellular carcinoma (HCC), its expression level in serum samples and the potential clinical values remain unknown. Materials and methods: The expression level of miR-424 in the serum clinical samples from HCC patients and healthy volunteers were determined by qRT-PCR. Then the association of serum miR-424 expression level with various important clinicopathological parameters and survival rates was evaluated. Multivariate Cox regression analysis was used to identify the independent risk factors for HCC. Results: The expression level of serum miR-424 was significantly decreased in patients with HCC compared with the healthy volunteers (P<0.01). Reduced expression of serum miR-424 was associated with serum AFP (P=0.048), vein invasion (P=0.006) and TNM stage (P=0.003). In addition, survival analysis showed that HCC patients with lower serum miR-424 expression suffered poorer overall survival (P=0.018) and disease free survival (P=0.008). Moreover, serum miR-424 was demonstrated to be an independent risk factor for HCC. Conclusions: Our findings provide the compelling evidence that the decreased expression of serum miR-424 may serve as a novel biomarker to predict the unfavorable prognosis of HCC patients.
Keywords: Hepatocellular carcinoma, miR-424, prognosis, serum
Introduction
Hepatocellular carcinoma (HCC), a primary malignancy of the liver, is the third leading cause of cancer-related death worldwide [1,2]. Despite great advance has been made in the field of diagnosis and multi-modality treatments, the clinical outcome of HCC remains unclear [3]. High rates of recurrence and metastasis are the major reasons responsible for the poor prognosis [4]. Therefore, detecting biomarkers that can identify HCC at an early clinical stage and predict the prognosis of HCC might not only help clinical treatments, but also significantly improve the outcome of this malignant disease.
MicroRNAs (miRNAs) are a group of short non-coding, highly conservative RNAs that regulate gene expression at the post-transcriptional level [5]. MiRNAs play important roles in most physiological processes such as cell proliferation, survival and differentiation [6,7]. Deregulation of miRNAs is closely associated with the initiation and progression of many types of cancers including HCC [8]. Zhai et al showed that miR-129 was down-regulated in HCC tissues and its decreased expression was correlated with tumor progression. In addition, enforced expression of miR-129 suppressed HCC cells proliferation and invasion, while induced apoptosis; indicating miR-129 might function as a tumor suppressor in HCC [9]. Ma et al revealed that the expression level of miR-24 was increased in HCC tissues and cell lines. Moreover, down-regulation of miR-24 could inhibit the proliferation, migration and invasion capacity of HCC cells by partly targeting sex-determining region Y (SRY)-box 7; suggesting miR-24 might be a positive regulator of HCC progression [10].
Aberrant expression of miR-424 is frequent in a number of cancers including HCC. Previous studies have demonstrated that miR-424 was down-regulated in HCC tissues and cell lines, and it might play a tumor suppressive role in the development of HCC [11,12]. However, whether miR-424 is up-regulated in serum samples from HCC patients and it has any potential clinical values are poorly known. Thus the aim of the current study was to explore the clinical significance of miR-424 for HCC.
Materials and methods
Study population and clinical samples
The study was approved by the Research Ethnic Committee of The First Affiliated Hospital of Shantou University Medical College. The written inform consent was obtained from all the participants. Serum samples were drawn from 95 patients with HCC and 40 healthy volunteers from the Department of Gastroenterology, The First Affiliated Hospital of Shantou University Medical College. The patients with HCC were pathologically diagnosed and they did not receive any kind of therapy before serum samples collection. The clinical feature of HCC patients was summarized in Table 1.
Table 1.
Correlations between serum miR-204 expression and clinicopathological features in HCC patients
| Parameters | n | Serum miR-204 expression | P | |
|---|---|---|---|---|
|
|
||||
| Low | High | |||
| Gender | ||||
| Male | 72 | 38 | 34 | 0.133 |
| Female | 23 | 8 | 15 | |
| Age | ||||
| <50 | 51 | 29 | 22 | 0.076 |
| ≥50 | 44 | 17 | 27 | |
| Tumor size (cm) | ||||
| <5 | 37 | 16 | 21 | 0.420 |
| ≥5 | 58 | 30 | 28 | |
| Serum AFP (µg/L) | ||||
| <400 | 65 | 27 | 38 | 0.048 |
| ≥400 | 30 | 19 | 11 | |
| Live cirrhosis | ||||
| No | 17 | 10 | 7 | 0.344 |
| Yes | 78 | 36 | 42 | |
| Vein invasion | ||||
| No | 27 | 7 | 20 | 0.006 |
| Yes | 68 | 39 | 29 | |
| Tumor number | ||||
| Single | 53 | 26 | 27 | 0.889 |
| Multiple | 42 | 20 | 22 | |
| TNM stage | ||||
| Ι-II | 46 | 15 | 31 | 0.003 |
| III-IV | 49 | 31 | 18 | |
| Tumor differentiation | ||||
| Well+Moderate | 43 | 18 | 25 | 0.247 |
| Poor | 52 | 28 | 24 | |
qRT-PCR
Approximately 5 mL of whole blood were collected from each participant. To remove the cellular components completely, the serum was separated by centrifugation at 1,200 × g at 4°C for 20 min and passed through a 13 mm serum filter (Thermo Fisher Scientific Inc.). QIAamp RNA Blood kit (Qiagen, Hilden, Germany) was used to extract total RNA from the cells and then Prime-Script RT reagent kit (TaKaRa, Dalian, China) was employed for reverse transcription and PCR reaction. The PCR reaction was performed on the Applied Biosystems 7500 Fast platform (Applied Biosystems, CA, USA). The average expression level of serum miR-424 was normalized against U6 using the 2-ΔCt method. Each experiment was performed in triplicate.
Statistical analysis
The expression level of serum miR-424 in HCC patients and healthy volunteers was compared by Mann-Whitney U test. Then the correlation between clinicopathological parameters and serum miR-424 expression level was evaluated using χ2 tests. The Kaplan-Meier method and log-rank test were used to analyze the association between serum miR-424 with OS as well as DFS. The Cox proportional hazards model was employed for the multivariate analysis. Statistical analyses were performed using SPSS 21.0 software (Chicago, Ill., USA) and the P<0.05 was indicated to be statistically significant.
Results
Serum miR-424 was downregulated in patients with HCC
The results showed that the expression level of serum miR-424 was significantly lower in patients with HCC compared with the healthy controls (P<0.01) (Figure 1).
Figure 1.
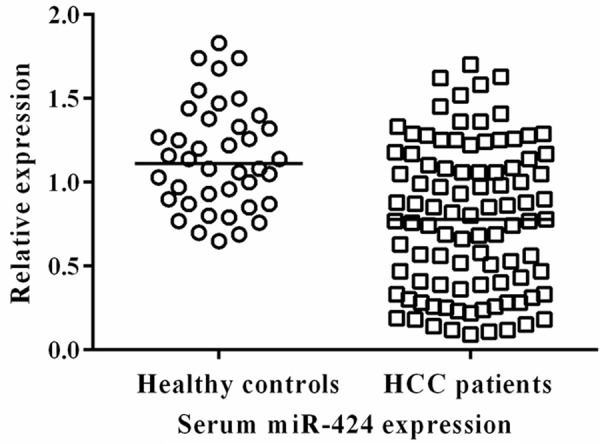
Serum miR-424 was down-regulated in patients with HCC.
Correlations between serum miR-424 and clinical features of HCC
The chi-square test revealed that serum miR-424 expression was associated with serum AFP (P=0.048), vein invasion (P=0.006) and TNM stage (P=0.003). However, it was not correlated with gender (P=0.133), age (P=0.076), tumor size (P=0.420), liver cirrhosis (P=0.344), tumor number (P=0.899) and tumor differentiation (P=0.247) (Table 1).
Low serum miR-424 was correlated with poor clinical outcome of HCC
The survival analysis showed that the 5-year OS rate of HCC patients in the low serum miR-424 expression group was 28.57%, which was significantly lower than that of patients (45.65%) in the high serum miR-424 expression group (P=0.018) (Figure 2).
Figure 2.
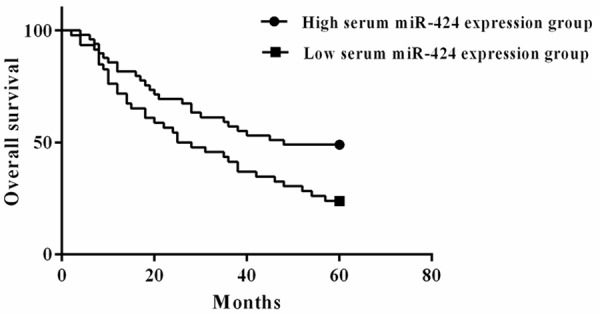
Low serum miR-424 was correlated with poor overall survival.
The 5 year DFS of the HCC patients in the low serum miR-424 expression group and high serum miR-424 expression group were16.32% and 34.78% respectively. Significant difference regarding to the DFS was detected between the two groups (P=0.008) (Figure 3).
Figure 3.
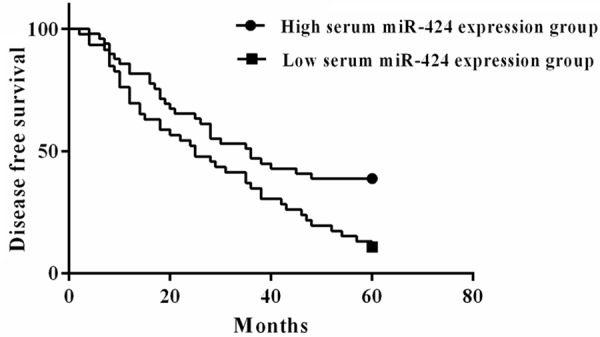
Low serum miR-424 was correlated with poor disease free survival.
Serum miR-424 was an independent risk factor for HCC
Multivariate analysis showed that vein invasion, TNM stage and serum miR-424 expression were independent predictors for OS and DFS of patients with HCC (P<0.05; P<0.01) (Table 2).
Table 2.
Multivariate analysis of parameters associated with OS and DFS of HCC patients
| Parameter | Overall survival | Disease-free survival | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| HR | 95% CI | P | HR | 95% CI | P | |
| Serum AFP | 2.185 | 0.956-5.362 | 0.054 | 1.928 | 0.912-5.093 | 0.061 |
| Vein invasion | 4.626 | 1.751-9.845 | 0.011 | 4.892 | 1.944-9.216 | 0.015 |
| TNM stage | 5.320 | 2.476-13.578 | 0.007 | 5.951 | 2.616-14.983 | 0.004 |
| Serum miR-204 expression | 3.864 | 1.281-7.950 | 0.031 | 4.465 | 1.579-8.511 | 0.024 |
Discussion
MiR-424 plays an important role in regulation of many important cellular processes such as cell proliferation, migration and differentiation [13-15]. Therefore, deregulation of miR-424 involves in a number of human diseases. The expression level of miR-424 in hair shaft was significantly upregulated only in patients with psoriasis compared with normal controls and those with atopic dermatitis, indicating miR-424 might participate in regulation of psoriasis [16]. Kim et al reported that miR-424 was downregulated in pulmonary arterial hypertension (PAH) and miR-424 might exert its function by regulating apelin and fibroblast growth factor 2 signaling. In addition, reconstitution of miR-424 could ameliorate pulmonary hypertension in animal models [17].
The results of current study revealed that serum miR-424 expression was decreased in HCC patients. In addition, serum miR-424 expression was associated with serum AFP, vein invasion and TNM stage. The HCC patients with lower serum miR-424 had both poorer 5 year OS and DFS. Moreover, serum miR-424 was demonstrated to be an independent predictor of HCC. Consistent with our study, Yu et al showed that down-regulation of miR-424 was found in HCC tissue samples and cell lines. Enforced expression of miR-424 resulted in reduced HCC cell proliferation, migration and invasion in vitro, and vice versa. In addition, c-Myb was identified as a direct target of miR-424; indicating miR-424 functioned as a tumor suppressor gene in HCC by downregulating the activity of c-Myb [11]. The expression level of miR-424-5p was significantly down-regulated in anoikis-resistant HCC cells. Ectopic overexpression of miR-424-5p in HCC cells could reverse the resistance to anoikis, suppress EMT process as well as inhibit malignant behaviors. ICAT/CTNNBIP1, which is a potent β-catenin inhibitor, was a direct target of miR-424-5p. Moreover, the clinical data revealed that miR-424-5p was significantly reduced in HCC tissues in comparison with that of the non-cancerous liver tissues. Decreased expression of miR-424-5p was significantly correlated with the progression of this malignant disease, suggesting the potential clinical value of miR-424-5p for HCC [12]. Similarly, Yang et al reported that miR-424 expression level was associated with various important HCC clinical parameters including tumor size, multiple nodules, vein invasion, TNM stage and overall survival; and Akt3/E2F3 axis was demonstrated to be regulated by miR-424 [18].
Similar to HCC, miR-424 functions as a tumor suppressor gene is other types of cancers. Li et al showed that the expression level of miR-424 was reduced in endometrial cancer and inhibition of miR-424 suppressed the growth of endometrial cancer cells by targeting E2F7 [19]. Hershkovitz-Rokah et al reported that the oncogenic BCR-ABL tyrosine kinase fusion gene was a direct target of miR-424 In addition; ectopic expression of miR-424 could not only inhibit proliferation and induce apoptosis of K562 cells, but also sensitize these cells to imatinib treatment; indicating miR-424 might be a promising therapeutic target of chronic myeloid leukemia [20]. Suppressing DNMT-1 increased the expression level of miR-424 and inverse correlations were observed between DNMT-1 and miR-424 in clinical bladder cancer specimens. The reduction of miR-424 expression was significantly associated with aggressive tumor growth, advanced clinical stage and poor prognosis in bladder cancer [21].
Each single miRNA might have hundreds of downstream targets and its biological functions are closely correlated with the targets. Therefore, it is common to see a specific miRNA might play different roles in different types of cancers or even in the same disease. Wu et al revealed that the expression level of miR-424-5p was significantly up-regulated in pancreatic cancer. In addition, overexpression of miR-424-5p could enhance proliferation, migration and invasion of pancreatic cancer cells, while inhibited cell apoptosis; suggesting that miR-424 might act as an oncogene in pancreatic cancer [22]. MiR-424 was also reported to be up-regulated in colon cancer in different studies, indicating miR-424 might be a positive regulator of colon cancer progression [23,24].
Taken together, our data demonstrated that serum miR-424 was decreased in patients with HCC. In addition, reduced serum miR-424 expression was associated with advanced clinical stage and poor prognosis of HCC. Therefore, serum miR-424 might be a novel prognostic biomarker for HCC.
Disclosure of conflict of interest
None.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz M, Roayaie S, Konstadoulakis M. Strategies for the management of hepatocellular carcinoma. Nat Clin Pract Oncol. 2007;4:424–32. doi: 10.1038/ncponc0844. [DOI] [PubMed] [Google Scholar]
- 3.Bodzin AS, Busuttil RW. Hepatocellular carcinoma: Advances in diagnosis, management, and long term outcome. World J Hepatol. 2015;7:1157–67. doi: 10.4254/wjh.v7.i9.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baek KK, Kim JH, Uhm JE, Park SH, Lee J, Park JO, Park YS, Kang WK, Lim HY. Prognostic factors in patients with advanced hepatocellular carcinoma treated with sorafenib: a retrospective comparison with previously known prognostic models. Oncology. 2011;80:167–74. doi: 10.1159/000327591. [DOI] [PubMed] [Google Scholar]
- 5.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–31. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 6.Bueno MJ, Pérez de Castro I, Malumbres M. Control of cell proliferation pathways by microRNAs. Cell Cycle. 2008;7:3143–8. doi: 10.4161/cc.7.20.6833. [DOI] [PubMed] [Google Scholar]
- 7.Ivey KN, Srivastava D. MicroRNAs as regulators of differentiation and cell fate decisions. Cell Stem Cell. 2010;7:36–41. doi: 10.1016/j.stem.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Garzon R, Calin GA, Croce CM. MicroRNAs in Cancer. Annu Rev Med. 2009;60:167–79. doi: 10.1146/annurev.med.59.053006.104707. [DOI] [PubMed] [Google Scholar]
- 9.Zhai J, Qu S, Li X, Zhong J, Chen X, Qu Z, Wu D. miR-129 suppresses tumor cell growth and invasion by targeting PAK5 in hepatocellular carcinoma. Biochem Biophys Res Commun. 2015;464:161–7. doi: 10.1016/j.bbrc.2015.06.108. [DOI] [PubMed] [Google Scholar]
- 10.Ma Y, She XG, Ming YZ, Wan QQ. miR-24 promotes the proliferation and invasion of HCC cells by targeting SOX7. Tumour Biol. 2014;35:10731–6. doi: 10.1007/s13277-014-2018-6. [DOI] [PubMed] [Google Scholar]
- 11.Yu L, Ding GF, He C, Sun L, Jiang Y, Zhu L. MicroRNA-424 is down-regulated in hepatocellular carcinoma and suppresses cell migration and invasion through c-Myb. PLoS One. 2014;9:e91661. doi: 10.1371/journal.pone.0091661. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Zhang Y, Li T, Guo P, Kang J, Wei Q, Jia X, Zhao W, Huai W, Qiu Y, Sun L, Han L. MiR-424-5p reversed epithelial-mesenchymal transition of anchorage-independent HCC cells by directly targeting ICAT and suppressed HCC progression. Sci Rep. 2014;4:6248. doi: 10.1038/srep06248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merlet E, Atassi F, Motiani RK, Mougenot N, Jacquet A, Nadaud S, Capiod T, Trebak M, Lompré AM, Marchand A. miR-424/322 regulates vascular smooth muscle cell phenotype and neointimal formation in the rat. Cardiovasc Res. 2013;98:458–68. doi: 10.1093/cvr/cvt045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mouillet JF, Donker RB, Mishima T, Cronqvist T, Chu T, Sadovsky Y. The unique expression and function of miR-424 in human placental trophoblasts. Biol Reprod. 2013;89:25. doi: 10.1095/biolreprod.113.110049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao X, Huang C, Zhao C, Gou X, Senavirathna LK, Hinsdale M, Lloyd P, Liu L. Regulation of myofibroblast differentiation by miR-424 during epithelial-to-mesenchymal transition. Arch Biochem Biophys. 2015;566:49–57. doi: 10.1016/j.abb.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsuru Y, Jinnin M, Ichihara A, Fujisawa A, Moriya C, Sakai K, Fukushima S, Ihn H. miR-424 levels in hair shaft are increased in psoriatic patients. J Dermatol. 2014;41:382–5. doi: 10.1111/1346-8138.12460. [DOI] [PubMed] [Google Scholar]
- 17.Kim J, Kang Y, Kojima Y, Lighthouse JK, Hu X, Aldred MA, McLean DL, Park H, Comhair SA, Greif DM, Erzurum SC, Chun HJ. An endothelial apelin-FGF link mediated by miR-424 and miR-503 is disrupted in pulmonary arterial hypertension. Nat Med. 2013;19:74–82. doi: 10.1038/nm.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang H, Zheng W, Shuai X, Chang RM, Yu L, Fang F, Yang LY. MicroRNA-424 inhibits Akt3/E2F3 axis and tumor growth in hepatocellular carcinoma. Oncotarget. 2015;6:27736–50. doi: 10.18632/oncotarget.4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q, Qiu XM, Li QH, Wang XY, Li L, Xu M, Dong M, Xiao YB. MicroRNA-424 may function as a tumor suppressor in endometrial carcinoma cells by targeting E2F7. Oncol Rep. 2015;33:2354–60. doi: 10.3892/or.2015.3812. [DOI] [PubMed] [Google Scholar]
- 20.Hershkovitz-Rokah O, Modai S, Pasmanik-Chor M, Toren A, Shomron N, Raanani P, Shpilberg O, Granot G. Restoration of miR-424 suppresses BCR-ABL activity and sensitizes CML cells to imatinib treatment. Cancer Lett. 2015;360:245–56. doi: 10.1016/j.canlet.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 21.Wu CT, Lin WY, Chang YH, Lin PY, Chen WC, Chen MF. DNMT1-dependent suppression of microRNA424 regulates tumor progression in human bladder cancer. Oncotarget. 2015;6:24119–31. doi: 10.18632/oncotarget.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu K, Hu G, He X, Zhou P, Li J, He B, Sun W. MicroRNA-424-5p suppresses the expression of SOCS6 in pancreatic cancer. Pathol Oncol Res. 2013;19:739–48. doi: 10.1007/s12253-013-9637-x. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Wang J, Ma H, Zhang J, Zhou X. Downregulation of miR-195 correlates with lymph node metastasis and poor prognosis in colorectal cancer. Med Oncol. 2012;29:919–27. doi: 10.1007/s12032-011-9880-5. [DOI] [PubMed] [Google Scholar]
- 24.Wang YX, Zhang XY, Zhang BF, Yang CQ, Chen XM, Gao HJ. Initial study of microRNA expression profiles of colonic cancer without lymph node metastasis. J Dig Dis. 2010;11:50–4. doi: 10.1111/j.1751-2980.2009.00413.x. [DOI] [PubMed] [Google Scholar]


