Abstract
We have explored the mechanism of adenovirus type 12 (Ad12) DNA integration because of its importance for viral oncogenesis and as an example of insertional recombination. We have used a fractionated cell-free system from nuclear extracts of hamster cells and have partly purified nuclear proteins that could catalyze in vitro recombination. As recombination partners, the 20,880- to 24,049-nucleotide Pst I D fragment of Ad12 DNA and the hamster preinsertion sequence p7 from the Ad12-induced tumor CLAC1 have proven to recombine at higher frequencies than randomly selected adenoviral or cellular DNA sequences. A preinsertion sequence might carry elements essential in eliciting recombination. Patch homologies between the recombination partners seem to play a role in the selection of sites for recombination in vivo and in the cell-free system. Nuclear extracts from BHK21 cells were prepared by incubating the nuclei in 0.42 M (NH4)2SO4 and fractionated by Sephacryl S-300 gel filtration, followed by chromatography on Mono S and Mono Q columns. The purified products active in recombination contained a limited number of different protein bands, as determined by polyacrylamide gel electrophoresis and silver staining. The most highly purified fraction IV had helicase and topoisomerase I activities. We used two different methods to assess the in vitro generation of hamster DNA-Ad12 DNA recombinants upon incubation with the purified protein fractions: (i) transfection of the recombination products into recA- strains of Escherichia coli and (ii) the polymerase chain reaction by using amplification primers unique for each of the two recombination partners. In p7 hamster DNA, the nucleotide sequence 5'-CCTCTCCG-3' or similar sequences served repeatedly as a preferred recombination target for Ad12 DNA in the tumor CLAC1 and in five independent cell-free recombination experiments.
Full text
PDF
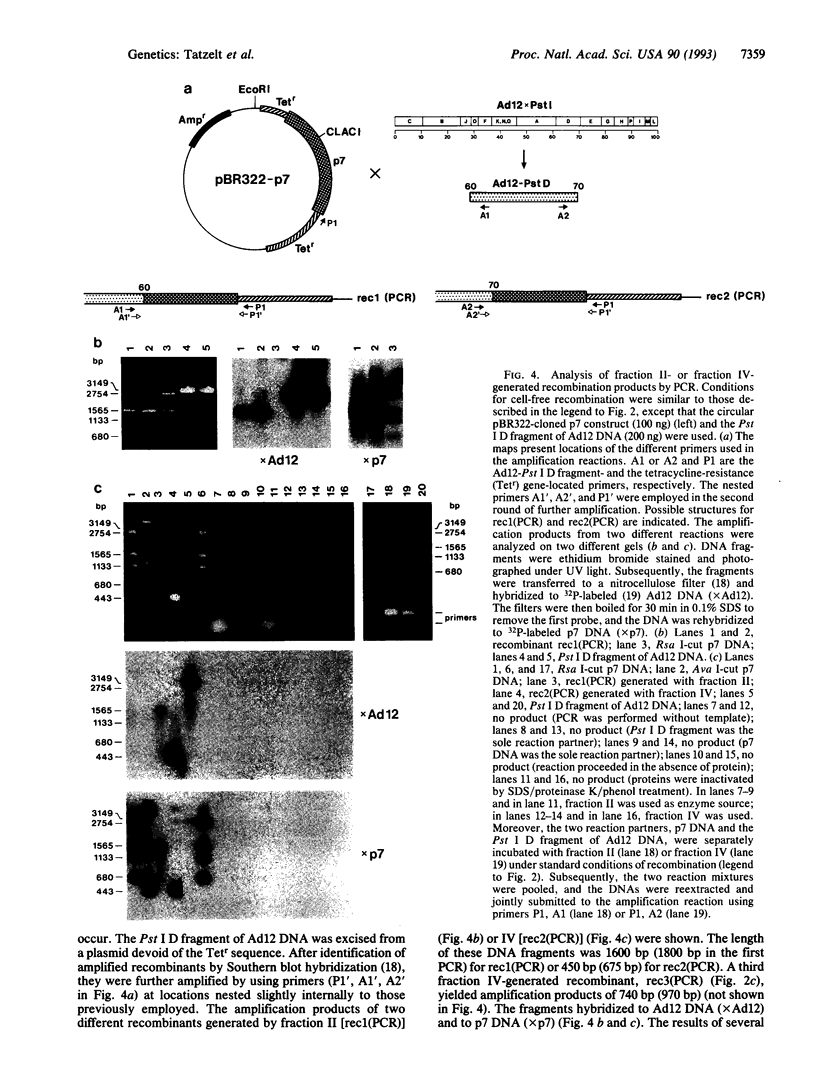
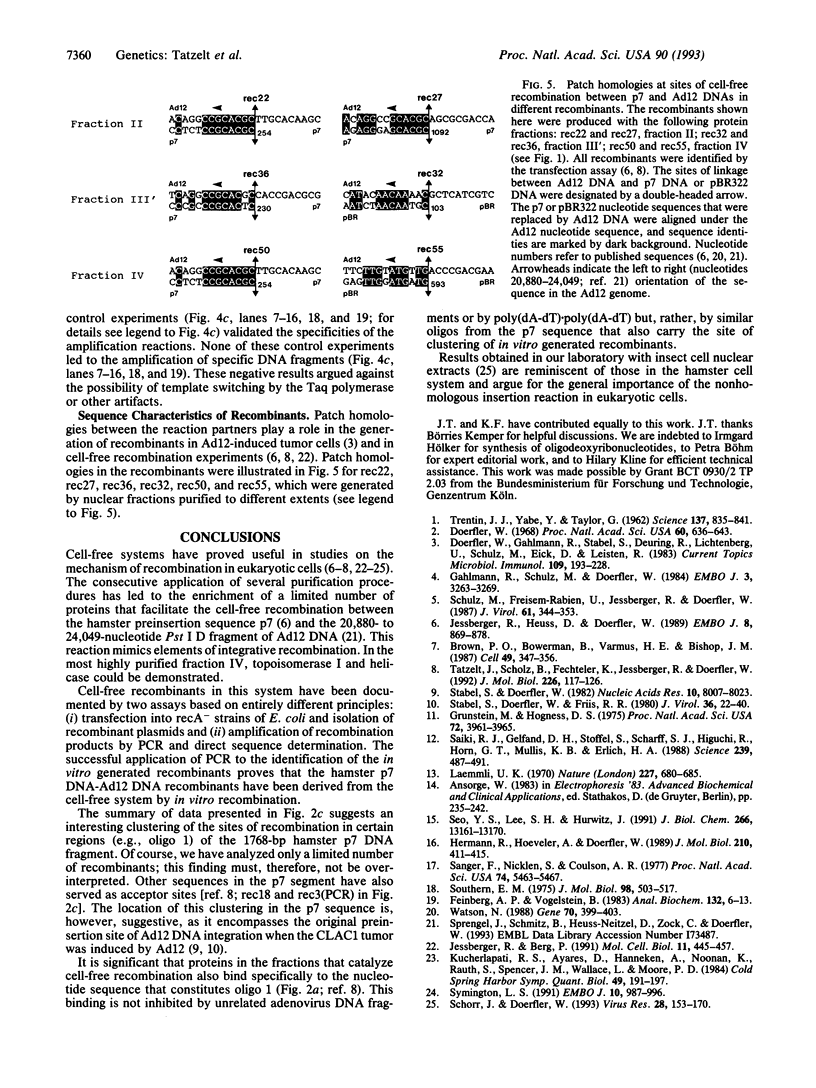
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown P. O., Bowerman B., Varmus H. E., Bishop J. M. Correct integration of retroviral DNA in vitro. Cell. 1987 May 8;49(3):347–356. doi: 10.1016/0092-8674(87)90287-x. [DOI] [PubMed] [Google Scholar]
- Doerfler W., Gahlmann R., Stabel S., Deuring R., Lichtenberg U., Schulz M., Eick D., Leisten R. On the mechanism of recombination between adenoviral and cellular DNAs: the structure of junction sites. Curr Top Microbiol Immunol. 1984;109:193–228. doi: 10.1007/978-3-642-69460-8_9. [DOI] [PubMed] [Google Scholar]
- Doerfler W. The fate of the DNA of adenovirus type 12 in baby hamster kidney cells. Proc Natl Acad Sci U S A. 1968 Jun;60(2):636–643. doi: 10.1073/pnas.60.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gahlmann R., Schulz M., Doefler W. Low molecular weight RNAs with homologies to cellular DNA at sites of adenovirus DNA insertion in hamster or mouse cells. EMBO J. 1984 Dec 20;3(13):3263–3269. doi: 10.1002/j.1460-2075.1984.tb02288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann R., Hoeveler A., Doerfler W. Sequence-specific methylation in a downstream region of the late E2A promoter of adenovirus type 2 DNA prevents protein binding. J Mol Biol. 1989 Nov 20;210(2):411–415. doi: 10.1016/0022-2836(89)90340-9. [DOI] [PubMed] [Google Scholar]
- Jessberger R., Berg P. Repair of deletions and double-strand gaps by homologous recombination in a mammalian in vitro system. Mol Cell Biol. 1991 Jan;11(1):445–457. doi: 10.1128/mcb.11.1.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger R., Heuss D., Doerfler W. Recombination in hamster cell nuclear extracts between adenovirus type 12 DNA and two hamster preinsertion sequences. EMBO J. 1989 Mar;8(3):869–878. doi: 10.1002/j.1460-2075.1989.tb03448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucherlapati R. S., Ayares D., Hanneken A., Noonan K., Rauth S., Spencer J. M., Wallace L., Moore P. D. Homologous recombination in monkey cells and human cell-free extracts. Cold Spring Harb Symp Quant Biol. 1984;49:191–197. doi: 10.1101/sqb.1984.049.01.022. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorr J., Doerfler W. Non-homologous recombination between adenovirus and AcNPV DNA fragments in cell-free extracts from insect Spodoptera frugiperda nuclei. Virus Res. 1993 May;28(2):153–170. doi: 10.1016/0168-1702(93)90133-8. [DOI] [PubMed] [Google Scholar]
- Schulz M., Freisem-Rabien U., Jessberger R., Doerfler W. Transcriptional activities of mammalian genomes at sites of recombination with foreign DNA. J Virol. 1987 Feb;61(2):344–353. doi: 10.1128/jvi.61.2.344-353.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo Y. S., Lee S. H., Hurwitz J. Isolation of a DNA helicase from HeLa cells requiring the multisubunit human single-stranded DNA-binding protein for activity. J Biol Chem. 1991 Jul 15;266(20):13161–13170. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stabel S., Doerfler W., Friis R. R. Integration sites of adenovirus type 12 DNA in transformed hamster cells and hamster tumor cells. J Virol. 1980 Oct;36(1):22–40. doi: 10.1128/jvi.36.1.22-40.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabel S., Doerfler W. Nucleotide sequence at the site of junction between adenovirus type 12 DNA and repetitive hamster cell DNA in transformed cell line CLAC1. Nucleic Acids Res. 1982 Dec 20;10(24):8007–8023. doi: 10.1093/nar/10.24.8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symington L. S. Double-strand-break repair and recombination catalyzed by a nuclear extract of Saccharomyces cerevisiae. EMBO J. 1991 Apr;10(4):987–996. doi: 10.1002/j.1460-2075.1991.tb08033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRENTIN J. J., YABE Y., TAYLOR G. The quest for human cancer viruses. Science. 1962 Sep 14;137(3533):835–841. doi: 10.1126/science.137.3533.835. [DOI] [PubMed] [Google Scholar]
- Tatzelt J., Scholz B., Fechteler K., Jessberger R., Doerfler W. Recombination between adenovirus type 12 DNA and a hamster preinsertion sequence in a cell-free system. Patch homologies and fractionation of nuclear extracts. J Mol Biol. 1992 Jul 5;226(1):117–126. doi: 10.1016/0022-2836(92)90128-7. [DOI] [PubMed] [Google Scholar]
- Watson N. A new revision of the sequence of plasmid pBR322. Gene. 1988 Oct 30;70(2):399–403. doi: 10.1016/0378-1119(88)90212-0. [DOI] [PubMed] [Google Scholar]