Abstract
Objective: In this study, we screened the different human osteosarcoma cell line MG-63 miRNAs after the treatment of curcumin and explored the effects of curcumin on MG-63 cells and its mechanism. Methods: Affemitrix miRNA chip was used to detect the changes of miRNA expression profile in MG-63 cells before and after curcumin treatment, and screen different expression of miRNAs. The target gene of miRNA was analyzed by bioinformatics. The expression levels of miRNA-138 target genes Smad4, NFκB p65 and cyclin D3 were detected. MTT and Transwell Cell invasion assays were used to observe the effects of curcumin on MG-63 cells. Results: Curcumin could significantly inhibit the proliferation of MG-63 cells and the expression levels of miRNA-138 target genes Smad4, NFκB p65 and cyclin D3 in MG-63 cells (P<0.05); overexpression of hsa-miR-138 down-regulated the expression levels of Smad4, NFκB p65 and cyclin D3 compared with the treatment of curcumin, while inhibition of hsa-miR-138 up-regulated the expression levels of Smad4, NFκB p65 and cyclin D3. Conclusions: Curcumin could increase the expression of hsa-miR-138, hsa-miR-138 inhibited cell proliferation and invasive ability by inhibition of its target genes.
Keywords: Curcumin, miRNAs, miR-138, MG-63 cells, proliferation, invasion
Introduction
Curcumin is the main turmeric compounds in the spice of turmeric. It is a kind of lipid soluble phenolic pigment which is extracted from the roots of the plant Curcuma longa Linn. Curcumin has many pharmacological activities such as anti-inflammatory, anti-oxidation, lowering blood fat, anti-tumor and so on. It can inhibit the growth of a variety of tumor cells and induce apoptosis so as to exert its anti-tumor activity. Previous studies showed that curcumin induced the apoptosis of tumor cells in vivo and in vitro, such as lung cancer, colon cancer, breast cancer, pancreatic cancer, ovarian cancer and leukemia [1-3]. The drug development and molecular mechanism of anti-tumor researches based on curcumin had become a hot spot in the research of natural anti-tumor drugs.
Osteosarcoma is a malignant tumor originating from the tissues of the leaves, its incidence accounts for about 35% of the primary tumor, it was the most common primary malignant bone tumor in children and adolescents [4]. The prognosis of patients with osteosarcoma is very poor because of the high degree of malignancy and the ability of invasion and metastasis [5].
MicroRNAs (miRNAs) is a kind of non-coding RNA which is about 17-25 nucleotides in length, it participates in many life processes such as cellular differentiation, proliferation, apoptosis and tumor development. Although its sequence only accounted for about 1% of the human genome, it participates in the regulation of about 30% gene expression [6,7]. Studies showed that miRNAs may be involved in the regulation of cell proliferation and differentiation by regulating the expression of target genes. So the expression profile of miRNAs could be a marker for early diagnosis and prognosis of cancer. Many abnormal expressions of miRNAs were found in osteosarcoma through miRNA expression profile recently [8-11].
In this study, we analyzed the effects of curcumin on miRNAs expression profile in human osteosarcoma cell line MG-63 and explored its mechanism.
Materials and methods
Cell culture and transfection
Human osteosarcoma cell line MG-63 was cultured with Eagle’s Minimum Essential Medium (ATCC-30-2003) containing 10% fetal calf serum at 37°C with 5% CO2. Transfection of miRNA mimic/inhibitor was performed using Amaxa Nucleofactor according to the manual. The foreign miRNA mimic/inhibitor was imported into the nucleus directly.
Detection of miRNA chip
The treated cells were harvested and counted, they were digested and miRNAs were isolated using miRNA isolation kit (mirVana, AM1561) according to the manual. The concentration and purity of miRNAs were detected with Qubit fluorometer. RNA was labeled Hy3 using Cancer MicroRNA Array kit (Signosis, AP-0003) according to the manual and hybridized in miRCUPYTM LNA chips. Microarray images were scanned using GeneChipR Scanner 3000 and analyzed using miRNA QC Tool software.
RNA extraction and real-time PCR
The harvested cells were washed with RNase free PBS. Total RNA and miRNAs were extracted using miRNA isolation kit and RNeasy Mini Kit (Qiagen) respectively according to the manufacturer’s protocol. Their concentration and purity were detected with Qubit fluorometer. 1 μg RNA was subjected to reverse transcription using reverse transcription kit (Promega). Real-time PCR were performed using SYNBR Green PCR Master Mix (Qiagen). At the end of each reaction, a melting curve analysis was performed to confirm the absence of primer dimmers. The primers used in this study were shown in Table 1, the synthetic system of PCR were shown in Table 2. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was used as an internal control for normalization of RNA quantity and quality differences in all samples. Quantifications of target genes mRNA was performed using the 2-ΔΔCt method.
Table 1.
Primers used in real-time PCR
| Gene | Accesion NO. | Primer (5’-3’) |
|---|---|---|
| Smad4 | NM_005359 | F: CTCATGTGATCTATGCCCGTC |
| R: AGGTGATACAACTCGTTCGTAGT | ||
| NFκB p65 | NM_001145138 | F: ATGTGGAGATCATTGAGCAGC |
| R: CCTGGTCCTGTGTAGCCATT | ||
| Cyclin D3 | NM_001136017 | F: TACCCGCCATCCATGATCG |
| R: AGGCAGTCCACTTCAGTGC | ||
| GAPDH | NM_002046 | F: GAAGGTGAAGGTCGGAGTC |
| R: GAAGATGGTGATGGGATTTC |
F: forward; R: reverse.
Table 2.
Synthetic system of PCR
| Components | Volume per reaction |
|---|---|
| SYBR Green Super mix | 5 μl |
| Forward primer (10 μM) | 0.3-0.55 μl (270-450 nM)2 |
| Reverse primer (10 μM) | 0.3-0.55 μl (270-450 nM) |
| cDNA template | 75-100 ng |
| Nuclease-free water | Up to 10 μl |
MTT assay
The cells were treated with 0 μM, 10 μM, 20 μM and 40 μM curcumin respectively, the tested cells were seeded at density 5000 cells/well in 24-well plates and measured in 1, 2 and 3 days after culture. Before measured the cells were added 20 μl MTT (5 mg/ml) and the cells were incubated for an additional 4 h at 37°C. The culture medium was removed, 100 ml of DMSO were added to each well. With shaking at low speed for 10 min, the MTT solution was aspirated and optical densities (OD) of the supernatant were read at 492 nm using a Microplate Reader (Thermolex, Molecular Device Co). The experiments were repeated three times and the negative control was conducted using only cell-free culture medium (means ± SD).
Cell invasion
Matrigel was melt at 4°C overnight and diluted with pre-cooling Eagle’s Minimum Essential medium to final concentration of 1 mg/ml. The 100 μl of 1 mg/ml Matrigel was added into the center of the upper chamber bottom of Transwell and incubate at 37°C to gelatine. 200 μl Eagle’s Minimum Essential medium was added to each well for gel reconstruction. The cells were detached, suspended, counted and cultured in upper chamber of Transwell, the lower chambers of Transwell were filled with medium with 10% FBS and incubated at 37°C. The liquid in upper chambers was discarded and the upper chambers were taken out. The cells not passing through the membrane were wiped off with cotton swab. The chambers were fixed with 4% paraformaldehyde at room temperature for 10 min and stained with 0.1% crystal violet. The cells were observed and counted under high magnification microscopy.
Protein extraction and western blotting determination
The cells were lysed with RIPA lysis buffer and total proteins of cells were extracted and analyzed with SDS-PAGE electrophoresis. Then it was electrotransferred to the PVDF membrane. After the transmembrane, PVDF membrane was rinsed with TBS for 10 to 15 min, placed in TBS/T blocking buffer containing 5% (w/v) skimmed milk powder and shaked at room temperature for one hour. It was incubated at room temperature for two hours after added with appropriate dilution degree of primary antibody (diluted with TBST containing 1% (w/v) skimmed milk powder). Then the membrane was rinsed with TBST for three times (5 to 10 minutes one time). The membrane was incubated at room temperature for one hour with HRP labeled secondary antibody (1:10000) diluted with TBST containing 0.05% (w/v) skimmed milk powder and rinsed for three times with TBST (5 to 10 minutes at a time). The protein bands were scanned and quantified as a ratio to GAPDH.
Statistical analysis
The results are expressed as mean ± S.D. and analyzed with SPSS 11.5 software; t-test was used to evaluate the differences between groups. A value of P<0.05 and P<0.01 was taken to denote statistical significance.
Results
Effects of curcumin on the growth of MG-63 cells
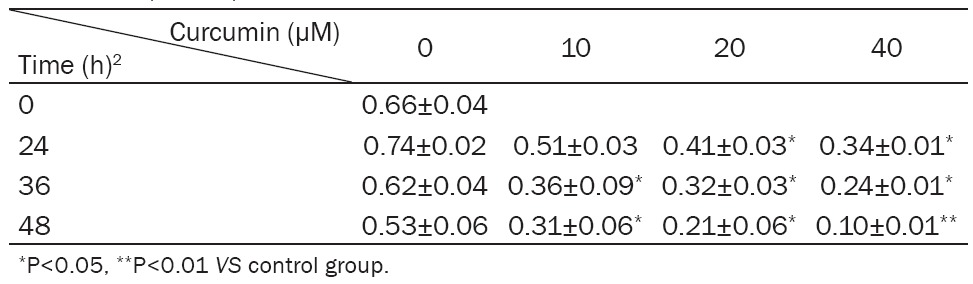
The proliferation of MG-63 cells treated by 0 μM, 10 μM, 20 μM and 40 μM curcumin was detected by MTT method after treatment for 24 h, 36 h and 48 h respectively. The results were shown in Table 3. We found that curcumin could significantly inhibit the proliferation of MG-63 cells (P<0.05).
Table 3.
The 440 nm OD of MG-63 treated with different concentration of curcumin (x̅±SD)
|
Effects of curcumin on the miRNAs expression profile in MG-63 cells
The effects of curcumin on the miRNAs expression profile in MG-63 cells were detected by miRNA chips. More than 1.5 times of differentially expressed miRNAs after the treatment of 20 μM curcumin for 24 h were shown in Table 4. The larger changed miRNAs were selected to confirm the chip results. Real-time PCR results of hsa-miR-138 and hsa-miR-186 were shown in Figure 1. It showed that hsa-miR-138 significantly increased after the treatment of 20 μM curcumin for 24 h while hsa-miR-186 significantly decreased after the treatment of 20 μM curcumin for 24 h.
Table 4.
Curcumin treatment induced differential expressed miRNAs (24 h)
| ProbeSet_Name | Fold_change (B1_vs_A1) | Regulation (B1_vs_A1) | median CV (%) | label |
|---|---|---|---|---|
| hsa-miR-149 | 2.4357 | Up | 5.32 | Cy3 |
| hsa-miR-138 | 2.6614 | Up | 6.12 | Cy3 |
| hsa-miR-181b | 1.8996 | Up | 6.34 | Cy3 |
| hsa-miR-193b | 2.0147 | Up | 6.58 | Cy3 |
| hsa-miR-339-5p | 2.1366 | Up | 7.11 | Cy3 |
| hsa-miR-22 | 1.9654 | Up | 6.04 | Cy3 |
| hsa-miR-671-5p | 1.7452 | Up | 6.23 | Cy3 |
| hsa-miR-124 | 1.8639 | Up | 5.99 | Cy3 |
| hsa-miR-744 | 2.1660 | Up | 6.10 | Cy3 |
| hsa-miR-455-3p | 1.8254 | Up | 6.38 | Cy3 |
| hsa-miR-494 | 1.6332 | Down | 7.46 | Cy3 |
| hsa-miR-186 | 2.4365 | Down | 8.20 | Cy3 |
| hsa-miR-100 | 2.3334 | Down | 6.09 | Cy3 |
| hsa-miR-154 | 1.7562 | Down | 7.13 | Cy3 |
A1: DMSO control group, B1: 20 μM Curcumin group.
Figure 1.
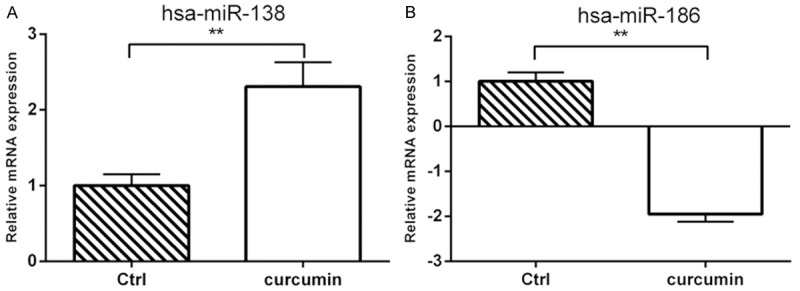
Effects of curcumin on the expression of hsa-miR-138 and hsa-miR-186 in MG-63 cells. A: hsa-miR-138; B: hsa-miR-186. **P<0.01.
Bioinformatics analysis of miRNA target genes
miRNA target gene prediction software of PicTar and TargetScan were used to predict the miRNA target genes. Some predicted target genes in the intersection of these two softwares were shown in Table 5. Cell differentiation and proliferation related genes Smad4, NFκB p65 (RELA) and cyclin D3 were selected from these genes.
Table 5.
Prediction of miRNA targets
| MicroRNA | Gene | RefseqID | Seed Length | Start | Sequence | End | Region | P value |
|---|---|---|---|---|---|---|---|---|
| hsa-miR-138 | LIPH | NM_139248 | 11 | 2076 | AGCUGGUGUUG | 2066 | 3 UTR | 0.0002 |
| PDE3A | NM_000921 | 11 | 3663 | AGCUGGUGUUG | 3653 | 3 UTR | 0.0002 | |
| KIAA0494 | NM_014774 | 11 | 5476 | AGCUGGUGUUG | 5466 | 3 UTR | 0.0008 | |
| CCND3 | NM_001136017 | 11 | 3305 | AGCUGGUGUUG | 3295 | 3 UTR | 0.0006 | |
| TSR1 | NM_018128 | 11 | 3863 | AGCUGGUGUUG | 3853 | 3 UTR | 0.0004 | |
| CEBPG | NM_001806 | 11 | 2542 | AGCUGGUGUUG | 2532 | 3 UTR | 0.0007 | |
| SMAD4 | NM_005359 | 11 | 1611 | AGCUGGUGUUG | 1601 | 3 UTR | 0.0004 | |
| MEOX1 | NM_004527 | 11 | 2054 | AGCUGGUGUUG | 2044 | 3 UTR | 0.0004 | |
| YBX2 | NM_015982 | 10 | 1278 | AGCUGGUGUU | 1269 | 3 UTR | 0.0004 | |
| DGKG | NM_001346 | 10 | 2934 | AGCUGGUGUU | 2925 | 3 UTR | 0.0028 | |
| RELA | NM_001145138 | 10 | 1443 | AGCUGGUGUU | 1434 | 3 UTR | 0.0014 | |
| ZAK | NM_133646 | 10 | 6184 | AGCUGGUGUU | 6175 | 3 UTR | 0.0054 |
Effects of curcumin on the expression of miRNA138 and its target genes
Western blotting results showed that curcumin significantly inhibit the expression levels of Smad4, NFκB p65 and cyclin D3 in MG-63 cells. RT-PCR results showed that compared with curcumin group, overexpression of hsa-miR-138 could down-regulate the expression levels of Smad4, NFκB p65 and cyclin D3 in MG-63 cells, while hsa-miR-138 inhibitor could up-regulate the expression levels of Smad4, NFκB p65 and cyclin D3 in MG-63 cells (Figure 2).
Figure 2.
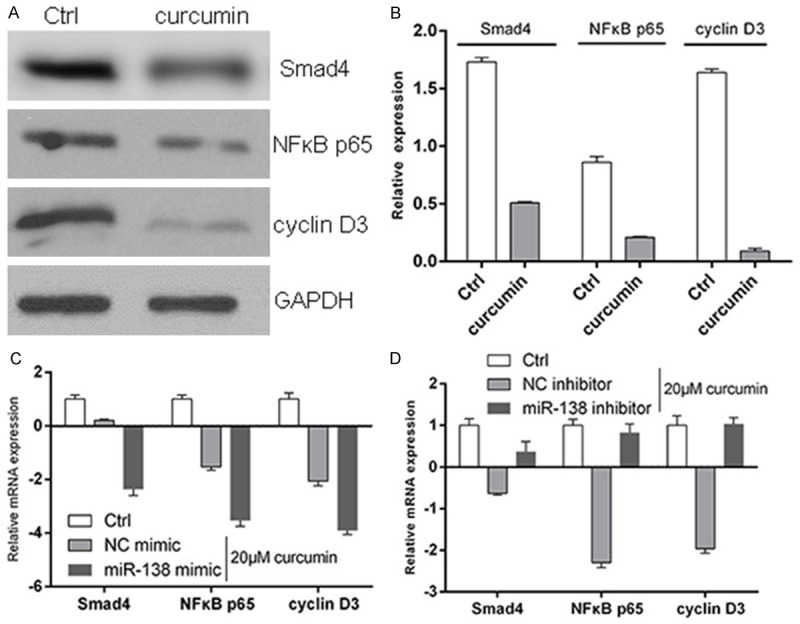
Effects of curcumin on the expression of miRNA138 and its target genes. A, B: Western blotting results showed that curcumin significantly inhibit the expression levels of Smad4, NFκB p65 and cyclin D3 in MG-63 cells; C: miR-138 mimic could down-regulate the expression levels of Smad4, NFκB p65 and cyclin D3 in MG-63 cells; D: miR-138 inhibitor could up-regulate the expression levels of Smad4, NFκB p65 and cyclin D3 in MG-63 cells.
Effects of curcumin on the invasion ability of MG-63 cells
The results of cell invasion experiment were shown in Table 6. It showed that cell numbers across the membrane decreased after the treatment of curcumin. The invasion ability of MG-63 cells decreased after the transfection of miR-138 mimic, while the invasion ability enhanced after the transfection of miR-138 inhibitor (P<0.05).
Table 6.
Results of cell invasion Experiment
| miRNAs mimic | miRNAs inhibitor | ||||
|---|---|---|---|---|---|
|
|
|||||
| Blank Control | 20 μM curcumin | ||||
|
|
|||||
| Control | miR-138 | Control | miR-138 | ||
| V1a | 172b | 122 | 96 | 134 | 186 |
| V2 | 178 | 137 | 99 | 120 | 193 |
| V3 | 194 | 129 | 108 | 117 | 182 |
| 181.33±11.37c | 129.33±7.51 | 101.00±6.24** | 123.67±9.07 | 187.00±5.57** | |
Field of vision;
Cell numbers through the basement membrane;
Mean ± SD.
Compared with control;
P<0.01.
Discussion
miRNAs has a variety of biological functions and its mechanism is also very complex. miRNAs and its target genes or other miRNAs combine to form a complex regulatory network to involve in the life processes, especially in the development of tumors [12-14]. A variety of abnormal expression of miRNAs was found in the osteosarcoma [8-11]. The application of miRNA chip makes it possible for large-scale screening of miRNA profile. The target genes of miRNA can be predicted by bioinformatics method after the abnormal expression of miRNAs was detected [15]. There are six miRNA target gene prediction softwares (miRanda; TargetScan; RNAhybrid; PicTar; RNA22; MicroInspector) used widely. The intersection of PicTar and TargetScan predicted well for the miRNA function site in 3’UTR region [16].
In this study, we screened the abnormal expression of miRNAs in MG-63 cells after the treatment of curcumin and explored the effects of curcumin on MG-63 cells and its mechanism using Affemitrix miRNA chip and bioinformatics analysis. We found that hsa-miR-138, hsa-miR-149, hsa-miR-181b, hsa-miR-193b, hsa-miR-339-5p, hsa-miR-671-5p, hsa-miR-22 and hsa-miR-124 up-regulated and hsa-miR-494, hsa-miR-186, hsa-miR-100 and hsa-miR-154 down-regulated after the treatment of curcumin. Smad4, NFκB p65 and cyclin D3 were predicted as the target genes of hsa-miR-138 by bioinformatics analysis. Smad4 is an intermediate in the TGF-beta signal pathway and abnormally expressed in many tumors, it could enhance the migration ability of tumor cells [17,18]. P65 is a member of the NF-κB transcription factor protein family. When cells are stimulated by extracellular signals, the activation IκB kinase (IKK) makes the phosphorylation of IκB and the nuclear localization site of NF-κB exposed. Free NF-κB shifted to the nucleus and combined with specific κB sequences to induce the transcription of related genes [19]. Cyclin D3 is an important regulatory factor from G1 to S phase of cell cycle, studies showed that it was the adverse prognostic factor of diffuse large B-cell lymphoma (DLBCL) [20]. We detected the expression levels of Smad4, NFκB p65 and cyclin D3 with western blotting method and found that they down-regulated after the treatment of curcumin. Transient transfection of hsa-miR-138 mimic and hsa-miR-138 inhibitor to change the expression of hsa-miR-138 showed that overexpression of hsa-miR-138 down-regulated the expression levels of Smad4, NFκB p65 and cyclin D3 compared with the treatment of curcumin, while inhibition of hsa-miR-138 up-regulated the expression levels of Smad4, NFκB p65 and cyclin D3. These suggested that curcumin could increase the expression of hsa-miR-138, hsa-miR-138 inhibited cell proliferation and invasive ability by inhibition of its target genes. This conclusion was also confirmed by Transwell cell invasion assay.
In a word, curcumin could increase the expression of hsa-miR-138, hsa-miR-138 inhibited cell proliferation and invasive ability by inhibition of its target genes.
Acknowledgements
The research was supported by the General Financial Grant from the China Postdoctoral Science Foundation (2014M562659).
Disclosure of conflict of interest
None.
References
- 1.Mudduluru G, George-William JN, Muppala S, Asangani IA, Kumarswamy R, Nelson LD, Allgayer H. Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer. Biosci Rep. 2011;31:185–197. doi: 10.1042/BSR20100065. [DOI] [PubMed] [Google Scholar]
- 2.Zhu GH, Dai HP, Shen Q, Ji O, Zhang Q, Zhai YL. Curcumin induces apoptosis and suppresses invasion through MAPK and MMP signaling in human monocytic leukemia SHI-1 cells. Pharm Biol. 2015;1:1–9. doi: 10.3109/13880209.2015.1060508. [DOI] [PubMed] [Google Scholar]
- 3.Ting CY, Wang HE, Yu CC, Liu HC, Liu YC, Chiang IT. Curcumin Triggers DNA Damage and Inhibits Expression of DNA Repair Proteins in Human Lung Cancer Cells. Anticancer Res. 2015;35:3867–3873. [PubMed] [Google Scholar]
- 4.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 5.Lafzi A, Vahabi S, Ghods S, Torshabi M. In vitro effect of mineralized and demineralized bone allografts on proliferation and differentiation of MG-63 osteoblast-like cells. Cell Tissue Bank. 2015 doi: 10.1007/s10561-015-9516-7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Chen B, Li H, Zeng X, Yang P, Liu X, Zhao X, Liang S. Roles of miRNA on cancer cell metabolism. J Transl Med. 2012;10:228. doi: 10.1186/1479-5876-10-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu L, Liu J, Cheng G. Role of miRNAs in schistosomes and schistosomiasis. Front Cell Infect Microbiol. 2014;4:165. doi: 10.3389/fcimb.2014.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maire G, Martin JW, Yoshimoto M, Chilton-MacNeill S, Zielenska M, Squire JA. Analysis of miRNA-gene expression-genomic profiles reveals complex mechanisms of microRNA deregulation in osteosarcoma. Cancer Genet. 2011;204:138–146. doi: 10.1016/j.cancergen.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Xu M, Jin H, Xu CX, Sun B, Mao Z, Bi WZ, Wang Y. Analysis of miRNA-gene expressiongenomic profiles reveals complex mechanisms of microRNA deregulation in osteosarcoma. Cancer Genet. 2011;204:138–146. doi: 10.1016/j.cancergen.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Fang Y, Zhang Z, Wang Q, Zhao J. Expression and clinical significance of cyclooxygenase-2 and microRNA-143 in osteosarcoma. Exp Ther Med. 2015;9:2374–2378. doi: 10.3892/etm.2015.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang J, Shi Y, Li H, Yang M, Liu G. MicroRNA-144 acts as a tumor suppressor by targeting Rho-associated coiled-coil containing protein kinase 1 in osteosarcoma cells. Mol Med Rep. 2015;12:4554–9. doi: 10.3892/mmr.2015.3937. [DOI] [PubMed] [Google Scholar]
- 12.Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–4. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagner A, Mayr C, Bach D, Illig R, Plaetzer K, Berr F, Pichler M, Neureiter D, Kiesslich T. MicroRNAs Associated with the Efficacy of Photodynamic Therapy in Biliary Tract Cancer Cell Lines. Int J Mol Sci. 2014;15:20134–20157. doi: 10.3390/ijms151120134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu L, Chen L, Xu Y, Li R, Du X. microRNA-195 promotes apoptosis and suppresses tumorigenicity of human colorectal cancer cells. Biochem Biophys Res Commun. 2010;400:236–240. doi: 10.1016/j.bbrc.2010.08.046. [DOI] [PubMed] [Google Scholar]
- 15.Wu D, Gantier MP. Normalization of Affymetrix miRNA Microarrays for the Analysis of Cancer Samples. Methods Mol Biol. 2015 doi: 10.1007/7651_2015_239. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Sethupathy P, Megraw M, Hatzigeorgiou AG. A guide through present computational approaches for the identification of mammalian microRNA targets. Nat Methods. 2006;3:881–886. doi: 10.1038/nmeth954. [DOI] [PubMed] [Google Scholar]
- 17.Lim J, Tu X, Choi K, Akiyama H, Mishina Y, Long F. BMP-Smad4 signaling is required for precartilaginous mesenchymal condensation independent of Sox9 in the mouse. Dev Biol. 2015;400:132–138. doi: 10.1016/j.ydbio.2015.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia X, Wu W, Huang C, Cen G, Jiang T, Cao J, Huang K, Qiu Z. SMAD4 and its role in pancreatic cancer. Tumour Biol. 2015;36:111–119. doi: 10.1007/s13277-014-2883-z. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Diao J, Colbert KN, Lai Y, Pfuetzner RA, Padolina MS, Vivona S, Ressl S, Cipriano DJ, Choi UB, Shah N, Weis WI, Brunger AT. Munc18a does not alter fusion rates mediated by neuronal SNAREs, synaptotagmin, and complexin. J Biol Chem. 2015;290:10518–10534. doi: 10.1074/jbc.M114.630772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goda AE, Erikson RL, Ahn JS, Kim BY. Induction of G1 Arrest by SB265610 Involves Cyclin D3 Down-regulation and Suppression of CDK2 (Thr160) Phosphorylation. Anticancer Res. 2015;35:3235–3243. [PubMed] [Google Scholar]


