Abstract
Background: Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of lymphoma with quite high mortality. PTEN/PI3K/AKT signal pathway is constitutively activated and plays an oncogenic role in most tumors including non-Hodgkin’s lymphoma (NHL). Since rituximab used in chemotherapy has been proved to improve the survival of DLBCL patients, rituximab resistance is a common clinical challenge in the treatment of DLBCL. The aims of the present study are to determine the different levels of several important biomarkers of PTEN/PI3K/AKT pathway in DLBCL patients who are resistant or sensitive to rituximab treatment, and investigate the potential clinical application of these biomarkers. Methods: 48 patients with DLBCL who were treated by rituximab-based chemotherapy were divided into 2 groups according to their reactions to rituximab. The expression of p-AKT, PTEN, and Ki-67 protein in 48 DLBCL tissues were detected using immunohistochemistry and analyzed for the clinical pathological significance and the resistance to rituximab. Meanwhile, PTEN gene deletion was detected also by FISH, and mutation of PIK3CA was performed by sequencing analysis. Results: Activation of p-AKT in 12 of 48 (25.0%) and loss expression of PTEN in 15 of 48 (31.3%) DLBCL species were observed. P-AKT activation (P<0.05) and loss of PTEN expression (P<0.05) were significantly associative with high Ki-67 index. P-AKT and PTEN expression showed a significant negative correlation in all 48 DLBCL patients (r=-0.450, P<0.05), and the Spearman correlation coefficient in the resistant group (r=-0.769, P<0.05) was greater than in the sensitive group (r=-0.691, P<0.05). Conclusion: Regulation of PTEN/PI3K/AKT signal pathway participates in the progression of DLBCL, and may be involved in the development of the resistance to rituximab for some DLBCL patients.
Keywords: PTEN, PI3K, AKT, rituximab resistance, lymphoma
Introduction
Non-Hodgkin’s lymphoma (NHL) is a heterogeneous disease which accounts for about 4% in all kinds of malignancies and ranks fifth in tumor incidence and mortality. Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of lymphoma and accounts for 30-40% in all lymphoma patients approximately [1]. Despite the fact that patients with DLBCL may be cured with combined chemotherapy, the disease represents a high fatality rate of 50% approximately [2]. Rituximab is a mouse-human chimeric monoclonal antibody, which can identify human-CD20 antigen and regulate its antitumor activity by multiple mechanisms including complement-dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC) and inducing apoptosis [3,4]. Rituximab seems not only to activate the inherent apoptotic program directly, but also to sensitize cells to chemotherapy-induced apoptosis by downregulating several pathways, in which PI3K/Akt is included [5,6]. Adding rituximab to chemotherapy regimens, such as the cyclophosphamide, vincristine, doxorubicin, and prednisolone (named as CHOP), has been shown to improve the survival of DLBCL patients [7]. Despite these advantages, responses to treatment of patients are heterogeneous and rituximab resistance is common in DLBCL patients. It has been reported that 30% of cases can not represent a positive response approximately [8,9]. The potential antitumor mechanism of rituximab has been reported by previous researches [10,11], but the exact mechanism of rituximab resistance remains poorly understood. Therefore, it is essential to clarify the mechanism of rituximab resistance to promote the chemotherapy regimens of DLBCL patients.
PI3K/AKT pathway, which is constitutively activated, plays an important role in most tumor cells as a carcinogenic factor, including B-NHL [12 13]. The serine-threonine kinase AKT in PI3K/AKT pathway can regulate multiple survival signals related to anti-apoptosis, proliferation, cell growth and angiogenesis. Various mechanisms have been reported to contribute to activating the AKT pathway, such as regulation of PTEN and PIP3 in upper stream [14,15]. PTEN, a tumor suppressor gene, can resist the function of PI3K and negatively regulate AKT activity, and its loss has been confirmed in various human cancers [16,17]. Additionally, mutations within the catalytic domain p110 of PIK3CA are also found in most cancers cells, and this also indicates the important role of PIK3CA in regulating this pathway negatively [18,19]. It has been reported that hyperactivation of the AKT pathway may be involved in the pathogenesis of NHL and the development of rituximab resistance [20]. However, very little is known about the prognostic role of proteins involved in AKT pathway in DLBCL patients, especially in those resistant to rituximab [21].
In this study, we detected the levels of several important biomarkers (including AKT activation, PTEN loss, and PIK3CA mutation) of PTEN/PI3K/AKT pathway in 48 DLBCL patients, who are resistant or sensitive to rituximab treatment. The correlations among clinical pathological features, p-AKT activation, PTEN loss, PIK3CA mutation, and rituximab resistance were also investigated to clarify the mechanism of PTEN/PI3K/AKT signal pathway in rituximab resistance and evaluate the value of these biomarkers in clinical application and prognosis.
Patients and methods
Approval of an appropriate ethics committee
All experimental research reported in this manuscript was approved by the Zhengzhou University Faculty of Medicine scientific local ethics committee and conducted in accordance with the ethical guidelines of the Helsinki Declaration. Informed consent was obtained from all the patients before enrollment in the study.
Patients
The patients were collected based on the following criteria: diagnosis of denovo DLBCL, availability of paraffin-embedded tissue obtained at diagnosis before the initiation of therapy, availability of follow-up and outcome data, receiving combined treatment of rituximab and chemotherapy for at least 6 weeks. Primary mediastinal DLBCL and primary central nervous system lymphomas were not included in this study. At last, the paraffin-embedded tissues of 48 patients between January 2007 and December 2010 were selected from the Department of Pathology of 1st Affiliated Hospital of Zhengzhou University. Meanwhile, 10 cases of normal tonsils or lymph nodes were also included in this study as control. Clinical information was obtained from the case histories of patients. The gender, age, level of LDH, stage, International Prognostic Index (IPI) factors were all summarized as shown in Table 1. Additionally, according to the molecular subtypes of DLBCL based on Hans’ algorithm [22], 20 cases (41.7%) were evaluated as GCB subtype and 28 cases (58.3%) were evaluated as non-GCB (ABC) subtype. And meanwhile, 27 of the tumor tissues (56.3%) were defined as low proliferation since the Ki-67 index was not more than 25%, while 21 tissues (43.7%) were defined as high proliferation with the Ki-67 index greater than 25%.
Table 1.
Clinical characteristics for the 48 DLBCL patients
| Clinical parameter | Total (No.) | Study population (n/%) | P value | ||
|---|---|---|---|---|---|
|
| |||||
| 48 | Resistant group (No.=20) | Sensitive group (No.=28) | |||
| Gender | Male | 22 | 8 (40%) | 14 (50%) | 0.874 |
| Female | 26 | 12 (60%) | 14 (50%) | ||
| Age | Median (range) | 54.9 | 58 (39-81) | 53 (24-81) | 0.665 |
| LDH (u/l) | <245 [26] | 15 | 6 (30%) | 9 (32.1%) | 0.536 |
| ≥245 | 23 | 14 (70%) | 19 (67.9%) | ||
| Stage | I-II | 24 | 4 (20%) | 20 (71.4%) | 0.001* |
| III-IV | 24 | 16 (80%) | 8 (28.6%) | ||
| IPI | 0-2 | 24 | 9 (45%) | 15 (53.6%) | 0.562 |
| 3-5 | 24 | 11 (55%) | 13 (46.4%) | ||
| Subtype | GCB | 20 | 8 (40%) | 12 (42.9%) | 0.845 |
| Not-GCB | 28 | 12 (60%) | 16 (57.1%) | ||
| Ki-67 index | ≤25% | 27 | 12 (60%) | 15 (53.6%) | 0.661 |
| >25% | 21 | 8 (40%) | 13 (46.4%) | ||
LDH, lactate dehydrogenase; IPI, International Prognostic Index.
P<0.05 was regarded as statistically significant.
Immunohistochemistry (IHC) for p-AKT and PTEN protein expression
Immunohistochemistry assays were performed on PV-6000 polymer detection system and the four-micrometer thick sections were obtained from the paraffin-embedded tissues. The sections were deparaffinized in xylene, rehydrated with graded alcohol, and then boiled in EDTA buffer (pH 9.0) for 2.5 minutes with an autoclave. The sections were incubated at 4°C overnight with different mouse monoclonal antibodies all at a dilution of 1:50 including, anti-phosphorylated AKT (p-AKT, Thr308) antibody (Cell Signaling Technology, Beverly, MA), anti-PTEN antibody (Cell Signaling Technology, Beverly, MA) and anti-Ki-67 antibody (Beijing ZhongShan Biotechnology Co. Ltd., Beijing, China). 3, 3’-diaminobenzidine (DAB) was used to perform the peroxidase reaction. PV-6000 system, antibodies, and DAB were obtained from Zhongshan Golden Bridge Biotechnology (China). Slides were also stained without any primary antibody as negative controls. The staining results were assessed by experienced pathologists.
The cells showing cytoplasm and/or nucleus staining were judged as positive. Scoring of AKT phosphorylation and PNET was based on distribution and intensity of staining. Distri-bution was scored as 0 (0%), 1 (1% to 50%), and 2 (51% to 100%) to indicate the percentage of positive cells of interest. The intensity of the signal was scored as 0 (no signal), 1 (weak), 2 (moderate), and 3 (strong). The final score was calculated by multiplying the proportion score with the staining intensity score. Scores less than 2 (including 2) were regarded as negative expression for p-AKT and loss for PTEN, and scores more than 2 were regarded as activation for p-AKT and positive expression for PTEN [23]. For Ki-67, the expression was defined as low (positive nuclei were less than 25%) or high (positive nuclei were more than 25%) based on the percentage of stained/unstained nuclei in the tumor cells.
Flurescence in situ hybridization (FISH) for PTEN deletion
Three-micrometer thick sections were obtained from the paraffin-embedded tissues for FISH analysis using the ZytoLight FISH-Tissue Implementation kit (ZytoVision, Bremerhaven, Germany). For the PTEN gene, 100 non-overlapping nuclei from the tumor were randomly selected and scored. PTEN gene loss was identified when the ratio of the gene probe/centromere probe was less than 0.8 [24].
Single nucleotide polymorphism (SNP) genotyping for PIK3CA mutations
Mutational analysis of for PIK3CA E542K and E545K (exon 9) and H1047R (exon 20) was performed since almost 80% somatic mutations were found in the helical (exon 9) and kinase (exon 20) domains [25]. Mutation testing for PIK3CA E542K and E545K (exon 9) and H1047R (exon 20) was accomplished with custom Taqman-MGB-SNP genotyping assays. DNA samples were extracted from paraffin-embedded tissues and duplex qRT-PCR of control DNA and mutant target DNA were completed at the same reaction. Samples run in duplicates under default conditions for Allelic Discrimination in an ABI7500 sequence detection system equipped with the SDS v1.4 software (Applied Biosystems). Sequencing validation was performed in selected cases (n=4 for each assay). Forward primer of PIK3CA exon 9: 5’-TCTGTAAATCATCTGTGAATCCAG-3’, and reverse: 5’-GCCAACTACCAATGTAGTATGATTT-3’. Forward primer of PIK3CA exon 20: 5’-GACCTGAAGGTATTAACATCATTTG-3’, reverse; 5’-GTGAGCTTTCATTTTCTCAGTTATC-3’.
Statistical analysis
All computations were carried out using the software of SPSS version 13.0 for Windows. Data are expressed as mean ± SD. The differences between groups were analyzed using the Student’s test or chi-square test. P<0.05 was regarded as statistically significant.
Results clinical pathological features of patients in two groups
As described above, all the 48 patients were treated with rituximab combined with CHOP-based chemotherapy. Patients with obvious disease progression during initial rituximab therapy were clearly defined as resistance (primary resistance). And patients with the decrease of tumor size, represented certain degree of rituximab sensitivity, and were also defined as “resistance” (secondary resistance) if they did not conform classical criteria in part or in whole. The 48 DLBCL patients were divided into 2 groups, resistant group (20 patients) and sensitive group (28 patients) based on the above standard.
Clinical characteristics of the 48 DLBCL patients were summarized in Table 1. The patients consisted of 22 males and 26 females ranging from 24 to 81 years old (median 54.9). Patients in the resistant group represented more advanced clinical stages (III-IV) (P=0.001) compared with the sensitive group. The number of GCB subtype in the resistant group (40.0%) was slightly less than sensitive group (42.9%). While no significant difference was found in age, sex, IPI, LDH levels and Ki-67 index between the two groups.
Evaluation of IHC for p-AKT
P-AKT protein expression was primarily positive in cytoplasm of cells (Figure 1C). 0 (0%) for p-AKT activation were detected among 10 cases of normal tonsils and lymph nodes. However, expression of p-AKT protein was observed in 12 patients (25.0%) of 48 DLBCL species. The expressions of p-AKT was statistically significant in patients compared with normal tonsils and lymph nodes (P<0.05). Additionally, 4 (20%) of 20 GCB subtype DLBCL species and 8 (28.6%) of 28 not-GCB subtype species displayed the p-AKT activation.
Figure 1.
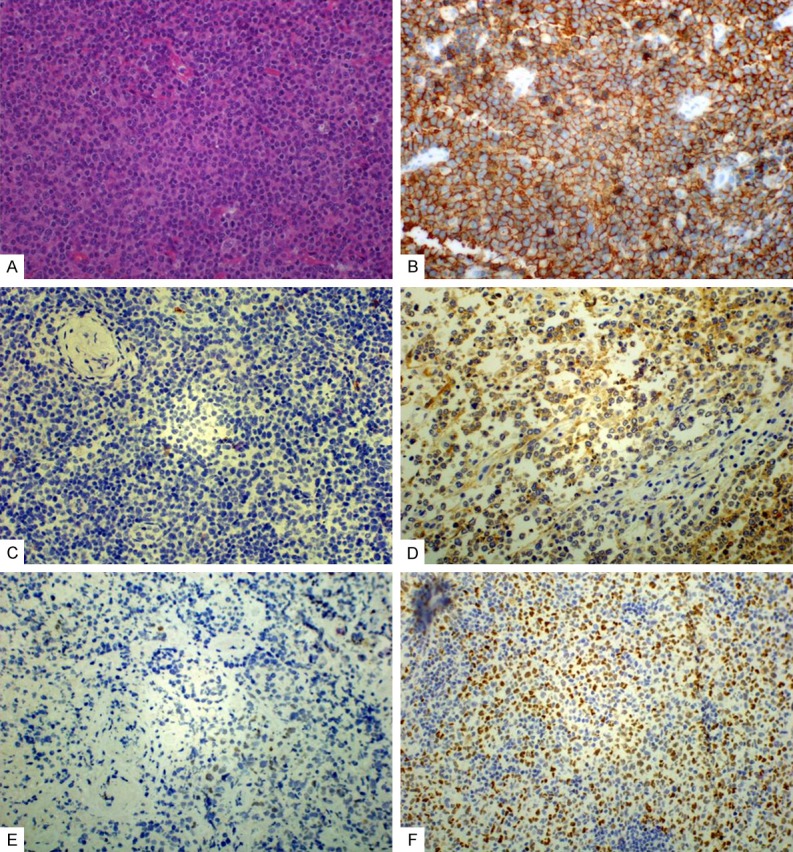
The expression of CD20, p-AKT and PTEN in DLBCL. A. HE of DLBCL (original magnification, ×400); B. Expression of CD20, located in the membrane (original magnification, ×400); C. negative expression of p-AKT (original magnification, ×400); D. Expression of p-AKT was primarily positive in cytoplasmic (original magnification, ×400); E. Loss expression of PTEN (original magnification, ×400); F. Expression of PTEN was primarily in nuclear and, less frequently in cytoplasmic of tumor cells (original magnification, ×400).
Evaluation of IHC and FISH for PTEN
PTEN protein detected by IHC was mainly expressed in nucleus rather than in cytoplasm of cells (Figure 1D). 10 cases (100%) for PTEN expression were detected among 10 cases of normal tonsils and lymph nodes. The expression of PTEN protein can be detected in more than 50% centrocytes and centroblasts in reactive follicular of the tonsils and normal lymph nodes. Meanwhile, for PTEN protein, more than 50% of the cells in the inter-follicular and peri-follicular areas can be stained. Loss expression of PTEN was detected in 15 (31.3%) of 48 DLBCL species. The expression of PTEN was significantly higher in patients compared with normal tonsils and lymph nodes (P<0.05). Additionally, 8 (40%) of 20 GCB subtype DLBCL species and 7 (25%) of 28 not-GCB subtype species displayed the loss of PTEN protein. PTEN gene status was evaluated by FISH in all the 48 cases, and only 3 cases displayed the deletion of PTEN (Figure 2). No significant correlation was observed between PTEN loss detected by IHC and its deletion by FISH.
Figure 2.
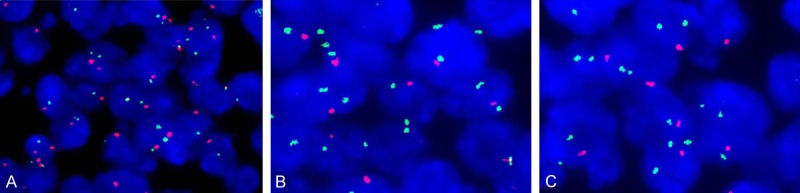
Flurescence in situ hybridization (FISH) for PTEN deletion (green signals represent centromere probe, and red signals represent the gene probe). A. No deletion of PTEN, the ratio of the gene probe/centromere probe was nearly 1:1; B, C. PTEN deletion, the ratio of the gene probe/centromere probe was less than 0.8.
Evaluation of mutation for PIK3CA gene
PIK3CA mutations were observed in 5/48 (10.4%), when mutations at exons 9 and 20 were combined. However, no clear correlation between resistant group and sensitive group was observed in the distribution of patients based on the mutation. And 4 of 5 PIK3CA mutation cases were not-GCB DLBCL.
Correlations between p-AKT activation, PTEN protein loss and PIK3CA gene mutation, and clinical pathological features in DLBCL patients
Among the 48 DLBCL patients, overexpression of p-AKT and loss expression of PTEN were not associated with age, gender, clinical stage and IPI, except for that loss of PTEN expression was dramatically associated with LDH levels (P=0.014, Table 2). PIK3CA mutations detected in 5 of 48 DLBCL patients displayed no significant correlation with the age, sex, LDH, clinical stage of patients. 3 of all 5 cases with PIK3CA mutation showed overexpression of p-AKT, and only one showed loss of PTEN expression simultaneously. As shown in Table 2, p-AKT activation and loss of PTEN expression was both significantly associated with high Ki-67 index (P=0.009, 0.001, respectively).
Table 2.
Status of p-AKT activation, PTEN loss and PIK3CA mutation, and their correlations with clinicopathological features in 48 DLBCL patients
| Clinical Parameter | p-AKT positive | PTEN negative | PIK3CA mutant | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| No. | % | P-value | No. | % | P-value | No. | % | P-value | |||
| Total (No.) | 48 | 12 | 25.3 | 15 | 31.3 | 5 | 10.4 | ||||
| Gender (No.) | Male | 22 | 6 | 27.3 | 0.463 | 8 | 36.4 | 0.435 | 2 | 9.1 | 0.678 |
| Female | 26 | 6 | 23.1 | 7 | 26.9 | 3 | 11.5 | ||||
| Age (years) | >50 | 27 | 7 | 25.9 | 0.473 | 9 | 33.3 | 0.650 | 5 | 18.5 | 0.079 |
| ≤50 | 21 | 5 | 23.8 | 6 | 28.6 | 0 | 0 | ||||
| LDH (u/l) | <245 [26] | 15 | 2 | 13.3 | 0.175 | 1 | 13.3 | 0.014* | 2 | 13.3 | 0.980 |
| ≥245 | 33 | 10 | 30.3 | 14 | 39.4 | 3 | 9.1 | ||||
| Stage | I-II | 24 | 5 | 20.8 | 0.855 | 8 | 33.3 | 0.758 | 1 | 4.2 | 0.882 |
| III-IV | 24 | 7 | 29.2 | 7 | 29.2 | 4 | 20.8 | ||||
| IPI | 0-2 | 24 | 7 | 29.2 | 0.509 | 6 | 25.0 | 0.575 | 3 | 12.5 | 0.944 |
| 3-5 | 24 | 5 | 20.8 | 9 | 37.5 | 2 | 8.3 | ||||
| Subtype | GCB | 20 | 4 | 20 | 0.503 | 8 | 40 | 0.274 | 1 | 5 | 0.304 |
| Not-GCB | 28 | 8 | 28.6 | 7 | 25 | 4 | 14.3 | ||||
| Ki-67 | ≤25% | 27 | 1 | 3.7 | 0.009* | 3 | 11.1 | 0.001* | 2 | 7.41 | 0.134* |
| >25% | 21 | 11 | 52.4 | 12 | 57.1 | 3 | 14.3 | ||||
| Therapeutic effect | Resistant group | 20 | 7 | 35.0 | 0.339 | 10 | 50.0 | 0.019* | 3 | 15.0 | 0.493 |
| Sensitive group | 28 | 5 | 17.9 | 5 | 17.9 | 2 | 7.1 | ||||
LDH, lactate dehydrogenase; IPI, International Prognostic Index;
P<0.05 was regarded as statistically significant.
Comparison of p-AKT, PTEN and PIK3CA status between two groups
As shown in Table 2, p-AKT was expressed in 7 of 20 patients in the resistant group (35.0%), which was slightly higher than 5 of 28 (17.9%) in the sensitive group. Loss of PTEN expression was detected in 10 cases in resistant group (50.0%), which was significantly greater than 5 cases in sensitive group (17.9%, P=0.019). Additionally, 15.0 % patients in the resistant group represented PIK3CA mutation, and it was slightly greater than the sensitive group. As shown in Table 3, the expression of p-AKT and PTEN in all 48 DLBCL patients showed a significant negative correlation (r=-0.450, P=0.003), and the Spearman correlation coefficient in the resistant group (r=-0.769) was greater than sensitive group (r=-0.691).
Table 3.
Correlation between the immunostaining of p-AKT and PTEN
| PTEN | r | P-value | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Positive | Negative | No. | ||||
| All patients | 33 | 15 | 48 | -0.450 | 0.003* | |
| p-AKT | Positive | 2 | 10 | 12 | ||
| Negative | 31 | 5 | 36 | |||
| Resistant group | 10 | 10 | 20 | -0.769 | 0.001* | |
| p-AKT | Positive | 0 | 7 | 7 | ||
| Negative | 10 | 3 | 13 | |||
| Sensitive group | 23 | 5 | 28 | -0.691 | 0.001* | |
| p-AKT | Positive | 2 | 3 | 5 | ||
| Negative | 21 | 2 | 23 | |||
P<0.05 was regarded as statistically significant.
Discussion
PI3K/AKT pathway has been proved to relate directly to several kinds of human cancers including NHL, and p-AKT is constitutively overexpressed in DLBCL which can be used as a prognostic factor [27]. AKT, a serine/threonine protein kinase, can regulate various downstream reactions of PI3K in PI3K/AKT pathway. The most common genetic regulations of this pathway include loss of the tumor suppressor gene PTEN, amplification of genomic region containing AKT, activation of AKT, and point mutations of PIK3CA [28,29]. In the present study, we demonstrated that loss expression of PTEN was significantly associated with the LDH level, and both of p-AKT activation and loss expression of PTEN were significantly associated with the proliferation index (Ki-67 index) in the DLBCL patients. In accordance with previous researches [30-32], our results indicates that the DLBCL patients with constitutive activation of the PI3K/AKT signaling pathway which included both the activation of AKT and loss expression of PTEN represent higher proliferation activity. The regulation of PI3K/AKT signaling pathway may play a role in the malignant progression of DLBCL.
In our study, patients in the resistant group showed more advanced clinical stages (III-IV), and the percentage of loss of PTEN expression in the resistant group was significantly higher than the sensitive group. And furthermore, the expression of p-AKT and PTEN represented significant negative correlation especially in the resistant group. We inferred that rituximab resistance may correlate with PTEN expression in DLBCL patients to a large extent, and loss of PTEN may be involved in the occurrence and development of rituximab resistance in some DLBCL patients.
PTEN gene level was also evaluated by FISH, and no significant correlation between the results of FISH and IHC was observed. It has been proved that loss of PTEN expression generally occurs as a result of mutation, deletion, or promoter methylation in cancer cells [33]. However, the molecular mechanism of PTEN silence in most DLBCL patient samples is still discovered. MicroRNAs (miRNAs) play a crucial role in the regulation of PTEN expression in DLBCLs and various miRNAs such as miR-17-92 and miR-21 has been reported to downregulate PTEN expression [34,35]. In our study, 31.3% of 48 DLBCLs patients showed loss expression of PTEN protein by IHC, while only 3 cases (6.3%) showed the loss of PTEN gene by FISH. The different evaluation of PTEN by two methods suggested that more complex mechanisms may exist in the expression of PTEN of DLBCLs patients besides deletion of PTEN. Certainly, we should note that the evaluation of FISH by using the ratio gene probe/centromere probe might lead to false-negative interpretation of a monosomy, and moreover, small deletions or mutations of PTEN cannot be detected by FISH. Hence, a mutation screening of the hot spots of this gene such as the C2 domain as described should be performed for those cases which do not harbor a PTEN loss as defined by FISH [27]. In consideration of the complex status of PTEN gene in tumors, we think that IHC may be a better method to evaluate the PTEN level compared with FISH, since IHC provides a means for identifying loss of PTEN protein expression resulting from any of these mechanisms [36]. Additionally, we found that PTEN represented more statistical significance in evaluating rituximab resistance compared with p-AKT. This result suggests that an AKT-independent mechanism may contribute to tumorigenesis and drug resistance. That is to say, p-AKT activation is necessary but not sufficient to trigger PTEN loss-dependent tumorigenesis and drug resistance in DLBCL which should be a combined effect of different signal pathways [37,38].
According to gene-expression profile, DLBCLs can be divided into 2 molecular subtypes, GCB and not-GCB. GCB DLBCLs express a molecular signature of normal germinal center B-cells with a more favorable overall survival, whereas not-GCB DLBCLs expresses genes that are distinctive of activated B-cells and plasma cells with a poor clinical outcome. Patients with different DLBCL subtypes have significantly different survival rates after chemotherapy, which may lead to misdiagnosis with distinct disease entities. It has been reported that more than 50% of primary GCB DLBCL patients were characterized by loss of PTEN protein expression, and in contrast, PTEN was expressed in most not-GCB DLBCLs [29]. Loss of PTEN in GCB DLBCLs was related to constitutive activation of the PI3K/AKT signaling pathway, and PI3K/AKT activation was rarely detected in PTEN-positive GCB DLBCLs [39]. These results indicate that loss of PTEN is possibly the predominant molecular mechanism of PI3K/AKT activation in GCB DLBCLs, but not in not-GCB DLBCLs. In our study, 40% of GCB and 25% of not-GCB DLBCLs showed the loss of PTEN protein expression. This result suggested that PTEN loss expression is more related to the GCB subtype of DLBCLs, which was similar to the reported 50% of GCB DLBCLs. We also analyzed the distribution of two molecular subtypes of DLBCLs, and found that 40% in the resistant group and 42.9% in the sensitive group patients were GCB DLBCLs, which showed no significant difference. These results indicate that the classification of molecular subtype of DLBCL is not closely related to rituximab resistance, although different subtypes represent different prognosis and response to rituximab in clinic. This conclusion is not in consistent with existing researches, which may be resulted by little number of cases in our study [40].
In addition, the relationship of PIK3CA mutation and PTEN loss was detected and analyzed. Among 5 cases with PIK3CA mutation, only one showed loss of PTEN expression, and the PIK3CA mutation was not associated with any clinical pathological parameter and Ki-67 index. However, mutation PIK3CA has been reported to be a potent activator of PI3K pathway by itself [41], both the low rate of PIK3CA mutation in DLBCL and the less number of cases in this study represent no statistical significance.
In conclusion, we evaluated p-AKT activation, PTEN loss, and PIK3CA mutation in rituximab-treated DLBCL patients, and confirmed that regulation of PTEN/PI3K/AKT signal pathway was involved in the progression of DLBCL, and maybe played a role in the development of rituximab resistance for some DLBCL patients. B-NHL cell line of the primary resistant to rituximab has been incubated for our follow-up study in vitro to further explore the specific mechanism of rituximab resistance to in DLBCL and explain the function of deregulation of PTEN/PI3K/AKT pathway at the molecular level more clearly.
Acknowledgements
The authors have received support from Natural Science Foundation of China (No. 81201793 and No. 81401936), and honoraria from the speakers bureau of different parts.
Disclosure of conflict of interest
None.
References
- 1.Coiffier B. Diffuse large cell lymphoma. Curr Opin Oncol. 2001;13:325–334. doi: 10.1097/00001622-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Lenz G, Wright G, Dave SS, Xiao W, Powell J, Zhao H, Xu W, Tan B, Goldschmidt N, Iqbal J, Vose J, Bast M, Fu K, Weisenburger DD, Greiner TC, Armitage JO, Kyle A, May L, Gascoyne RD, Connors JM, Troen G, Holte H, Kvaloy S, Dierickx D, Verhoef G, Delabie J, Smeland EB, Jares P, Martinez A, Lopez-Guillermo A, Montserrat E, Campo E, Braziel RM, Miller TP, Rimsza LM, Cook JR, Pohlman B, Sweetenham J, Tubbs RR, Fisher RI, Hartmann E, Rosenwald A, Ott G, Muller-Hermelink HK, Wrench D, Lister TA, Jaffe ES, Wilson WH, Chan WC, Staudt LM Lymphoma/Leukemia Molecular Profiling Project. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med. 2008;359:2313–2323. doi: 10.1056/NEJMoa0802885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith MR. Rituximab (monoclonal anti-CD20 antibody): mechanisms of action and resistance. Oncogene. 2003;22:7359–7368. doi: 10.1038/sj.onc.1206939. [DOI] [PubMed] [Google Scholar]
- 4.Fanale MA, Younes A. Monoclonal antibodies in the treatment of non-Hodgkin’s lymphoma. Drugs. 2007;67:333–350. doi: 10.2165/00003495-200767030-00002. [DOI] [PubMed] [Google Scholar]
- 5.Bonavida B. What Signals Are Generated by Anti-CD20 Antibody Therapy? Curr Hematol Malig Rep. 2006;1:205–213. doi: 10.1007/s11899-006-0001-z. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki E, Umezawa K, Bonavida B. Rituximab inhibits the constitutively activated PI3K-Akt pathway in B-NHL cell lines: involvement in chemosensitization to drug-induced apoptosis. Oncogene. 2007;26:6184–693. doi: 10.1038/sj.onc.1210448. [DOI] [PubMed] [Google Scholar]
- 7.Cheson BD, Leonard JP. Drug therapy: monoclonal antibody therapy for B-cell non-Hodgkin’s lymphoma. N Engl J Med. 2008;359:613–626. doi: 10.1056/NEJMra0708875. [DOI] [PubMed] [Google Scholar]
- 8.Stolz C, Schuler M. Molecular mechanisms of resistance to Rituximab and pharmacologic strategies for its circumvention. Leuk Lymphoma. 2009;50:873–885. doi: 10.1080/10428190902878471. [DOI] [PubMed] [Google Scholar]
- 9.Rezvani AR, Maloney DG. Rituximab resistance. Best Pract Res Clin Haematol. 2011;24:203–216. doi: 10.1016/j.beha.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonavida B. Rituximab-induced inhibition of antiapoptotic cell survival pathways: implications in chemo/immunoresistance, rituximab unresponsiveness, prognostic and novel therapeutic interventions. Oncogene. 2007;26:3629–3636. doi: 10.1038/sj.onc.1210365. [DOI] [PubMed] [Google Scholar]
- 11.Toker A, Yoeli-Lerner M. Akt signaling and cancer: surviving but not moving on. Cancer Res. 2006;66:3963–3966. doi: 10.1158/0008-5472.CAN-06-0743. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki T, Kuniyasu H. Significance of AKT in gastric cancer (Review) Int J Oncol. 2014;45:2187–2192. doi: 10.3892/ijo.2014.2678. [DOI] [PubMed] [Google Scholar]
- 13.Uddin S, Bu R, Ahmed M, Hussain AR, Ajarim D, Al-Dayel F, Bavi P, Al-kuraya KS. Leptin receptor expression and its association with PI3K/AKT signaling pathway in diffuse large B-cell lymphoma. Leuk Lymphoma. 2010;51:1305–1314. doi: 10.3109/10428191003802365. [DOI] [PubMed] [Google Scholar]
- 14.Kang S, Bader AG, Vogt PK. Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proc Natl Acad Sci U S A. 2005;102:802–807. doi: 10.1073/pnas.0408864102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Razis E, Bobos M, Kotoula V, Eleftheraki AG, Kalofonos HP, Pavlakis K, Papakostas P, Aravantinos G, Rigakos G, Efstratiou I, Petraki K, Bafaloukos D, Kostopoulos I, Pectasides D, Kalogeras KT, Skarlos D, Fountzilas G. Evaluation of the association of PIK3CA mutations and PTEN loss with efficacy of trastuzumab therapy in metastatic breast cancer. Breast Cancer Res Treat. 2011;128:447–456. doi: 10.1007/s10549-011-1572-5. [DOI] [PubMed] [Google Scholar]
- 16.Carracedo A, Alimonti A, Pandolfi PP. PTEN level in tumour suppression: How much is too little? Cancer Res. 2011;71:629–633. doi: 10.1158/0008-5472.CAN-10-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blanco-Aparicio C, Renner O, Leal JF, Carnero A. PTEN, more than the AKT pathway. Carcinogenesis. 2007;28:1379–1386. doi: 10.1093/carcin/bgm052. [DOI] [PubMed] [Google Scholar]
- 18.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, Willson JK, Markowitz S, Kinzler KW, Vogelstein B, Velculescu VE. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 19.Pérez-Tenorio G, Alkhori L, Olsson B, Waltersson MA, Nordenskjöld B, Rutqvist LE, Skoog L, Stål O. PIK3CA Mutations and PTEN Loss Correlate with Similar Prognostic Factors and Are Not Mutually Exclusive in Breast Cancer. Clin Cancer Res. 2007;13:3577–3584. doi: 10.1158/1078-0432.CCR-06-1609. [DOI] [PubMed] [Google Scholar]
- 20.Cordo Russo RI, García MG, Alaniz L, Blanco G, Alvarez E, Hajos SE. Hyaluronan oligosaccharides sensitize lymphoma resistant cell lines to vincristine by modulating P-glycoprotein activity and PI3K/Akt pathway. Int J Cancer. 2008;122:1012–1018. doi: 10.1002/ijc.23122. [DOI] [PubMed] [Google Scholar]
- 21.Hasselblom S, Hansson U, Olsson M, Torén L, Bergström A, Nilsson-Ehle H, Andersson PO. High immunohistochemical expression of p-AKT predicts inferior survival in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Br J Haematol. 2010;149:560–568. doi: 10.1111/j.1365-2141.2010.08123.x. [DOI] [PubMed] [Google Scholar]
- 22.Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, Powell JI, Yang L, Marti GE, Moore T, Hudson J Jr, Lu L, Lewis DB, Tibshirani R, Sherlock G, Chan WC, Greiner TC, Weisenburger DD, Armitage JO, Warnke R, Levy R, Wilson W, Grever MR, Byrd JC, Botstein D, Brown PO, Staudt LM. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 23.Tsurutani J, Fukuoka J, Tsurutani H, Shih JH, Hewitt SM, Travis WD, Jen J, Dennis PA. Evaluation of two phosphorylation sites improves the prognostic significance of Akt activation in non-small-cell lung cancer tumors. J. Clin. Oncol. 2006;24:306–314. doi: 10.1200/JCO.2005.02.4133. [DOI] [PubMed] [Google Scholar]
- 24.Christodoulou C, Kostopoulos I, Kalofonos HP, Lianos E, Bobos M, Briasoulis E, Gogas H, Razis E, Skarlos DV, Fountzilas G Study of the Hellenic Cooperative Oncology Group. Trastuzumab combined with pegylated liposomal doxorubicin in patients with metastatic breast cancer. Phase II Study of the Hellenic Cooperative Oncology Group (HeCOG) with biomarker evaluation. Oncology. 2009;76:275–285. doi: 10.1159/000207504. [DOI] [PubMed] [Google Scholar]
- 25.Wu G. Recent progess in phosphoinositide 3-kinases: oncogenic properties and prognostic and therapeutic implications. Curr Protein Pept Sci. 2010;11:425–35. doi: 10.2174/138920310791824156. [DOI] [PubMed] [Google Scholar]
- 26.Lin S, YuJun L, XiaoMing X, WenWen R. Expression and significance of leptin receptor, p-STAT3 and p-AKT in diffuse large B-cell lymphoma. Acta Histochem. 2014;116:126–130. doi: 10.1016/j.acthis.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Pfeifer M, Grau M, Lenze D, Wenzel SS, Wolf A, Wollert-Wulf B, Dietze K, Nogai H, Storek B, Madle H, Dörken B, Janz M, Dirnhofer S, Lenz P, Hummel M, Tzankov A, Lenz G. PTEN loss defines a PI3K/AKT pathway-dependent germinal center subtype of diffuse large B-cell lymphoma. Proc Natl Acad Sci U S A. 2013;110:12420–12425. doi: 10.1073/pnas.1305656110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 29.Nishimura R, Arima N, Toyoshima S, Ohi Y, Anan K, Sagara Y, Mitsuyama S, Tamura K. Evaluation of PTEN loss and PIK3CA mutations and their correlation with efficacy of trastuzumab treatment in HER2-positive metastatic breast cancer: A retrospective study (KBC-SG 1001) Mol Clin Oncol. 2013;1:47–52. doi: 10.3892/mco.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfeifer M, Lenz G. PI3K/AKT addiction in subsets of diffuse large B-cell lymphoma. Cell Cycle. 2013;12:3347–3348. doi: 10.4161/cc.26575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ocana A, Vera-Badillo F, Al-Mubarak M, Templeton AJ, Corrales-Sanchez V, Diez-Gonzalez L, Cuenca-Lopez MD, Seruga B, Pandiella A, Amir E. Activation of the PI3K/mTOR/AKT Pathway and Survival in Solid Tumors: Systematic Review and Meta-Analysis. PLoS One. 2014;9:e95219. doi: 10.1371/journal.pone.0095219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abbott RT, Tripp S, Perkins SL, Elenitoba-Johnson KS, Lim MS. Analysis of the PI-3-Kinase-PTEN-AKT Pathway in Human Lymphoma and Leukemia Using a Cell Line. Mod Pathol. 2003;16:607–612. doi: 10.1097/01.MP.0000067423.83712.74. [DOI] [PubMed] [Google Scholar]
- 33.Leslie NR, Foti M. Non-genomic loss of PTEN function in cancer: not in my genes. Trends Pharmacol Sci. 2011;32:131–140. doi: 10.1016/j.tips.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 34.Zhu H, Han C, Lu D, Wu T. MiR-17-92 cluster promotes cholangiocarcinoma growth: evidence for PTEN as downstream target and IL-6/Stat3 as upstream activator. Am J Pathol. 2014;184:2828–39. doi: 10.1016/j.ajpath.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ren W, Qiang C, Gao L, Li SM, Zhang LM, Wang XL, Dong JW, Chen C, Liu CY, Zhi KQ. Circulating microRNA-21 (MIR-21) and phosphatase and tensin homolog (PTEN) are promising novel biomarkers for detection of oral squamous cell carcinoma. Biomarkers. 2014;19:590–596. doi: 10.3109/1354750X.2014.955059. [DOI] [PubMed] [Google Scholar]
- 36.Sangale Z, Prass C, Carlson A, Tikishvili E, Degrado J, Lanchbury J, Stone S. A Robust Immunohistochemical Assay for Detecting PTEN Expression in Human Tumors. Appl Immunohistochem Mol Morphol. 2011;19:173–183. doi: 10.1097/PAI.0b013e3181f1da13. [DOI] [PubMed] [Google Scholar]
- 37.Hafsi S, Pezzino FM, Candido S, Ligresti G, Spandidos DA, Soua Z, McCubrey JA, Travali S, Libra M. Gene alterations in the PI3K/PTEN/AKT pathway as a mechanism of drug-resistance (Review) Int J Oncol. 2012;40:639–644. doi: 10.3892/ijo.2011.1312. [DOI] [PubMed] [Google Scholar]
- 38.Abubaker J, Bavi PP, Al-Harbi S, Siraj AK, Al-Dayel F, Uddin S, Al-Kuraya K. PIK3CA mutations are mutually exclusive with PTEN loss in diffuse large B-cell lymphoma. Leukemia. 2007;21:2368–2370. doi: 10.1038/sj.leu.2404873. [DOI] [PubMed] [Google Scholar]
- 39.Seki R, Ohshima K, Fujisaki T, Uike N, Kawano F, Gondo H, Makino S, Eto T, Moriuchi Y, Taguchi F, Kamimura T, Tsuda H, Ogawa R, Shimoda K, Yamashita K, Suzuki K, Suzushima H, Tsukazaki K, Higuchi M, Utsunomiya A, Iwahashi M, Imamura Y, Tamura K, Suzumiya J, Yoshida M, Abe Y, Matsumoto T, Okamura T. Prognostic impact of immunohistochemical biomarkers in diffuse large B-cell lymphoma in the rituximab era. Cancer Sci. 2009;100:1842–1847. doi: 10.1111/j.1349-7006.2009.01268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xia Y, Li ZM, Shi YX, Xia ZJ, Jiang WQ, Huang HQ. Short-term efficacy of rituximab-CHOP and CHOP regimens on two subtypes of diffuse large B-cell lymphoma. Ai Zheng. 2009;28:146–149. [PubMed] [Google Scholar]
- 41.Chen Y, Hou Q, Yan W, Luo J, Chen D, Liu Z, He S, Ding X. PIK3CA is critical for the proliferation, invasiveness, and drug resistance of human tongue carcinoma cells. Oncol Res. 2011;19:563–571. doi: 10.3727/096504012x13340632812677. [DOI] [PubMed] [Google Scholar]


