Abstract
Activation of human telomere reverse transcriptase (hTERT) is associated with the tumorigenic role of Akt. We aimed to evaluate the significance of Akt phosphorylation and hTERT expression on the prognosis of epithelial ovarian cancer (EOC). Between 2005 and 2012, 92 EOC patients treated at the Seoul National University Hospital were included in this study. Paraffin-embedded sections from the tumors of EOC patients were stained with anti-hTERT and anti-phosphorylated Akt (pAkt) antibodies. Correlations of pAkt and hTERT expression with the clinicopathological factors were analyzed using SPSS software, version 17.0 (IBM, Chicago, IL, USA). In 92 EOC tissues, pAkt and hTERT were not associated with any clinicopathological factors. However, the disease-free survival rate of patients exhibiting coexpression of pAkt and hTERT was poor compared with that of the other patients (P = 0.013). Coexpression of pAkt and hTERT is a poor prognostic marker for EOC.
Keywords: Ovarian neoplasm, AKT, hTERT, prognosis
Introduction
Epithelial ovarian cancer (EOC) is the malignancy with the highest mortality rate in women. Despite the low prevalence rate, most patients with advanced disease experience tumor recurrence and die eventually [1]. EOCs consist of a group of molecularly heterogeneous neoplasia with diverse sets of genetic and epigenetic changes, and can be classified into two groups, type I and II, proposed recently [2]. Despite enormous molecular diversity among EOC types, there is little information available on the mechanism of ovarian carcinogenesis.
Normal human cells are characterized by a finite lifespan partly due to a progressive shortening of their telomeres. Meanwhile, carcinogenesis can be triggered through activation of the enzyme responsible for lengthening telomeres, telomerase, which is present in approximately 90% of human cancers [3]. Human telomerase reverse transcriptase (hTERT) is the catalytic subunit of telomerase responsible for telomerase activity and telomere elongation, and increased activity of hTERT is observed in more than 90% of ovarian cancers [4,5].
Akt, a serine/threonine protein kinase, is a major downstream effector of the phosphatidylinositide-3-kinase (PI3K) pathway that is activated through phosphorylation at key amino acid residues Thr-308 and Ser-473 [6]. Akt plays a central role in the coordination of multiple signal transduction processes involved in transcriptional regulation including glucose metabolism, angiogenesis modulation, cell survival, and cell cycle arrest. Because of this regulatory role, Akt is a key factor in many types of cancer [7,8]. One of the substrates of Akt is hTERT, which contains two Akt consensus sites, phosphorylation of which by Akt leads to increased telomerase activity in cancer cells [9].
Expression of the activated forms of Akt and hTERT has been used as a prognostic factor in several types of cancer including EOC. However, the relationship between pAkt and hTERT in carcinogenesis is not fully understood, and the significance of pAkt and hTERT expression has not been thoroughly evaluated in EOC. In the current study, we investigated the expression of pAkt and hTERT, evaluated their relationship with each other, and determined their prognostic value in EOC.
Materials & methods
Patients
Patients who underwent standard treatment, debulking surgery followed by platinum-based chemotherapy, for EOC between 2005 and 2012 at Seoul National University Hospital were selected for this retrospective study. Ninety-two formalin-fixed, paraffin-embedded patient samples were retrieved. Available data included the patients’ age, tumor stage, tumor grade, tumor histology, and clinical response (Table 1).
Table 1.
Characteristics of the epithelial ovarian cancer patients
| Number of patients (%) | |
|---|---|
| Mean age (year, range) | 49.8 (15-79) |
| FIGO stage | |
| I | 26 (28.3) |
| II | 6 (6.5) |
| III | 51 (55.4) |
| IV | 9 (9.8) |
| Histology | |
| Serous | 52 (56.5) |
| Mucinous | 12 (13.0) |
| Endometrioid | 16 (17.4) |
| Clear cell | 8 (8.7) |
| Others | 4 (4.3) |
| Grade | |
| 1 | 13 (14.1) |
| 2 | 20 (21.7) |
| 3 | 59 (64.1) |
| Clinical response | |
| Complete response | 64 (69.6) |
| Partial response | 11 (12.0) |
| Stable disease | 9 (9.8) |
| Progressive disease | 8 (8.7) |
Immunohistochemical analysis for pAkt and hTERT
Serial sections from paraffin-embedded EOC tissues were cut at 4 µm for hematoxylin and eosin staining and subsequent pAkt and hTERT immunohistochemical analyses. Samples were deparaffinized in xylene, rehydrated with graded ethanol, and washed in PBS. The sections were then placed in 10 mM citrate buffer (pH 6.0) and boiled in a microwave for epitope retrieval. Endogenous peroxidase activity was quenched by incubating tissue sections in 3% H2O2 for 10 min. Prepared sections were incubated with a primary rabbit anti-hTERT antibody (Rockland, Gilbertsville, PA, USA) at a dilution of 1:200 and a primary rabbit anti-pAkt antibody (pSer/Thr, Cell Signaling, Danvers, MA, USA) at a dilution of 1:200 in a humidifying chamber at 4°C overnight. Sections were then washed in PBS for 5 min at room temperature and subsequently stained by the labeled streptavidin biotin (LSAB) method using a Dako LSAB kit (Dako) and visualized using 3,3’-diaminobenzidine. The sections were then counterstained with hematoxylin.
The negative controls were stained using the same method, except that the primary antibody incubation step was omitted. Microscopic fields from each stained section were randomly sampled. For immunohistochemistry (IHC) scoring of hTERT, only nuclear staining for telomerase was considered positive. The IHC scores were determined as follows: 0 = no staining (negative), +1 = 1%-10% of the cells were positive, +2 = 11%-30% of the cells were positive, and +3 = > 30% of the cells were positive. Cytoplasmic or cell membrane staining for pAkt was considered positive. Each case was interpreted as pAkt-positive by IHC if staining was observed in more than 10% of all neoplastic cells [10].
Statistical analysis
The relationships between categorical variables were assessed using χ2 tests and ANOVA, and continuous variables were evaluated using a Spearman test. Disease-free survival (DFS) and overall survival (OS) were estimated using the Kaplan-Meier method, and the differences in survival were compared using the log-rank test. Multivariate analysis was performed using the Cox regression method. A P-value < 0.05 was considered statistically significant. The data were analyzed using SPSS software, version 17.0 (IBM, Chicago, IL, USA).
Results
Patient characteristics
The mean age of the patients was 49.8 years, and more than half (n = 60, 65.2%) were diagnosed with advanced stage EOC (FIGO III and IV). The most common histology was serous type (56.5%). Although the majority of patients (n = 75, 81.6%) experienced a clinical response, 50 patients (54%) experienced a relapse during a median follow-up period of 35 months (range, 5-112 months). Detailed characteristics of the patients are described in Table 1.
Expression of pAkt and hTERT in EOC and clinicopathological significance
We evaluated 92 tumors with respect to pAkt and hTERT expression and patient clinical outcome. In total, 73.9% (n = 68) and 56.5% (n = 52) of tumors were considered positive for pAkt and hTERT expression, respectively (Figure 1). Although there was no significant association between the expression of pAkt and hTERT (Table 2), the expression of pAkt correlated with the histological tumor grade (P = 0.045). However, no significant correlation was identified between pAkt or hTERT expression and other clinicopathological parameters (Table 3).
Figure 1.
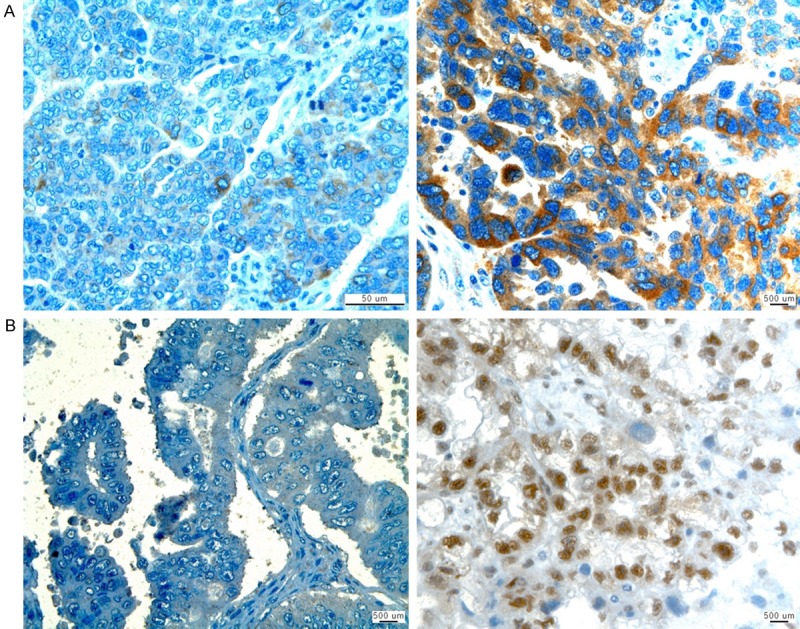
Immunohistochemical negative (left) and positive (right) staining of (A) pAkt and (B) hTERT in epithelial ovarian cancer tissue. ×400 original magnification.
Table 2.
Correlation between hTERT and pAkt expression in epithelial ovarian cancer patients
| hTERT (+) | hTERT (-) | P-value | |
|---|---|---|---|
| pAkt (+) | 37 | 31 | 0.633 |
| aAkt (-) | 15 | 9 |
Table 3.
Correlation between hTERT and pAkt expression, and the clinicopathological parameters in epithelial ovarian cancer patients
| hTERT expression (%) | pAkt expression (%) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| (+) | (-) | P-value | (+) | (-) | P-value | |
| Age | ||||||
| < 55 | 34 (37.0) | 26 (28.3) | 0.969 | 42 (45.7) | 18 (19.6) | 0.242 |
| ≥ 55 | 18 (19.6) | 14 (15.2) | 26 (28.3) | 6 (6.5) | ||
| FIGO stage | ||||||
| Early (I, II) | 17 (18.5) | 15 (16.3) | 0.631 | 24 (26.1) | 8 (8.7) | 0.862 |
| Advanced (III, IV) | 35 (38.0) | 25 (27.2) | 44 (47.8) | 16 (17.4) | ||
| Histology | ||||||
| Serous | 30 (32.6) | 19 (20.7) | 0.331 | 37 (40.2) | 12 (13.0) | 0.710 |
| Non-serous | 22 (23.9) | 21 (22.8) | 31 (33.7) | 12 (13.0) | ||
| Grade | ||||||
| 1 | 8 (8.7) | 5 (5.4) | 0.694 | 2 (2.2) | 11 (12.0) | 0.045 |
| 2 or 3 | 44 (47.8) | 35 (38.0) | 66 (71.7) | 13 (14.1) | ||
Prognostic value of pAkt and hTERT expression in EOC patients
Expression of pAkt or hTERT was not significantly associated with the DFS or OS of the patients after a median follow-up period of 35 months. However, patients with coexpression of pAkt and hTERT exhibited a significantly shorter DFS (P = 0.013). By contrast, coexpression of pAkt and hTERT was not associated with OS (P = 0.448) (Figure 2). By univariate analysis, other clinicopathological parameters were associated with patient survival including tumor stage, histology, and tumor grade. Subsequently, a multivariate Cox regression analysis indicated that the following factors significantly and independently contributed to a decrease in DFS: coexpression of pAkt and hTERT (P = 0.002), advanced tumor stage (P < 0.001), and high tumor grade (P = 0.044) (Table 4).
Figure 2.
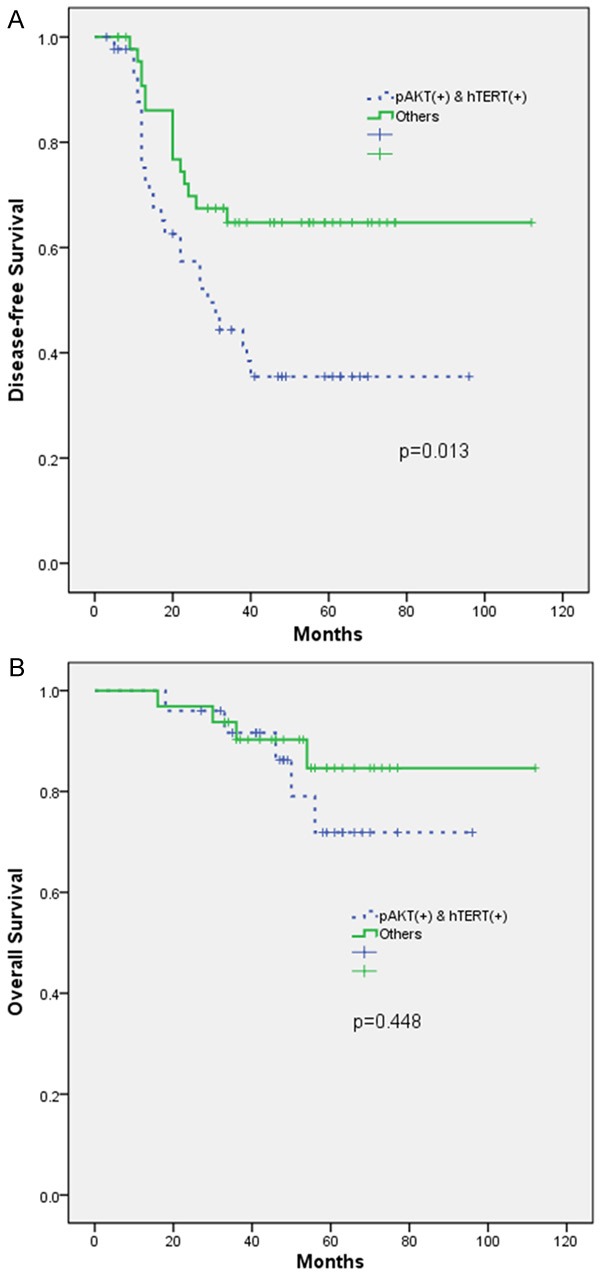
Kaplan-Meier analyses for (A) disease-free survival (DFS) and (B) overall survival in epithelial ovarian cancer patients. High expression of both pAkt and hTERT was significantly associated with shorter DFS (P = 0.013).
Table 4.
Multivariate Cox regression analyses for the parameters associated with disease-free survival
| Relative risk | 95% CI | P-value | |
|---|---|---|---|
| FIGO stage | 23.231 | 5.166-104.469 | < 0.001 |
| Histology | 1.101 | 0.512-2.368 | 0.805 |
| Grade | 2.282 | 1.023-5.087 | 0.044 |
| pAkt/hTERT | 2.864 | 1.463-5.606 | 0.002 |
Discussion
In this study, expression of pAkt or hTERT showed little association with clinicopathological parameters or survival; however, the coexpression of pAkt and hTERT significantly correlated with prognosis of EOC patients.
Overexpression of the PI3K/Akt/mTOR pathway proteins is well known in several cancers [11]. Although mutation of Akt is rare (< 5%) in serous EOC, immunohistochemical analyses have shown that the PI3K/Akt/mTOR pathway is activated in half of these tumors [12,13]. In support of this, the current study demonstrated activation of Akt in over half of the EOC patient samples; however, it did not demonstrate a significant association between pAkt expression and any clinicopathological parameter except for tumor grade. Although some studies have indicated the clinical value of pAkt in ovarian cancers, others, including the current study, have not [14]. The results of previous studies regarding the prognostic value of pAkt expression in EOC patients are conflicting. For example, the expression of pAkt was found to negatively correlate with the prognosis of EOC [15], which is in contrast to the results demonstrated by other researchers [14,16]. This study demonstrated that pAkt expression was not associated with DFS or OS in EOC patients.
Although telomerase is considered an important factor in the carcinogenesis of EOC, the prognostic value of its expression is still unclear. Overexpression of hTERT occurs in the majority of serous ovarian carcinomas, and is associated with a poor prognosis [17]. However, the immunohistochemical detection of telomerase may not provide meaningful information related to the typing of ovarian tumors [18]. Furthermore, Widschwendter et al. reported that quantitative hTERT expression was not associated with EOC patient survival [19]. Although telomerase expression does not directly cause cancer, it contributes to the long life span of cancer cells and subsequently, increased tumor formation [20].
Telomerase comprises hTERT, the human telomerase RNA component (TERC), and the protein dyskerin. Among them, hTERT is considered the rate-limiting component, and is regulated by both transcriptional and post-transcriptional factors [4]. Transcriptionally, hTERT is phosphorylated at various sites by different kinases, including Akt, phosphorylation by which can increase the activity of telomerase [9,21]. Therefore, interaction between Akt and hTERT could play an important role in cancer cell survival. Supporting this, the phosphorylation of Akt was reported to be a key event in the induction of telomerase activity in human ovarian cancer cells [22].
The current study indicated that individual expression of pAkt and hTERT did not significantly correlate with patient survival. However, coexpression of pAkt and hTERT positively correlated with an increased risk of tumor relapse. Similarly, coexpression of pAkt and hTERT is associated with a poor prognosis in gastric cancer despite no association between clinicopathological parameters and the individual expression of these proteins [23]. To date, data regarding the prognostic value of pAkt and hTERT in EOC are inconclusive. In our study, we found that coexpression of pAkt and hTERT was an independent prognostic factor of poor DFS for EOC patients. These data are the first to document a prognostic role for coexpression of pAkt and hTERT in EOC.
In conclusion, we report the frequent expression of pAkt and hTERT in EOC on IHC. The coexpression of pAkt and hTERT is associated with poor prognosis, suggesting that Akt and hTERT could be potential therapeutic targets in EOC.
Acknowledgements
All experiments were approved by the Seoul National University Hospital IRB (1008-119-329). This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (2013R1A1A3012912), Cancer Control program by the Korean Ministry of Health and Welfare (1220210), and Seoul National University Hospital Fund (0420070620).
Disclosure of conflict of interest
None.
References
- 1.Chan JK, Cheung MK, Husain A, Teng NN, West D, Whittemore AS, Berek JS, Osann K. Patterns and progress in ovarian cancer over 14 years. Obstet Gynecol. 2006;108:521–528. doi: 10.1097/01.AOG.0000231680.58221.a7. [DOI] [PubMed] [Google Scholar]
- 2.Kurman RJ, Shih IeM. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer--shifting the paradigm. Hum Pathol. 2011;42:918–31. doi: 10.1016/j.humpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shay JW, Zou Y, Hiyama E, Wright WE. Telomerase and cancer. Hum Mol Genet. 2001;10:677–685. doi: 10.1093/hmg/10.7.677. [DOI] [PubMed] [Google Scholar]
- 4.Cong YS, Wright WE, Shay JW. Human telomerase and its regulation. Microbiol Mol Biol Rev. 2002;66:407–425. doi: 10.1128/MMBR.66.3.407-425.2002. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–791. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 6.Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol. 1998;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- 7.Bellacosa A, Kumar CC, Di Cristofano A, Testa JR. Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv Cancer Res. 2005;94:29–86. doi: 10.1016/S0065-230X(05)94002-5. [DOI] [PubMed] [Google Scholar]
- 8.Cicenas J. The potential role of Akt phosphorylation in human cancers. Int J Biol Markers. 2008;23:1–9. doi: 10.1177/172460080802300101. [DOI] [PubMed] [Google Scholar]
- 9.Kang SS, Kwon T, Kwon DY, Do SI. Akt protein kinase enhances human telomerase activity through phosphorylation of telomerase reverse transcriptase subunit. J Biol Chem. 1999;274:13085–90. doi: 10.1074/jbc.274.19.13085. [DOI] [PubMed] [Google Scholar]
- 10.Sangawa A, Shintani M, Yamao N, Kamoshida S. Phosphorylation status of Akt and caspase-9 in gastric and colorectal carcinomas. Int J Clin Exp Pathol. 2014;7:3312–3317. [PMC free article] [PubMed] [Google Scholar]
- 11.Markman B, Atzori F, Perez-Garcia J, Tabernero J, Baselga J. Status of PI3K inhibition and biomarker development in cancer therapeutics. Ann Oncol. 2010;21:683–691. doi: 10.1093/annonc/mdp347. [DOI] [PubMed] [Google Scholar]
- 12.Altomare DA, Wang HQ, Skele KL, De Rienzo A, Klein-Szanto AJ, Godwin AK, Testa JR. AKT and mTOR phosphorylation is frequently detected in ovarian cancer and can be targeted to disrupt ovarian tumor cell growth. Oncogene. 2004;23:5853–5857. doi: 10.1038/sj.onc.1207721. [DOI] [PubMed] [Google Scholar]
- 13.Verhaak RG, Tamayo P, Yang JY, Hubbard D, Zhang H, Creighton CJ, Fereday S, Lawrence M, Carter SL, Mermel CH, Kostic AD, Etemadmoghadam D, Saksena G, Cibulskis K, Duraisamy S, Levanon K, Sougnez C, Tsherniak A, Gomez S, Onofrio R, Gabriel S, Chin L, Zhang N, Spellman PT, Zhang Y, Akbani R, Hoadley KA, Kahn A, Kobel M, Huntsman D, Soslow RA, Defazio A, Birrer MJ, Gray JW, Weinstein JN, Bowtell DD, Drapkin R, Mesirov JP, Getz G, Levine DA, Meyerson M. Prognostically relevant gene signatures of high-grade serous ovarian carcinoma. J Clin Invest. 2013;123:517–525. doi: 10.1172/JCI65833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woenckhaus J, Steger K, Sturm K, Munstedt K, Franke FE, Fenic I. Prognostic value of PIK3CA and phosphorylated AKT expression in ovarian cancer. Virchows Arch. 2007;450:387–395. doi: 10.1007/s00428-006-0358-3. [DOI] [PubMed] [Google Scholar]
- 15.Jia W, Chang B, Sun L, Zhu H, Pang L, Tao L, Zou H, Du J, Dong Y, Qi Y, Jiang J, Liang W, Li F, Zhao X. REDD1 and p-AKT over-expression may predict poor prognosis in ovarian cancer. Int J Clin Exp Pathol. 2014;7:5940–5949. [PMC free article] [PubMed] [Google Scholar]
- 16.Rahman M, Nakayama K, Rahman MT, Nakayama N, Ishikawa M, Katagiri A, Iida K, Nakayama S, Otsuki Y, Shih IeM, Miyazaki K. Clinicopathologic and biological analysis of PIK3CA mutation in ovarian clear cell carcinoma. Hum Pathol. 2012;43:2197–2206. doi: 10.1016/j.humpath.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Brustmann H. Immunohistochemical detection of human telomerase reverse transcriptase (hTERT) and c-kit in serous ovarian carcinoma: a clinicopathologic study. Gynecol Oncol. 2005;98:396–402. doi: 10.1016/j.ygyno.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 18.Ozer H, Yenicesu G, Arici S, Cetin M, Tuncer E, Cetin A. Immunohistochemistry with apoptotic-antiapoptotic proteins (p53, p21, bax, bcl-2), c-kit, telomerase, and metallothionein as a diagnostic aid in benign, borderline, and malignant serous and mucinous ovarian tumors. Diagn Pathol. 2012;7:124. doi: 10.1186/1746-1596-7-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Widschwendter A, Muller HM, Hubalek MM, Wiedemair A, Fiegl H, Goebel G, Mueller-Holzner E, Marth C, Widschwendter M. Methylation status and expression of human telomerase reverse transcriptase in ovarian and cervical cancer. Gynecol Oncol. 2004;93:407–416. doi: 10.1016/j.ygyno.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 20.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 21.Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002;14:381–395. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 22.Kimura A, Ohmichi M, Kawagoe J, Kyo S, Mabuchi S, Takahashi T, Ohshima C, Arimoto-Ishida E, Nishio Y, Inoue M, Kurachi H, Tasaka K, Murata Y. Induction of hTERT expression and phosphorylation by estrogen via Akt cascade in human ovarian cancer cell lines. Oncogene. 2004;23:4505–4515. doi: 10.1038/sj.onc.1207582. [DOI] [PubMed] [Google Scholar]
- 23.Sasaki T, Kuniyasu H, Luo Y, Kitayoshi M, Tanabe E, Kato D, Shinya S, Fujii K, Ohmori H, Yamashita Y. AKT activation and telomerase reverse transcriptase expression are concurrently associated with prognosis of gastric cancer. Pathobiology. 2014;81:36–41. doi: 10.1159/000351721. [DOI] [PubMed] [Google Scholar]


