Abstract
Objective: Fibroblast growth factor-21 (FGF-21) has been discovered as a strong hormone, plays an important role in lipid metabolism, glucose metabolism, associated with several diseases such as obesity, metabolic syndrome, diabetes mellitus, and cardiovascular events; however, no evidence is available concerning the relationship of FGF-21 and atrial fibrosis in patients with atrial fibrillation (AF) and rheumatic heart disease (RHD). Methods: Twenty-four rheumatic heart disease patients were divided into two groups, 12 cases with AF and 12 cases with sinus rhythm (SR). Clinical characteristics and blood samples were collected before surgery; right atrial appendage samples were taken in the surgery of valve replacement. HE staining was performed to determine cross-sectional area of atrial myocytes; Masson stained sections and mRNA levels of cardiac fibrosis biomarkers were used to evaluate the degree of cardiac fibrosis; the level of FGF-21 was evaluated via enzyme-linked immunosorbent assay (ELISA), immunohistochemistry, and real-time polymerase chain reaction (PCR). Results: Compared with SR group, cross-sectional area of atrial myocytes and collagen volume fraction were significantly increased in the atrial tissue of AF group. The distribution of FGF-21 in the AF group was remarkably higher than SR group. In addition, plasma and mRNA levels of FGF-21 in atrial tissue of AF showed the same trend as the result of immunohistochemistry. Using linear correlation analysis, the expression level of FGF-21 was found to be positively related to the degree of atrial fibrosis. Conclusion: FGF-21 might involve in the development and maintenance of atrial fibrosis in atrial fibrillation with rheumatic heart disease, and FGF-21 could be used as a novel biomarker to evaluate myocardial fibrosis in the future.
Keywords: Fibroblast growth factor-21, atrial fibrosis, atrial fibrillation, rheumatic heart disease
Introduction
Atrial fibrillation (AF) is the most common persistent arrhythmia in clinic, which is the common manifestation of cardiovascular diseases including rheumatic heart disease, coronary heart disease, hypertension, cardiomyopathy, congenital heart disease and pericardial diseases [1-3]. The pathogenesis of atrial fibrillation is complex, which is considered the result of the interactions among atrial electrical remodeling, atrial structural remodeling, autonomic nervous system changes and abnormal calcium current, and atrial remodeling is the central link [4,5]. It is showed that atrial fibrillation is associated with progressive atrial structural remodeling and atrial fibrosis is the most prominent feature of atrial structural remodeling. Atrial fibrosis leads to atrial electrical conduction and excitability, which provides an important pathological basis for the occurrence and maintenance of atrial fibrillation [6,7]. It is showed that atrial fibrosis in patients with atrial fibrillation was more serious than that of patients with sinus rhythm. The degree of atrial fibrosis in patients with atrial fibrillation was significantly more than that of isolated atrial fibrillation, and the increase of atrial collagen deposition in patients with isolated atrial fibrillation was significantly increased when compared with patients with sinus rhythm [8]. Therefore, to inhibit atrial fibrosis may be a better way to prevent the development and deterioration of atrial fibrillation. Fibroblast growth factor-21 (FGF-21) is discovered as a new type of cytokine in fibroblast growth factor (FGF) family that regulates glucose and lipid metabolism in the recent [9,10]. It showed that FGF-21 can improve the endothelial function in early stage of atherosclerosis, and prevent the development of coronary heart disease [11]. Clinical studies showed that FGF-21 increased in plasma of patients with coronary heart disease and was related to lipid metabolism and glucose metabolism [12]. Recently, Han et al. demonstrated that serum FGF-21 levels was elevated in AF patients and associated with left atrial diameter, which indicated that FGF-21 may be an independent risk factor for AF [13]. However, whether the levels of plasma and cardiac FGF-21 could reflect the degree of myocardial fibrosis respectively, whether associated with atrial fibrosis as a biological indicator in patients with atrial fibrillation and rheumatic heart disease is still unclear. Therefore, this study aimed to investigate the relationship of FGF-21 and atrial fibrosis in patients with atrial fibrillation and rheumatic heart disease.
Materials and methods
Human right myocardium samples collection and processing
This study was approved by the Ethics Committee of Renmin Hospital of Wuhan University (Wuhan, China) and the samples were obtained according to the regulations of Renmin Hospital of Wuhan University. Twenty-four patients aged from 30 to 70 years with rheumatic heart disease (RHD) who underwent valve replacement were served as subjects in the present study. They were divided into two groups: 12 in the sinus rhythm (SR) group and 12 in the atrial fibrillation (AF) group. Exclusion criteria in the study including: infective endocarditis, hyperthyroidism, serious liver, kidney, lung dysfunction, malignant tumor, coronary atherosclerotic heart disease and chronic pulmonary heart disease. About 200 mg myocardium samples were collected by the surgeon from the right atrium of patients. Each sample was divided into two parts after removed connective tissue and residual blood, one part was quickly put into liquid nitrogen jar for molecular biological detection, the other part was immediately washed with saline solution and fixed with 10% neutral buffered formalin for paraffin section.
Materials
Human FGF-21 Quantikine ELISA Kit (DF2100) was obtained from R&D Systems. Primary antibody against FGF-21 (ab171941) was purchased from Abcam. TRIzol was purchased from Invitrogen Life Technologies (#15596018). Transcriptor First Strand cDNA Synthesis Kit (#04896866001) was purchased from Roche. Power SYBR® Green PCR Master Mix (#4367659) was purchased from Life Technologies.
Hematoxylin-eosin (HE) staining
HE staining was performed to determine cross-sectional area of atrial myocytes. Briefly, myocardium tissues were fixed in 10% neutral buffered formalin for at least 24 hours, and embed in paraffin wax according to embedding machine manufactures instructions. Prepare 8 µm sections on the microtome and place on clean, positively-charged microscope slides. Heat in tissue-drying oven for 1 hour at 60°C. After deparaffinization and rehydration through 100% alcohol, 95% alcohol and 70% alcohol, the slides were washed in distilled water for 5min; stain nuclei with the hematoxylin, rinse the slides in running tap water; and then differentiate with 0.3% acid alcohol, rinse the slides in running tap water; rinse the slides in Scott’s tap water substitute, rinse them in tap water; stain with eosin for 1 min, and then dehydrate, clear and mount.
Masson staining
Evidence of interstitial collagen deposition was visualized using Masson staining, and collagen volume (%) was measured using an image quantitative digital analysis system (Image-Pro Plus 6.0). As mentioned above, after fixed, embed, sectioned, deparaffinization and rehydration, the slides were washed in distilled water, and then re-fixed in Bouin’s solution for 1 hour at 56°C to improve staining quality although this step is not absolutely necessary. Rinse the slides in running tap water for 10 minutes to remove the yellow color. Firstly, stain in Weigert’s iron hematoxylin working solution for 10 minutes; rinse them in running warm tap water for 10 minutes and wash in distilled water. And then stain in Biebrich scarlet-acid fuchsin solution for 10 minutes and wash in distilled water. Differentiate in phosphomolybdic-phosphotungstic acid solution for 10 minutes until collagen is not red. Transfer sections directly to aniline blue solution and stain for 5 minutes; rinse them briefly in distilled water and differentiate in 1% acetic acid solution for 2 minutes, wash in distilled water. Dehydrate very quickly through 95% ethyl alcohol, absolute ethyl alcohol and clear in xylene, lastly mount with resinous mounting medium. Collagen was stained for blue, but muscle and cytoplasm were stained for red.
Immunohistochemistry
Deposition of FGF-21 in myocardium tissues was measured by the method of immunohistochemistry. As mentioned above, after fixed, embed, sectioned, deparaffinization and rehydration, we chose the most common method of antigen retrieval just as heat-mediated retrieval in citrate buffer. And add 100 µl blocking solution for per slide; incubate 30 minutes at room temperature. Drain the blocking solution from slides; apply 100 µl per slide of diluted primary antibody against FGF-21 at recommended concentration of 1:100; incubate overnight at 4°C. After washed with TBST, apply 100 µl per slide of diluted conjugated secondary antibody and incubate for 30 minutes at room temperature. Apply color development by the method of enzyme substrate for 30 minutes, wash slides in TBST and distilled water, and then dehydrate and mount slides. Brown-yellow expression in myocardium tissues meant a positive result.
Enzyme-linked immunosorbent assay (ELISA)
Plasma FGF-21 in this study was detected by the method of enzyme-linked immunosorbent assay (ELISA). Briefly, add 100 μl of each standard and sample into appropriate wells. Cover well and incubate for 2.5 hours at room temperature with gentle shaking. Discard the solution and wash with Wash Solution by using a multi-channel Pipette. After the last wash, remove any remaining Wash Buffer by aspirating. Invert the plate and blot it against clean paper towels. Add 100 μl prepared biotinylated antibody to each well; incubate for 1 hour at room temperature with gentle shaking. Discard the solution and repeat the wash as before. Add 100 μl of prepared streptavidin solution to each well and incubate for 45 minutes at room temperature with gentle shaking. Discard the solution and repeat the wash as before. Add 100 μl of TMB One-Step Substrate Reagent to each well; incubate for 30 minutes at room temperature in the dark with gentle shaking. Lastly, Add 50 μl of Stop Solution to each well and read at 450 nm immediately. For data calculation, calculate the mean absorbance for each set of duplicate standards, controls and samples, and subtract the average zero standard optical density. Plot the standard curve on log-log graph paper, with standard concentration on the x-axis and absorbance on the y-axis. Draw the best-fit straight line through standard points.
Real-time polymerase chain reaction (PCR) analysis
Expression levels of fibrosis biomarkers including TGF-β1, CTGF, Collagen I and III were measured by real-time polymerase chain reaction (PCR) analysis. Total RNA was extracted from the myocardium tissues using TRIzol reagent according to the manufacturer’s instructions and the cDNA was synthesized using oligo (dT) primers with the transcriptor first-strand cDNA synthesis kit. Selected gene differences were confirmed by real-time PCR using SYBR-Green and the results were normalized against GAPDH gene expression. The sequences of all primers used in this study are presented as follows: GAPDH: 5’-GAGTCAACGGATTTGGTCGT-3’ and 5’-TTGATTTTGGAGGGATCTCG-3’; CTGF: 5’-TACCAATGACAACGCCTCCT-3’ and 5’-CCGTCGGTACATACTCCACA-3’; Collagen I: 5’-CCCCAGCCACAAAGAGTCTA-3’ and 5’-TACCTGAGGCCGTTCTGTAC-3’; Collagen III: 5’-GAAGGGCAGGGAACAACTTG-3’ and 5’-TTTGGCATGGTTCTGGCTTC-3’. FGF-21: 5’-CCTTGAAGCCGGGAGTTATT-3’ and 5’-GGCTTCGGACTGGTAAACAT-3’.
Statistical analysis
The data are expressed as the means ± standard deviation (SD). Differences between two groups were performed by an unpaired Student’s t-test using SPSS 19.0 statistical software. Spearman correlation tests were performed to linear correlation analysis between FGF-21 and the degree of atrial fibrosis. A value of P < 0.05 was considered to indicate statistically significant difference.
Results
Larger left atrial diameter (LAD) showed in AF patients with RHD
All subjects were received detailed physical examination, preoperative routine laboratory testing, electrocardiographic including left atrial diameter (LAD), right atrial diameter (RAD) and left ventricular ejection fraction (LVEF) and additional clinical data and so on. General clinical characteristics included name, age, gender, past history, duration time of AF were recorded for all patients. There was no statistically difference in terms of gender, age, classification of cardiac function, LVEF and RAD between SR and AF groups (P > 0.05). However, AF patients had a larger LAD than that of SR group (P < 0.05) (Table 1). These above results indicated that there was expansive LAD in the AF patients with RHD.
Table 1.
Larger left atrial diameter (LAD) showed in AF patients with RHD
| Clinical characteristics | SR (n = 12) | AF (n = 12) |
|---|---|---|
| Gender (m/w) | 6/6 | 6/6 |
| Age (y) | 50.08±7.20 | 49.25±6.84 |
| AF time (m) | -- | 15.75±4.16 |
| NYHA (I/II/III) | 2/7/3 | 2/6/4 |
| LAD (mm) | 34.17±3.64 | 51.25±4.96* |
| RAD (mm) | 37.08±2.50 | 36.92±3.20 |
| LVEF (%) | 57.58±3.37 | 54.75±3.74 |
Note: Gender and grade of heart function were compared by using Fisher exact probability method; m/w = male/female; y = year; m = month; NYHA = New York heart function classification; LAD = left atrial diameter; RAD = right atrial diameter; LVEF = left ventricular ejection fraction;
P < 0.05.
Expansive myocardial cells and accumulated collagen deposited in the AF patients with RHD
To investigate the role of atrial fibrillation in morphology of AF patients with rheumatic heart disease, atrial fibrosis was evaluated by visualizing surface area of the myocardial cells and the total amount of collagen present in the interstitial spaces and determining the collagen volume. As shown in the present study, the arrangement of myocardial cells in SR group was relatively neat, and there was a very small amount of collagen fibers scattered in the SR group; however, myocardial hypertrophy, interstitial collagen fibers arranged disorder, irregular cell morphology coexisted in the AF group. To further detect the severity of atrial fibrosis, we found that when compared with SR group, the mRNA levels of cardiac fibrosis biomarkers CTGF, Collagen I and III were significantly increased in atrial tissues of AF group (P < 0.05) (Figure 1). These above results indicated that there was expansive myocardial cells and more collagen deposited in the myocardial interstitial of AF patients.
Figure 1.
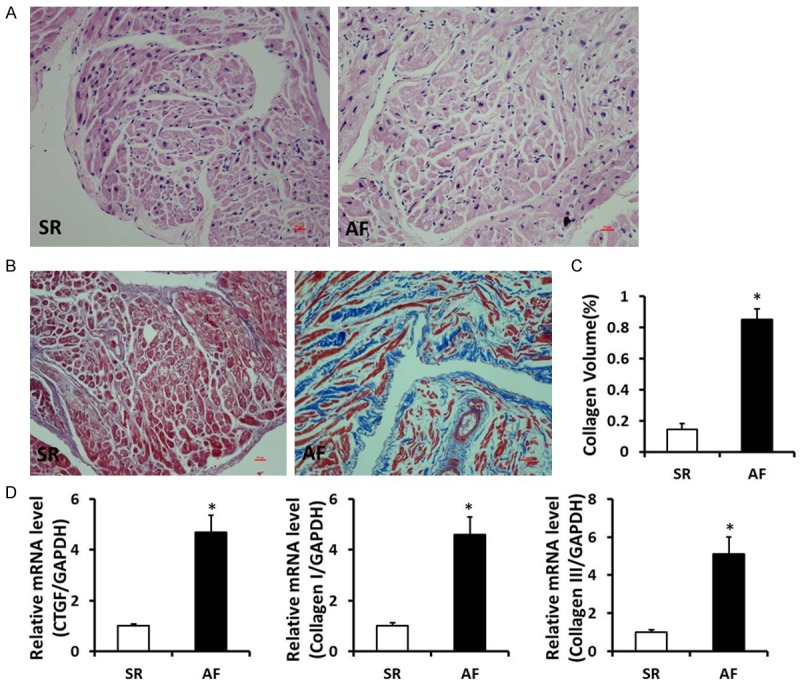
Expansive myocardial cells and accumulated collagen deposited in the myocardial interstitial of AF patients with RHD. A. HE staining in right atrium of SR and AF patients with rheumatic heart disease. B. Masson staining in right atrium of SR and AF patients with rheumatic heart disease (Representative image, collagen was stained for blue). C. Quantification of the total collagen volume in SR and AF patients with rheumatic heart disease. D. The mRNA levels of CTGF, Collagen I and III in the atrial tissues of SR and AF patients with rheumatic heart disease. *P < 0.05 vs. SR group.
Elevated FGF-21 levels in plasma and atrial tissue of AF patients with RHD
To detect the level of FGF-21, we performed the experiments of ELISA, Immunohistochemistry staining and real-time PCR. When compared with SR group, the plasma FGF-21 level was remarkably increased in the AF patients (P < 0.05). Immunohistochemistry staining was performed in tissue sections for accessing the distribution of FGF-21, the results showed that distribution of FGF-21 in atrial tissues of AF group was markedly higher than that of the SR group. Similar to these above data, the mRNA level of FGF-21 in atrial tissues was also significantly higher than that of SR patients (Figure 2). These data demonstrated that the FGF-21 level was elevated in plasma and myocardiac tissues of AF patients, which might involve in the development and maintenance of atrial fibrosis in patients with atrial fibrillation and rheumatic heart disease.
Figure 2.
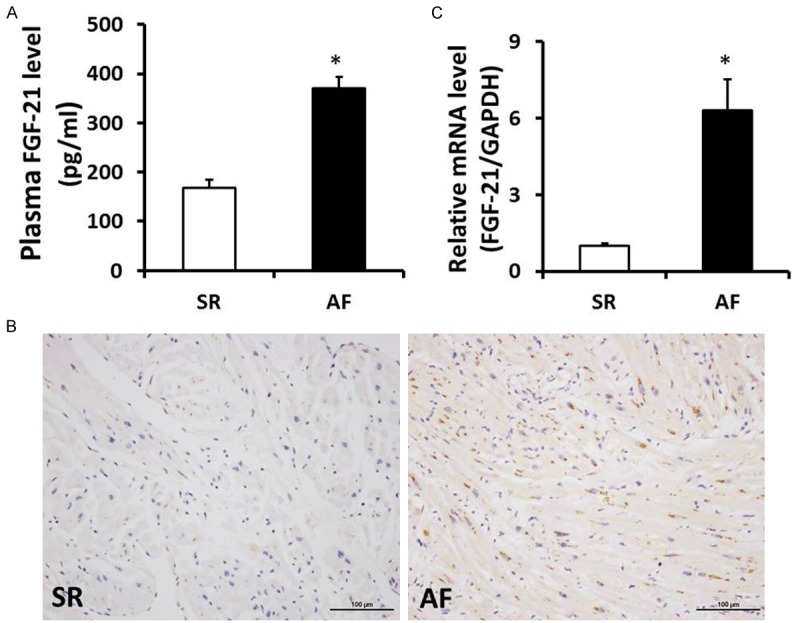
FGF-21 level was elevated in plasma and atrial tissues of AF patients with RHD. A. Level of FGF-21 in plasma was significantly increased in AF patients with rheumatic heart disease. B. Representative immunohistochemistry staining images of FGF-21 in SR and AF patients with rheumatic heart disease. C. The mRNA levels of FGF-21 in the atrial tissues of SR and AF patients with rheumatic heart disease. *P < 0.05 vs. SR group.
FGF-21 positively associated with the degree of atrial fibrosis in AF patients with RHD
To explore the relationship of FGF-21 and atrial fibrosis in AF patients with RHD, we performed the linear correlation analysis between SR and AF groups. We found that the plasma and mRNA level of FGF-21 in atrial tissues were all positively related to the parameters of atrial fibrosis including LAD, collagen volume and fibrosis biomarkers in the AF group (Table 2). Therefore, these results confirmed that the potential relationship between FGF-21 and atrial fibrosis in AF patients, and FGF-21 might be used as a novel biomarker to evaluate myocardial fibrosis for AF patients with rheumatic heart disease in the future.
Table 2.
FGF-21 positively associated with the degree of atrial fibrosis in AF patients with RHD (r, correlation coefficient)
| Plasma FGF-21 | mRNA of FGF-21 | |
|---|---|---|
| Collagen volume | 0.780 | 0.773 |
| CTGF | 0.745 | 0.770 |
| Collagen I | 0.615 | 0.678 |
| Collagen III | 0.594 | 0.846 |
Discussion
Atrial fibrillation is one of the most common malignant arrhythmia in clinical, and the present researches have showed that the pathogenesis of atrial fibrillation is multifactorial [3,14,15]. Clinical and basic studies have confirmed that atrial electrical and structural remodeling, which is the mechanism of development and maintenance of atrial fibrillation, and which is also the characteristic of occurrence and development process of atrial fibrillation [4,15,16]. Within the first few hours, electrophysiological changes occurred rapidly in myocardial tissue of continuous atrial tachycardia patients, while the atrial structural remodeling is a slow process refers to morphology changes in cell or subcellular level, including atrial myocyte hypertrophy, glycogen accumulation in nuclear, sarcomere disappear, structural protein abnormal expression, sarcoplasmic reticulum, changes of mitochondrial morphology, interstitial fibrosis and so on [17,18]. In brief, occurrence and maintenance of atrial fibrillation are closely related to atrial remodeling which includes electrical remodeling and structural remodeling, and atrial fibrosis is the most important part of the structural remodeling [19]. In the present study, we observed that the level of atrial fibrosis remarkably increased via HE staining, Masson staining and fibrosis biomarkers respectively in atrial fibrillation patients with rheumatic heart disease; however, there were only fewer collagen fibers in the sinus rhythm group of patients, which consistent with previous views that atrial structural remodeling were closely related to the generation and maintenance of atrial fibrillation.
Fibroblast growth factor-21 (FGF-21) is a newly discovered factor which is related to glucose metabolism and lipid metabolism [20,21]. Previous studies found that FGF-21 could specifically regulate the uptake of glucose by 3T3-L1 adipocytes and human adipocytes [22]; could lower blood glucose, triglyceride, glucagon and fructose concentration, improve the function of pancreatic beta cells, protect the survival of pancreatic beta cells and improve insulin resistance [21]; could reduce the body weight and body fat content in obese mice induced by high fat diet [23]; could significantly decreased serum lipid levels and reverse hepatic steatosis which might be related to inhibiting nuclear sterol regulatory element binding transcription factor 1 (SREBP-1) and a series of gene expression including some genes involved in fatty acid and triglyceride synthesis [24]. Atrial fibrillation is found to be associated with diabetes, coronary artery disease etc. cardiovascular diseases which are all related to increased FGF-21 level [25]. In addition to this, atrial fibrillation is an obesity associated disease, systemic adiposity, regional adipose tissue deposition, epicardial adipose tissue volume have emerged as potent risk factors for atrial fibrillation. Both human and animal studies suggest that epicardial adipose tissue may play an important role in the pathogenesis of atrial fibrillation [26]. Interestingly, it is found that serum FGF-21 level progressively increases with hepatic fat content; and increased level of serum FGF-21 is associated with non-alcoholic fatty liver disease [27]. These findings all suggest a possible link between FGF-21 and atrial fibrillation. Therefore, FGF-21 as a unique member of the FGF family, which has a number of molecular characteristics that differ from the classical FGF family and show its unique biological function, which is speculated that it may be involved in the occurrence and development of atrial fibrillation. In this study, our result firstly proved that compared with SR group, more FGF-21 diffusely distributed in the myocardium of atrial fibrillation patients, and the plasma level of FGF-21 was also significantly elevated in atrial fibrillation group which is consistent with the result of Han et al., who reported that serum FGF-21 levels were elevated in atrial fibrillation patients and associated with LAD, independent of established risk factors such as C-reactive protein [13]. Most importantly, we deeply found that the plasma and mRNA level of FGF-21 in atrial tissues were all positively related to the degree of atrial fibrosis in the atrial fibrillation patients, which would be expected to become one of the important biomarkers for predicting atrial fibrosis in patients with atrial fibrillation and rheumatic heart disease.
In conclusion, we show that FGF-21 might involve in the development and maintenance of atrial fibrosis in patients with atrial fibrillation and rheumatic heart disease, and FGF-21 could be used as a novel biomarker to evaluate atrial fibrosis in patients with atrial fibrillation and rheumatic heart disease in the future. However, there were still some limits in the present study as follows: 1) need further expand the sample size to increase the intensity of the results; 2) explore the mechanism of elevated FGF-21, and to find a new target for the intervention of atrial fibrosis in patients with atrial fibrillation and rheumatic heart disease.
Acknowledgements
We are grateful to all of the members of the Department of Cardiology and the Cardiovascular Research Institute of Renmin Hospital of Wuhan University for their expert advice. This work was supported by grants from the National Natural Science Foundation of China (No. 81170307) and Specialized Research Fund for the Doctoral Program of Higher Education (No. 20120141110013).
Disclosure of conflict of interest
None.
References
- 1.Shiran A, Sagie A. Tricuspid regurgitation in mitral valve disease incidence, prognostic implications, mechanism, and management. J Am Coll Cardiol. 2009;53:401–408. doi: 10.1016/j.jacc.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 2.Patel NJ, Patel A, Agnihotri K, Pau D, Patel S, Thakkar B, Nalluri N, Asti D, Kanotra R, Kadavath S, Arora S, Patel N, Patel A, Sheikh A, Patel N, Badheka AO, Deshmukh A, Paydak H, Viles-Gonzalez J. Prognostic impact of atrial fibrillation on clinical outcomes of acute coronary syndromes, heart failure and chronic kidney disease. World J Cardiol. 2015;7:397–403. doi: 10.4330/wjc.v7.i7.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirsh BJ, Copeland-Halperin RS, Halperin JL. Fibrotic atrial cardiomyopathy, atrial fibrillation, and thromboembolism: mechanistic links and clinical inferences. J Am Coll Cardiol. 2015;65:2239–2251. doi: 10.1016/j.jacc.2015.03.557. [DOI] [PubMed] [Google Scholar]
- 4.Poudel P, Xu Y, Cui Z, Sharma D, Tian B, Paudel S. Atrial fibrillation: recent advances in understanding the role of microRNAs in atrial remodeling with an electrophysiological overview. Cardiology. 2015;131:58–67. doi: 10.1159/000375403. [DOI] [PubMed] [Google Scholar]
- 5.Bunch TJ, Day JD. Adverse Remodeling of the Left Atrium in Patients with Atrial Fibrillation: When Is the Tipping Point in Which Structural Changes Become Permanent? J Cardiovasc Electrophysiol. 2015;26:606–607. doi: 10.1111/jce.12678. [DOI] [PubMed] [Google Scholar]
- 6.Wu H, Xie J, Li GN, Chen QH, Li R, Zhang XL, Kang LN, Xu B. Possible involvement of TGF-beta/periostin in fibrosis of right atrial appendages in patients with atrial fibrillation. Int J Clin Exp Pathol. 2015;8:6859–6869. [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L, Huang B, Scherlag BJ, Ritchey JW, Embi AA, Hu J, Hou Y, Po SS. Structural changes in the progression of atrial fibrillation: potential role of glycogen and fibrosis as perpetuating factors. Int J Clin Exp Pathol. 2015;8:1712–1718. [PMC free article] [PubMed] [Google Scholar]
- 8.Boldt A, Wetzel U, Lauschke J, Weigl J, Gummert J, Hindricks G, Kottkamp H, Dhein S. Fibrosis in left atrial tissue of patients with atrial fibrillation with and without underlying mitral valve disease. Heart. 2004;90:400–405. doi: 10.1136/hrt.2003.015347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Habegger KM, Stemmer K, Cheng C, Muller TD, Heppner KM, Ottaway N, Holland J, Hembree JL, Smiley D, Gelfanov V, Krishna R, Arafat AM, Konkar A, Belli S, Kapps M, Woods SC, Hofmann SM, D’Alessio D, Pfluger PT, Perez-Tilve D, Seeley RJ, Konishi M, Itoh N, Kharitonenkov A, Spranger J, DiMarchi RD, Tschop MH. Fibroblast growth factor 21 mediates specific glucagon actions. Diabetes. 2013;62:1453–1463. doi: 10.2337/db12-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angelin B, Larsson TE, Rudling M. Circulating fibroblast growth factors as metabolic regulators--a critical appraisal. Cell Metab. 2012;16:693–705. doi: 10.1016/j.cmet.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Zhu W, Wang C, Liu L, Li Y, Li X, Cai J, Wang H. Effects of fibroblast growth factor 21 on cell damage in vitro and atherosclerosis in vivo. Can J Physiol Pharmacol. 2014;92:927–935. doi: 10.1139/cjpp-2014-0227. [DOI] [PubMed] [Google Scholar]
- 12.Shen Y, Ma X, Zhou J, Pan X, Hao Y, Zhou M, Lu Z, Gao M, Bao Y, Jia W. Additive relationship between serum fibroblast growth factor 21 level and coronary artery disease. Cardiovasc Diabetol. 2013;12:124. doi: 10.1186/1475-2840-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han X, Chen C, Cheng G, Xie C, Yang M, Shou X, Sun C. Serum fibroblast growth factor 21 levels are increased in atrial fibrillation patients. Cytokine. 2015;73:176–180. doi: 10.1016/j.cyto.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 14.Dzeshka MS, Lip GY, Snezhitskiy V, Shantsila E. Cardiac fibrosis in patients with atrial fibrillation: mechanisms and clinical implications. J Am Coll Cardiol. 2015;66:943–959. doi: 10.1016/j.jacc.2015.06.1313. [DOI] [PubMed] [Google Scholar]
- 15.Akoum N, Marrouche N. Assessment and impact of cardiac fibrosis on atrial fibrillation. Curr Cardiol Rep. 2014;16:518. doi: 10.1007/s11886-014-0518-z. [DOI] [PubMed] [Google Scholar]
- 16.Voigt N, Heijman J, Wang Q, Chiang DY, Li N, Karck M, Wehrens XH, Nattel S, Dobrev D. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation. 2014;129:145–156. doi: 10.1161/CIRCULATIONAHA.113.006641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014;114:1453–1468. doi: 10.1161/CIRCRESAHA.114.303211. [DOI] [PubMed] [Google Scholar]
- 18.Kallergis EM, Goudis CA, Vardas PE. Atrial fibrillation: a progressive atrial myopathy or a distinct disease? Int J Cardiol. 2014;171:126–133. doi: 10.1016/j.ijcard.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi N, Kume O, Wakisaka O, Fukunaga N, Teshima Y, Hara M, Saikawa T. Novel strategy to prevent atrial fibrosis and fibrillation. Circ J. 2012;76:2318–2326. doi: 10.1253/circj.cj-12-1099. [DOI] [PubMed] [Google Scholar]
- 20.Ohta H, Konishi M, Itoh N. FGF10 and FGF21 as regulators in adipocyte development and metabolism. Endocr Metab Immune Disord Drug Targets. 2011;11:302–309. doi: 10.2174/187153011797881166. [DOI] [PubMed] [Google Scholar]
- 21.Cuevas-Ramos D, Aguilar-Salinas CA, Gomez-Perez FJ. Metabolic actions of fibroblast growth factor 21. Curr Opin Pediatr. 2012;24:523–529. doi: 10.1097/MOP.0b013e3283557d22. [DOI] [PubMed] [Google Scholar]
- 22.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim KH, Lee MS. FGF21 as a Stress Hormone: The Roles of FGF21 in Stress Adaptation and the Treatment of Metabolic Diseases. Diabetes Metab J. 2014;38:245–251. doi: 10.4093/dmj.2014.38.4.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Xu Y, Hu Y, Wang G. The role of fibroblast growth factor 21 in the pathogenesis of non-alcoholic fatty liver disease and implications for therapy. Metabolism. 2015;64:380–390. doi: 10.1016/j.metabol.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Al-Rawahi M, Proietti R, Thanassoulis G. Pericardial fat and atrial fibrillation: Epidemiology, mechanisms and interventions. Int J Cardiol. 2015;195:98–103. doi: 10.1016/j.ijcard.2015.05.129. [DOI] [PubMed] [Google Scholar]
- 26.Hatem SN, Sanders P. Epicardial adipose tissue and atrial fibrillation. Cardiovasc Res. 2014;102:205–213. doi: 10.1093/cvr/cvu045. [DOI] [PubMed] [Google Scholar]
- 27.Liu WY, Huang S, Shi KQ, Zhao CC, Chen LL, Braddock M, Chen YP, Feng WK, Zheng MH. The role of fibroblast growth factor 21 in the pathogenesis of liver disease: a novel predictor and therapeutic target. Expert Opin Ther Targets. 2014;18:1305–1313. doi: 10.1517/14728222.2014.944898. [DOI] [PubMed] [Google Scholar]


