Abstract
We undertook a retrospective analysis to evaluate the C-reactive protein/albumin (CRP/Alb) ratio for its prognostic value in patients with clear cell renal cell carcinoma (CCRCC). The study comprised 406 CCRCC patients undergoing nephrectomy between 2003 and 2012 in our hospital. The correlations among the pretreatment CRP/Alb ratio, clinicopathological parameters, and overall survival (OS) were evaluated. An elevated CRP/Alb ratio was associated with older age at surgery (P=0.007), more advanced TNM stage (P<0.001), more presence of tumor necrosis (P<0.001) and lymphovascular invasion (P<0.001), lower concentration of hemoglobin (P<0.001) and calcium (P=0.005), and shorter OS (P<0.001). The multivariate analysis confirmed that the CRP/Alb ratio independently predicted the OS of patients with CCRCC (P<0.001), the Glasgow Prognostic Score (GPS) (P=0.001) and modified GPS (mGPS) (P=0.019) were independent prognostic factors also. At last, we evaluated the prognostic value of the CRP/Alb ratio compared with the similar inflammation-based prognostic scores GPS and mGPS using the area under the curve (AUC). Although the differences were not statistically significant, the AUC value of the CRP/Alb ratio (continuous, categorical) was higher compared with the GPS and mGPS, except that the AUC value for the CRP/Alb ratio (categorical) at 3 years was lower than that for the GPS. The CRP/Alb ratio could take the place of the GPS and mGPS in terms of predicting prognosis in CCRCC.
Keywords: Clear cell renal cell carcinoma, C-reactive protein, albumin, prognosis
Introduction
Kidney cancer is one of the most common malignancies in the urological system. Clear cell renal cell carcinoma (CCRCC) is the most common subtype of kidney cancer and accounts for 70%-80% of all. Although advances in technology have improved the efficacy in patients with CCRCC, 20%-30% of patients undergoing curative nephrectomy will have a relapse during follow-up [1]. In order to assess patients’ postoperative risk, and draw their attention to the possible relapse, several prognostic factors and models have been established to predict CCRCC patient’s clinical outcome, such as TNM stage and Fuhrman grade and so on. Last few years, investigators have demonstrated that the presence of systemic inflammatory response is associated with a poor outcome in patients with various types of cancer [2-4]. As the established inflammation-based prognostic scores, GPS and mGPS determined based on the serum levels of C-reactive protein (CRP) and albumin have been proved to have prognostic value in various types of cancer. Previous studies have demonstrated that the GPS and mGPS are reliable and effective scoring system for outcome prediction in patients with CCRCC [5,6]. Moreover, Lucca indicated that the GPS is superior to other inflammation-based prognostic scores in terms of its prognostic ability in patients with CCRCC [7]. Recently, based on the same index, the ratio of CRP to albumin, which names the CRP/albumin (CRP/Alb) ratio, have been proved to show outstanding prognostic value in hepatocellular carcinoma and esophageal squamous cell carcinoma compared with other established inflammation-based prognostic scores [8,9]. However, whether the CRP/Alb ratio is associated with outcome in CCRCC patients has not yet been elucidated. Therefore, in this study we aim to explore the prognostic performance of the CRP/Alb ratio in Chinese patients with CCRCC. We also evaluated the prognostic value of the CRP/Alb ratio compared with the GPS and mGPS.
Materials and methods
Patients
This retrospective study included data from 461 CCRCC patients, who underwent radical or partial nephrectomy in The Third Affiliated Hospital of Soochow University from 2003 to 2012. 27 patients had been lost to follow-up, and 22 patients whose clinicopathological parameters were not complete were excluded. Patients who had a history of previous anti-cancer therapies and other malignancies, who showed evidence of acute infection or chronic inflammatory disease were also excluded. After excluding 55 patients, the remaining 406 patients were finally evaluated, who underwent potentially curative resection.
Clinical and laboratory data
For each patient, the following clinical and pathologic information was gathered: age at surgery, gender, TNM stage, presence of tumor necrosis and lymphovascular invasion, and concentration of serum hemoglobin and calcium. TNM stage was assigned according to the 2010 TNM classification. Tumor necrosis was defined as microscopic coagulative necrosis [10,11]. Lymphovascular invasion was defined as the presence of the invasion of cancer cells into blood vessels or the lymphatic system without underlying muscular walls [12,13]. The laboratory data, levels of CRP, albumin, hemoglobin, calcium and so on were obtained within 1 week before surgery. All of the clinicopathological data above were retrieved from Medical records inquiry system of The Third Affiliated Hospital of Soochow University. The CRP/Alb ratio was calculated by dividing the serum CRP level by the serum albumin level. The GPS was calculated as follows: Patients had both an elevated C-reactive protein serum levels (>10 mg/L) and low albumin (<35 g/L) were allocated a score of 2, and patients with 1 or no abnormality value were allocated a score of 1 or 0, respectively [5]. The mGPS was constructed as follows: Patients had both an elevated C-reactive protein serum levels (>10 mg/L) and hypoalbuminemia (<35 g/L) were allocated a score of 2; patients in whom only C-reactive protein was elevated (>10 mg/L) were allocated a score of 1 and those with a normal C-reactive protein were allocated a score of 0 [14].
Statistical analyses
For the description of clinical and pathological characteristics of patients, categorical variables were presented as numbers and percentages, continuous variables were allocated in groups according to the optimal cut-off value. Receiver operating characteristics (ROC) analysis was done to identify the cutoff point of continuous variables. OS was calculated from date of surgery to individuals’ death of any cause or last follow-up. The OS rates were calculated using the Kaplan-Meier method, and compared using the log-rank test. Univariate analysis of the potential factors related to survival was conducted with the help of Mantel-Cox regression methodology. The Cox proportional hazards model was used for the multivariate analysis to identify independent prognostic factors associated with OS. ROC analysis was also used to measure and compare the areas under the curve (AUC), and then to evaluate the discriminatory ability of the inflammation-based prognostic scores. The Chi square test was used to detect the differences between groups. All statistical tests were two-sided and a P-value <0.05 was considered statistically significant. All data analyses were performed with SPSS Statistics 17.0.
Results
The median age of patients was 58 (range 24-80) years. Overall, 253 (62.3%) patients were males and 153 (37.7%) were females. The mean postoperative follow-up was 63 (range 1-151) months.
Table 1 shows the correlations of the CRP/Alb ratio with the clinicopathological characteristics. The cut off level of CRP/Alb ratio to divide the three groups (CRP/Alb ratio <0.060 (n=163), CRP/Alb ratio ≥0.060 and <0.115 (n=150), CRP/Alb ratio ≥0.115 (n=93)) was determined after receiver operating characteristics (ROC) analysis. In order to analysis the continuous variables, we divide them into groups according to the cut off level. The cut off level of age at operation, hemoglobin, calcium were 57.5, 116.05, and 2.405 respectively. An elevated CRP/Alb ratio was associated with older age at surgery (P=0.007), more advanced TNM stage (P<0.001), more presence of tumor necrosis (P<0.001), more presence of lymphovascular invasion (P<0.001), lower concentration of hemoglobin (P<0.001) and calcium (P=0.005). There was no statistical difference in the distribution of sex and lymph node metastasis compared with the CRP/Alb ratio. The results of the Kaplan-Meier method were present as Figure 1, the OS rates were significantly lower in the high CRP/Alb ratio group compared with the low group (P<0.001).
Table 1.
Correlation of the CRP/Alb ratio with the clinicopathological characteristics of patients
| Variable | No. of Patients (%) | |||
|---|---|---|---|---|
|
|
P value | |||
| CRP/Alb ratio | ||||
|
| ||||
| <0.060 (n=163) | 0.060-0.115 (n=150) | ≥0.115 (n=93) | ||
| Age (years) | 0.007* | |||
| <57.5 | 91 (22.4) | 75 (18.5) | 33 (8.1) | |
| ≥57.5 | 72 (17.7) | 75 (18.5) | 60 (14.8) | |
| Sex | 0.221 | |||
| Male | 99 (24.4) | 89 (21.9) | 65 (16.0) | |
| Female | 64 (15.8) | 61 (15.0) | 28 (6.9) | |
| T stage | <0.001* | |||
| 1 | 144 (35.5) | 135 (33.3) | 61 (15.0) | |
| 2 | 15 (3.7) | 11 (2.7) | 13 (3.2) | |
| 3 | 4 (1.0) | 4 (1.0) | 18 (4.4) | |
| 4 | 0 (0.0) | 0 (0.0) | 1 (0.2) | |
| N stage | 0.163 | |||
| 0 | 159 (39.1) | 148 (36.5) | 88 (21.7) | |
| 1 | 4 (1.0) | 2 (0.5) | 5 (1.2) | |
| M stage | 0.034* | |||
| 0 | 163 (40.1) | 150 (37.0) | 91 (22.4) | |
| 1 | 0 (0.0) | 0 (0.0) | 2 (0.0) | |
| TNM stage | <0.001* | |||
| I | 142 (35.0) | 134 (33.0) | 59 (14.5) | |
| II | 15 (3.7) | 11 (2.7) | 12 (3.0) | |
| III | 6 (1.5) | 5 (1.2) | 19 (4.7) | |
| IV | 0 (0.0) | 0 (0.0) | 3 (0.7) | |
| Tumor necrosis | <0.001* | |||
| Absent | 155 (38.2) | 142 (35.0) | 71 (17.5) | |
| Present | 8 (2.0) | 8 (2.0) | 22 (5.4) | |
| Lymphovascular invasion | <0.001* | |||
| Absent | 160 (39.4) | 146 (36.0) | 80 (19.7) | |
| Present | 3 (0.7) | 4 (1.0) | 13 (3.2) | |
| Hemoglobin (g/L) | <0.001* | |||
| <116.05 | 14 (3.4) | 13 (3.2) | 30 (7.4) | |
| ≥116.05 | 149 (36.7) | 137 (33.7) | 63 (15.5) | |
| Ca (mmol/L) | 0.005* | |||
| <2.40 | 71 (17.5) | 73 (18.0) | 59 (14.5) | |
| ≥2.40 | 91 (22.4) | 75 (18.5) | 32 (7.9) | |
indicates that the difference was statistically significant.
Abbreviation: TNM tumor-node-metastasis, CRP/Alb the C-reactive protein/Albumin ratio, Ca calcium.
Figure 1.
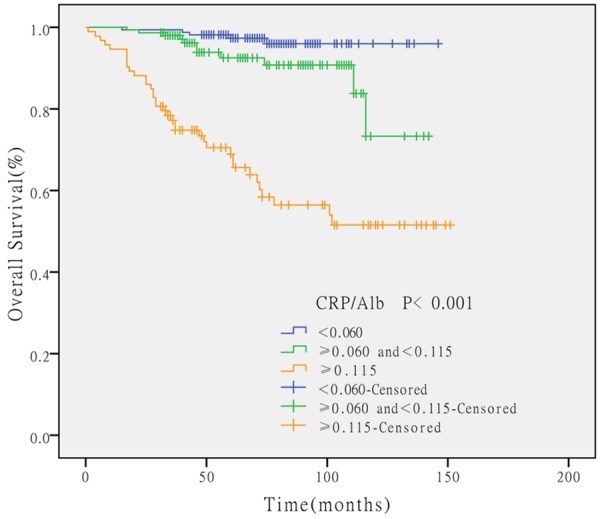
The Kaplan-Meier survival curves according to the preoperative CRP/Alb ratio. The OS rates were significantly lower in the high CRP/Alb ratio group compared with the low group (P<0.001).
The results of the univariate and multivariable analysis are shown in Table 2. Univariate analysis identified age (P=0.001), high TNM stage (P<0.001), tumor necrosis (P<0.001), lymphovascular invasion (P<0.001), lower concentration of hemoglobin (P<0.001) and calcium (P<0.001), and a higher CRP/Alb ratio (P<0.001), GPS (P<0.001), mGPS (P<0.001) as prognosticators of patients’ poor outcome, whereas SEX (P=0.110) was not statistically significantly associated with OS. Considering the original correlations among the CRP/Alb ratio, GPS, and mGPS, we conducted the multivariate analysis respectively. The age, TNM stage, tumor necrosis, lymphovascular invasion, hemoglobin and calcium were the common variables tested in the multivariate analysis. Multivariate analysis revealed that the CRP/Alb ratio (P<0.001), age (P=0.008), TNM stage (P=0.002), hemoglobin (P=0.001) and calcium (P=0.009) were independently associated with OS. The GPS (P=0.001) and mGPS (P=0.019) were independent prognostic factors also.
Table 2.
Overall survival, univariate and multivariate analyses
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age (years) | 0.001* | 0.008*,a | ||
| <57.5 | reference | reference | ||
| ≥57.5 | 2.66 (1.46-4.85) | 2.39 (1.26-4.56) | ||
| Sex | 0.110 | |||
| Female | reference | |||
| Male | 1.65 (0.89-3.04) | |||
| TNM stage | <0.001* | 0.002*,a | ||
| I | reference | reference | ||
| II | 2.68 (1.21-5.95) | 0.015* | 2.09 (0.86-5.12) | 0.105a |
| III | 11.13 (6.00-20.66) | <0.001* | 4.46 (2.07-9.59) | <0.001*,a |
| IV | 11.04 (2.57-47.33) | 0.001* | 2.40 (0.38-15.09) | 0.351a |
| Tumor necrosis | <0.001* | 0.982a | ||
| Absent | reference | reference | ||
| Present | 3.26 (1.75-6.05) | 1.01 (0.48-2.10) | ||
| Lymphovascular invasion | <0.001* | 0.907a | ||
| Absent | reference | reference | ||
| Present | 5.64 (2.74-11.60) | 1.06 (0.40-2.78) | ||
| Hemoglobin (g/L) | <0.001* | 0.001*,a | ||
| ≥116.05 | reference | reference | ||
| <116.05 | 5.89 (3.40-10.20) | 2.74 (1.48-5.07) | ||
| Ca (mmol/L) | <0.001* | 0.009*,a | ||
| ≥2.40 | reference | reference | ||
| <2.40 | 3.33 (1.80-6.19) | 2.52 (1.26-5.05) | ||
| CRP/Alb ratio | <0.001* | <0.001*,a | ||
| <0.060 | reference | reference | ||
| ≥0.060 and <0.115 | 2.92 (1.01-8.42) | 0.047* | 3.13 (1.07-9.13) | 0.037*,a |
| ≥0.115 | 15.49 (6.06-39.62) | <0.001* | 7.45 (2.81-19.73) | <0.001*,a |
| GPS | <0.001* | 0.001*,b | ||
| 0 | reference | reference | ||
| 1 | 6.64 (3.56-12.37) | <0.001* | 3.57 (1.79-7.15) | <0.001*,b |
| 2 | 13.26 (6.47-27.17) | <0.001* | 2.540 (1.03-6.29) | 0.044*,b |
| mGPS | <0.001* | 0.019*,c | ||
| 0 | reference | reference | ||
| 1 | 5.30 (2.65-10.59) | <0.001* | 2.98 (1.36-6.53) | 0.006*,c |
| 2 | 10.96 (5.47-21.94) | <0.001* | 1.94 (0.81-4.62) | 0.135c |
Indicates that the difference was statistically significant.
Abbreviation: HR Hazard ratio, CI confidence interval, TNM tumornode-metastasis, CRP/Alb the C-reactive protein/Albumin ratio, Ca calcium , GPS the Glasgow Prognostic Score, mGPS the modified Glasgow Prognostic Score.
Adjustment for age, TNM stage, tumor necrosis, lymphovascular invasion, hemoglobin, Ca, CRP/Alb ratio;
Adjustment for age, TNM stage, tumor necrosis, lymphovascular invasion, hemoglobin, Ca, GPS;
Adjustment for age, TNM stage, tumor necrosis, lymphovascular invasion, hemoglobin, Ca, mGPS.
To assess the discrimination ability of the CRP/Alb ratio compared with the GPS and mGPS, we generated ROC curves for the survival status at 3 years and 5 years follow-up examinations which was shown in Figure 2. Table 3 showed the comparison of discrimination ability of the CRP and albumin based inflammation-based prognostic score. As showed in the table, the AUC value of the CRP/Alb ratio (continuous) was always higher than that of the GPS and mGPS at both 3 years and 5 years. At the follow-up of 5 years, the AUC value of CRP/Alb ratio (categorical) remained to be the highest. When at the follow-up of 3 years, the AUC value of the GPS was higher than the CRP/Alb ratio (categorical). However with the help of the statistics software MedCalc12.7.0, we confirmed that none of the differences had statistical significance.
Figure 2.
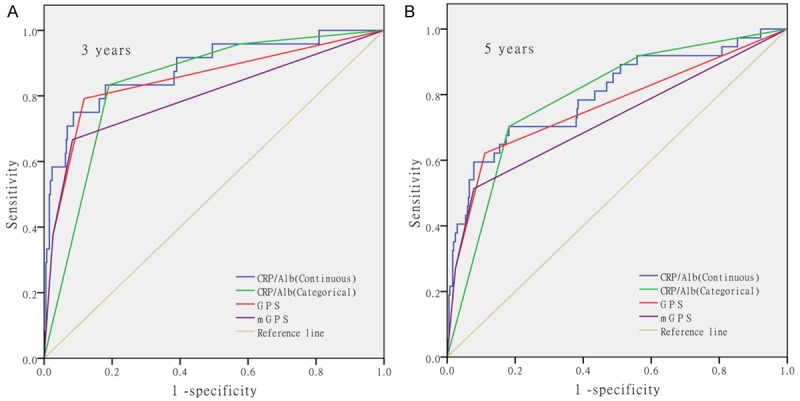
The ROC curves of inflammation-based prognostic indexes at 3 years and 5 years of follow-up. This figure showed the ROC curves of the CRP/Alb ratio (continuous), CRP/Alb ratio (categorical), GPS (categorical) and mGPS (categorical) for the survival status at 3 year and 5 years of follow-up.
Table 3.
Comparison of the discriminatory ability of the inflammation-based prognostic scores
| Period | AUC | 95% CI | p value |
|---|---|---|---|
| 3-year | |||
| CRP/Alb ratio | |||
| Continuous | 0.88 | 0.85-0.91 | <0.001* |
| Categorical | 0.84 | 0.80-0.87 | <0.001* |
| GPS | 0.85 | 0.81-0.88 | <0.001* |
| mGPS | 0.80 | 0.76-0.84 | <0.001* |
| 5-year | |||
| CRP/Alb ratio | |||
| Continuous | 0.80 | 0.76-0.84 | <0.001* |
| Categorical | 0.79 | 0.75-0.83 | <0.001* |
| GPS | 0.76 | 0.72-0.80 | <0.001* |
| mGPS | 0.72 | 0.68-0.77 | <0.001* |
Indicates that the difference was statistically significant.
Abbreviation: AUC area under the curves, CI confidence interval, CRP/Alb the C-reactive; protein/Albumin ratio, GPS the Glasgow Prognostic Score, mGPS the modified Glasgow Prognostic Score.
Discussion
In the nineteenth century, Virchow at all originally found the connection between cancer and inflammation [15]. Gradually, the opinion that inflammation has an important role in carcinogenesis was widely recognized. Except the secretion of inflammatory cells, cancer-related inflammation consist of inflammatory cytokines produced by cancer cells themselves and tumor-associated leukocytes during the process of tissue remodeling, tissue repair and angiogenesis [16]. Thus, except peritumoral zone microenvironment changes , inflammatory cytokines such as tumor necrosis factor (TNF), interleukin (IL)-1, -6, and -8, and vascular endothelial growth factor (VEGF) will have a great change in peripheral blood, which facilitate cancer process [15-17]. For the reason that the peripheral circulation may reflect the complex array of inflammatory cells and inflammatory mediators produced in tumor microenvironment. Thus, several inflammation-based prognostic scores have been developed to predict patient outcome in various types of tumor. As for renal carcinoma, NLR [18], PLR [19], LMR [20], PNI [21], GPS [5] and mGPS [6] were proved to be effective inflammation-based prognostic scores. With the help of these marks, clinicians can roughly estimate outcome of cancer patients.
In this study, we focus on the prognosis value of CRP/Alb ratio in CCRCC. The prognosis value of CRP/Alb ratio was primarily found in the outcome of acute exacerbations of chronic disease by Fairclough et al. [22]. Another study showed that CRP/albumin ratio was an independent risk factor for mortality at 90 days in septic patients [23]. When it comes to tumor, CRP/Alb ratio also proved to be efficacious in hepatocellular carcinoma and esophageal squamous cell carcinoma [8,9]. According to their articles, the CRP/Alb ratio is an independent prognostic marker, and compared with other established inflammation-based prognostic scores, the CRP/Alb ratio may have a better prognostic ability.
In our study, as shown on Table 1, an elevated CRP/Alb ratio is associated with higher TNM stage, more presence of tumor necrosis and lymphovascular invasion, lower concentration of hemoglobin and calcium. The multivariate analysis confirmed that the CRP/Alb ratio independently predicted the overall survival of patients with CCRCC (P<0.001). These results indicated that the CRP/Alb ratio was an effective and promising inflammation-based prognostic score in CCRCC.
As a sensitive and reliable prognostic marker for systemic inflammation, CRP has been shown to be significant in the prediction of outcomes of urological cancers, most intensively in renal cell carcinoma [24,25]. The albumin that indicates both the inflammation and the nutritional status, had been proved to be an independent prognostic factor for patients with metastatic renal cell carcinoma [26]. The CRP/Alb ratio and GPS, mGPS, combining the two efficacious indexes, CRP and albumin, should have greater predictive value. Numbers of articles had indicated the prognosis value of GPS, mGPS in different types of cancer, include CCRCC [5,6]. As a new prognostic indicator, the CRP/Alb ratio showed outstanding performance in the prognosis of CCRCC according to the univariate and multivariable analysis of this article. Thus, we also pay special attention to compare the prognostic value of the three related prognostic scores. The ROC analysis has demonstrated that the CRP/Alb ratio (continuous) had a higher AUC value than GPS and mGPS at 3 years and 5 years examined. Meanwhile, the AUC values for the CRP/Alb ratio (categorical) at 5 years were higher than those for the GPS and mGPS, but, the AUC values for the CRP/Alb ratio (categorical) at 3 years were lower than those for the GPS. Although the differences above were not statistically significant, the CRP/Alb ratio is comparable to GPS and mGPS in terms of its prognostic ability on the basis of the result above.
The CRP/Alb ratio, GPS, mGPS, originated from the same variables, were all useful for the prognosis of CCRCC, but due to the continuous variable in its nature, the CRP/Alb ratio could stratify the patient outcomes more strictly. Meanwhile, GPS, mGPS calculated the serum CRP and albumin levels separately, which ignored the relationship between the two indexes. In contrast, the CRP/Alb ratio blended the two together, highlight the interlinkages between CRP and albumin, can be more convincing theoretically. What further caught our attention was that, when considering the distinguish range, there were 84.2% and 88.2% of patients classified in the group of a score of 0 of the GPS and mGPS respectively, numbers of patients who were high-risk groups were classified in the group of a score of 0, which meant that the GPS and mGPS couldn’t distinguish the survival differences of most of the patients compared with the CRP/Alb ratio in this study.
There are some possible limitations associated with this study. We evaluated a relatively small number of patients, instead of a multi-center study, the prognostic value of the CRP/Alb ratio should be confirmed in a validation cohort. Meanwhile, this study was a retrospective study, some criteria for the research methods were not uniform. A multi-center, prospective study should be performed to confirm our findings.
In summary, to the best of our knowledge, this is the first study to indicate that CRP/Alb ratio can predict the overall survival in CCRCC. This study demonstrated that the CRP/Alb ratio, a novel inflammation-based prognostic score, is an independent predictor of overall survival for patients with CCRCC undergoing radical or partial nephrectomy, and it is comparable to the similar inflammation-based prognostic score GPS and mGPS. The CRP/Alb ratio might be a reliable model to predict survival after nephrectomy in CCRCC patients.
Disclosure of conflict of interest
None.
References
- 1.Ljungberg B, Campbell SC, Choi HY, Jacqmin D, Lee JE, Weikert S, Kiemeney LA. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60:615–621. doi: 10.1016/j.eururo.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 2.Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6:149–163. doi: 10.2217/fon.09.136. [DOI] [PubMed] [Google Scholar]
- 3.Qu JL, Qu XJ, Li Z, Zhang JD, Liu J, Teng YE, Jin B, Zhao MF, Yu P, Shi J, Fu LY, Wang ZN, Liu YP. Prognostic Model Based on Systemic Inflammatory Response and Clinicopathological Factors to Predict Outcome of Patients with Node-Negative Gastric Cancer. PLoS One. 2015;10:e0128540. doi: 10.1371/journal.pone.0128540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shibutani M, Maeda K, Nagahara H, Ohtani H, Iseki Y, Ikeya T, Sugano K, Hirakawa K. The prognostic significance of a postoperative systemic inflammatory response in patients with colorectal cancer. World J Surg Oncol. 2015;13:194. doi: 10.1186/s12957-015-0609-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramsey S, Lamb GW, Aitchison M, Graham J, McMillan DC. Evaluation of an inflammation-based prognostic score in patients with metastatic renal cancer. Cancer. 2007;109:205–212. doi: 10.1002/cncr.22400. [DOI] [PubMed] [Google Scholar]
- 6.Lamb GW, Aitchison M, Ramsey S, Housley SL, McMillan DC. Clinical utility of the Glasgow Prognostic Score in patients undergoing curative nephrectomy for renal clear cell cancer: basis of new prognostic scoring systems. Br J Cancer. 2012;106:279–283. doi: 10.1038/bjc.2011.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lucca I, de Martino M, Hofbauer SL, Zamani N, Shariat SF, Klatte T. Comparison of the prognostic value of pretreatment measurements of systemic inflammatory response in patients undergoing curative resection of clear cell renal cell carcinoma. World J Urol. 2015;33:2045–52. doi: 10.1007/s00345-015-1559-7. [DOI] [PubMed] [Google Scholar]
- 8.Kinoshita A, Onoda H, Imai N, Iwaku A, Oishi M, Tanaka K, Fushiya N, Koike K, Nishino H, Matsushima M. The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann Surg Oncol. 2015;22:803–810. doi: 10.1245/s10434-014-4048-0. [DOI] [PubMed] [Google Scholar]
- 9.Wei XL, Wang FH, Zhang DS, Qiu MZ, Ren C, Jin Y, Zhou YX, Wang DS, He MM, Bai L, Wang F, Luo HY, Li YH, Xu RH. A novel inflammationbased prognostic score in esophageal squamous cell carcinoma: the C-reactive protein/albumin ratio. BMC Cancer. 2015;15:350. doi: 10.1186/s12885-015-1379-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito K, Seguchi K, Shimazaki H, Takahashi E, Tasaki S, Kuroda K, Sato A, Asakuma J, Horiguchi A, Asano T. Tumor necrosis is a strong predictor for recurrence in patients with pathological T1a renal cell carcinoma. Oncol Lett. 2015;9:125–130. doi: 10.3892/ol.2014.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sengupta S, Lohse CM, Leibovich BC, Frank I, Thompson RH, Webster WS, Zincke H, Blute ML, Cheville JC, Kwon ED. Histologic coagulative tumor necrosis as a prognostic indicator of renal cell carcinoma aggressiveness. Cancer. 2005;104:511–520. doi: 10.1002/cncr.21206. [DOI] [PubMed] [Google Scholar]
- 12.Belsante M, Darwish O, Youssef R, Bagrodia A, Kapur P, Sagalowsky AI, Lotan Y, Margulis V. Lymphovascular invasion in clear cell renal cell carcinoma-association with disease-free and cancer-specific survival. Urol Oncol. 2014;32:30, e23–38. doi: 10.1016/j.urolonc.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Katz MD, Serrano MF, Humphrey PA, Grubb RL 3rd, Skolarus TA, Gao F, Kibel AS. The role of lymphovascular space invasion in renal cell carcinoma as a prognostic marker of survival after curative resection. Urol Oncol. 2011;29:738–744. doi: 10.1016/j.urolonc.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 14.Proctor MJ, Morrison DS, Talwar D, Balmer SM, O’Reilly DS, Foulis AK, Horgan PG, McMillan DC. An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: a Glasgow Inflammation Outcome Study. Br J Cancer. 2011;104:726–734. doi: 10.1038/sj.bjc.6606087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 16.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 17.Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13:759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- 18.Pichler M, Hutterer GC, Stoeckigt C, Chromecki TF, Stojakovic T, Golbeck S, Eberhard K, Gerger A, Mannweiler S, Pummer K, Zigeuner R. Validation of the pre-treatment neutrophil-lymphocyte ratio as a prognostic factor in a large European cohort of renal cell carcinoma patients. Br J Cancer. 2013;108:901–907. doi: 10.1038/bjc.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunduz S, Mutlu H, Tural D, Yildiz O, Uysal M, Coskun HS, Bozcuk H. Platelet to lymphocyte ratio as a new prognostic for patients with metastatic renal cell cancer. Asia Pac J Clin Oncol. 2015;11:288–92. doi: 10.1111/ajco.12358. [DOI] [PubMed] [Google Scholar]
- 20.Chang Y, An H, Xu L, Zhu Y, Yang Y, Lin Z, Xu J. Systemic inflammation score predicts postoperative prognosis of patients with clear-cell renal cell carcinoma. Br J Cancer. 2015;113:626–633. doi: 10.1038/bjc.2015.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeon HG, Choi DK, Sung HH, Jeong BC, Seo SI, Jeon SS, Choi HY, Lee HM. Preoperative Prognostic Nutritional Index is a Significant Predictor of Survival in Renal Cell Carcinoma Patients Undergoing Nephrectomy. Ann Surg Oncol. 2016;23:321–7. doi: 10.1245/s10434-015-4614-0. [DOI] [PubMed] [Google Scholar]
- 22.Fairclough E, Cairns E, Hamilton J, Kelly C. Evaluation of a modified early warning system for acute medical admissions and comparison with C-reactive protein/albumin ratio as a predictor of patient outcome. Clin Med (Lond) 2009;9:30–33. doi: 10.7861/clinmedicine.9-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ranzani OT, Zampieri FG, Forte DN, Azevedo LC, Park M. C-reactive protein/albumin ratio predicts 90-day mortality of septic patients. PLoS One. 2013;8:e59321. doi: 10.1371/journal.pone.0059321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saito K, Kihara K. Role of C-reactive protein in urological cancers: a useful biomarker for predicting outcomes. Int J Urol. 2013;20:161–171. doi: 10.1111/j.1442-2042.2012.03121.x. [DOI] [PubMed] [Google Scholar]
- 25.Huang J, Baum Y, Alemozaffar M, Ogan K, Harris W, Kucuk O, Master VA. C-reactive protein in urologic cancers. Mol Aspects Med. 2015;45:28–36. doi: 10.1016/j.mam.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Stenman M, Laurell A, Lindskog M. Prognostic significance of serum albumin in patients with metastatic renal cell carcinoma. Med Oncol. 2014;31:841. doi: 10.1007/s12032-014-0841-7. [DOI] [PubMed] [Google Scholar]


